Abstract
Drug resistance is always a great obstacle in any endocrine therapy of breast cancer. Although the combination of endocrine therapy and targeted therapy has been shown to significantly improve prognosis, refractory endocrine resistance is still common. Dysregulation of the Hippo pathway is often related to the occurrence and the development of many tumors. Targeted therapies of this pathway have played important roles in the study of triple negative breast cancer (TNBC). Targeting the Hippo pathway in combination with chemotherapy or other targeted therapies has been shown to significantly improve specific antitumor effects and reduce cancer antidrug resistance. Further exploration has shown that the Hippo pathway is closely related to endocrine resistance, and it plays a “co-correlation point” role in numerous pathways involving endocrine resistance, including related pathways in breast cancer stem cells (BCSCs). Agents and miRNAs targeting the components of the Hippo pathway are expected to significantly enhance the sensitivity of breast cancer cells to endocrine therapy. This review initially explains the possible mechanism of the Hippo pathway in combating endocrine resistance, and it concludes by recommending endocrine therapy in combination with therapies targeting the Hippo pathway in the study of endocrine-resistant breast cancers.
Similar content being viewed by others
Introduction
The incidence of breast cancer ranks first among all female malignancies [1]. Most breast cancers are surely estrogen receptor-positive (ER +) and can depend on estrogen for tumor cell growth. The major treatment strategy for endocrine therapies focused on ER + breast cancer is estrogen deprivation. The current drugs to this end can be classed as aromatase inhibitors, selective estrogen receptor degraders, and selective estrogen receptor modulators. However, about one-third of patients still develop recurrence after long-term endocrine therapy [2]. Drug resistance to endocrine therapy has become a pivotal obstacle to treatment, although endocrine therapy is effective in reducing mortality and improving survival rates [3]. The reasons for drug resistance are multiple and complex, and they involve various molecules. Currently, targeted therapies combined with endocrine therapy have been shown to be effective for combating endocrine resistance, as illustrated in Table 1, which includes targets of Cyclin-dependent kinases (CDKs) 4 and 6, mammalian targets of rapamycin (mTOR) and phosphoinositide 3-kinase (PI3K). Their combination as first or second-line treatments for hormone receptor-positive metastatic breast cancer has been recommended by AMA guidelines, which was corroborated by a network meta-analysis [4]. Unfortunately, the combination of CDK 4 and 6 inhibitors still does not completely overcome drug resistance [5, 6]. The Hippo pathway, a newly targeted pathway, has been found to be related to multiple malignancies and modulation of this pathway may be able to effectively overcome endocrine resistance. In this review, the therapeutic potential of the Hippo pathway in the promotion of endocrine therapy is addressed, which provides theoretical references for the design of further studies and clinical consequences.
Mechanism of endocrine resistance in breast carcinoma
The complex reasons of endocrine resistance involves the estrogen receptor (ER) pathway, the growth factor receptor (GFR) pathways, and the CDK 4 and 6 pathway, as well as epigenetic modification. In recent years, the interdependence between BCSCs and drug resistance has emerged, and it often involves BCSC-related pathways, offering new insight into endocrine resistance.
Refractory resistance facilitated by escaped BCSCs
BCSC-related markers, such as CD44+CD24−/low [12], ALDH [13], CD133 [14],Nanog [15], Sox2 [12], and Sox9 [16], were found to be enriched or positive in tamoxifen-resistant [12,13,14,15,16], letrozole-treated [17], and fulvestrant-treated cells [18]. The proportion of cells bearing stem cell markers in resistant cell line stronger increased than that of non-resistant [12]. On the one hand, dormant and self-renewal deficient BCSC populations are generated during long-term endocrine therapy, leading to endocrine resistance after these cells exit from metabolic dormancy [19]. On the other hand, ER activity affects the enrichment of BCSCs via mutations in the ESR1 gene (estrogen receptor-gene), including Y537N, Y537S, and D598G, which are involved in ligand-independent activation of ER [20]. Moreover, ER-α36 [21], ERβ [22], and G protein-coupled estrogen receptor (GPER) [23] are all involved in the promotion or maintenance of breast cancer stem cell character. Moreover, BCSC populations are considered to be estrogen receptor negative (ER −) and can regulate ER expression [24, 25], giving them the ability to generate ER + cells [26] or differentiate into cells that have lost ERα expression [27]. Therefore, the core reason for drug resistance is the stem cell behavior of breast cancers.
The interaction between ER signaling, GFR signaling, and BCSC-related pathways
BCSC-related pathways include the Hippo pathway, and those that signal via Hedgehog (Hh) signaling, transforming growth factor β (TGF-β) signaling, Notch signaling, PI3K/Akt/mTOR pathway, Wnt pathway, epidermal growth factor receptor (EGFR) pathway, ER signaling, and mitogen-activated protein kinase (MAPK) pathway. GFR signaling includes human epidermal growth factor receptor 2 (HER2) signaling, fibroblast growth factor receptor 1 (FGFR) signaling, and PI3K/Akt pathway, and they are three BCSC-related pathways. And other GFR signaling pathways are insulin-like growth factor 1 receptor (IGF-1R) signaling, MAPK signaling, and EGFR pathway. ER signaling is considered to be the main source of driving forces for the growth of ER + breast carcinomas. Changes in ER signaling are the first step for acquiring endocrine resistance, and this can include downregulation of ERα expression or loss of ERα expression caused by mutation or methylation of ERα-related genes [28]. These driving forces convert from ER signaling to GFR signaling [29, 30], as described in Fig. 1. The cross talk between GFR signaling and BCSC-related pathways may be the next step that leads to refractory resistance [28, 31, 32]. The preliminary relationship between part of BCSC-related pathways and endocrine resistance is diverse and complex, as illustrated in Table 2. And several signaling pathways of GFR are also BCSC-related pathways in this table. This reveals the dual roles of PI3K/Akt/mTOR, EGFR and MAPK pathways involve in endocrine resistance and breast cancer stem cell character. Moreover, it also reveals the extensive and complex role about BCSCs and BCSC-related pathways for endocrine resistance. BCSCs can depend on the GFR signaling pathway to survive, and escape from estrogen deprivation based on their ER − status [29, 33, 34]. Eventually, BCSCs will become the root cause of refractory resistance and escort breast cancers toward “permanent survival”. The combinations of targeted therapies focused on IGF-1R [35], HER2 [36, 37], or epidermal growth factor receptor [38,39,40] and endocrine therapy, even dual-targeting therapies plus endocrine therapy, have been shown not to reverse endocrine resistance or significantly enhance the effects of endocrine therapy. It has been suggested that breast cancers can still maintain endocrine resistance through the synergy of other pathways after inhibiting a small portion of these pathways. In conclusion, the process of endocrine resistance in breast carcinomas is the synergy of multiple mechanism. A new solution needs to be conceived.
Illustration of preliminary mechanisms driving endocrine resistance in breast cancer. ER signaling drives cell growth in breast cancer. These driving forces convert from ER signaling to GFR signaling, with the interruption or the gradual reduction of ER signaling and the increase of GFR signaling. Interactions between GFR signaling and BCSC-related pathways will form stronger driving forces and lead to refractory breast cancer
The Hippo pathway
The Hippo pathway is also called the Salvador/Warts/Hippo pathway, and can precisely control the number of cells and stop organism growth in a timely manner during the development of mammals.
Hippo pathway and its dysregulation
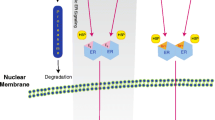
When the phosphorylation cascades of the Hippo pathway become blocked, these cells will differentiate abnormally and gradually develop into malignancies [70]. Then, inhibited or disabled Hippo phosphorylation cascades of Hippo pathway will further facilitate the invasion and migration of tumor cells [71, 72]. The mechanism of the canonical Hippo pathway and its dysregulation is described in Fig. 2. The activated Hippo pathway participates in the reasonable regulation of apoptosis and cell growth, when transcriptional coactivator with PDZ-binding motif (TAZ), and homologous component the yes-associated protein (YAP) are inactivated via their phosphorylation cascade. A variety of upstream signals activate mammalian sterile20-like (MST) kinases, and then MST kinases phosphorylate large tumor suppressor (LATS) kinases [73]. The activated LATS1/2 kinases can change the status of the phosphorylation and distribution of YAP/TAZ [74] resulting in the arrest of the cell cycle [75, 76]. LATS [77]and MST [73] are considered to have antitumor effects, and play negative roles in the regulation of TAZ and YAP. The entry of phosphorylated YAP/TAZ into the nucleus is restricted, and YAP/TAZ will be degraded after transferring into the cytoplasm from the nucleus, which can turn off antiapoptotic gene transcriptions and the cell cycle progression [78]. Conversely, when the Hippo pathway is dysregulated, the expression of downstream target genes of the Hippo pathway will promote cell proliferation and inhibit apoptosis genes, facilitating malignancies [79]. Dysregulation of the Hippo pathway showed results in excessive activation [80] or increased nuclear localization of YAP/TAZ by relatively decreasing YAP/TAZ phosphorylation [81], inhibiting LATS [82] and MST [83, 84]. Therefore, activated YAP and homologous TAZ are the core regulators of this pathway, since both of them exert oncogenic roles [85, 86] in the presence of a dysregulated Hippo pathway. Moreover, the low expression of MST and LATS will lead to the loss of control of YAP and TAZ [87]. Beyond this, additional non-canonical roles of the Hippo pathway in breast cancer have gradually emerged in recent years.
Illustration of the canonical Hippo pathway and its dysregulation. MST1/2 and LATS1/2 are kinases. SAV1 (WW45) and MOB1A/B are adaptors. The phosphorylation cascade in the Hippo pathway can negatively regulate YAP/TAZ and inhibit the proliferation-promoting effects of YAP/TAZ. Dysregulation leads to proliferation, invasion, and migration of malignancies via the nuclear translocation of YAP and TAZ
Breast cancer stem cell character suppression by the Hippo pathway
It has been confirmed that TAZ, YAP, LATS, and TEA domain family members (TEAD) are involved in the regulation of BCSCs. Cordenonsi et al. [88] first linked the Hippo pathway to the concept of BCSC proliferation. Since then, studies have confirmed that BCSCs can be facilitated by TAZ [89,90,91,92,93]. YAP [94,95,96,97], as a homology of TAZ, the same as LATS [98] or TEAD [99], can also regulate BCSCs. Liu et al. [90] showed the restoration of sensitivity to tamoxifen and suppressed BCSCs by inhibiting TAZ expression. Moreover, dysregulation of the Hippo pathway is one of the prerequisites for the progress of an epithelial-mesenchymal transition (EMT) [100], which enables tumor to maintain the stem cell character [88]. MiR-520b activates the Hippo/YAP signaling pathways by targeting LATS, increasing the mRNA level of BCSC markers, such as CD133, CD44, and ALDH1, and the EMT marker N-cadherin in breast cancer [94]. Therefore, reactivating the Hippo pathway can effectively combat endocrine resistance and the expression of several oncogenes facilitated by BCSC transition.
The cross talk between the Hippo pathway and multiple pathways involved in endocrine resistance
Breast cancer can rely on ER signaling to promote cell proliferation, which is different from other tumors. The Hippo pathway is indeed associated with almost all pathways related to endocrine resistance, including ER signaling. Researchers have found partial cross talk between the Hippo pathway and ER signaling. Recent studies have found that targeting and activating the Hippo pathway can negatively regulate BCSCs and overcome endocrine resistance. Moreover, it also seems to be involved in the integration of GFR signaling pathways and Hippo pathway. This indicates that the Hippo pathway may make an unexpected contribution to endocrine therapy in ER + breast cancers.
The correlation between the Hippo pathway and the ER signaling pathway
Preliminary non-canonical roles between the Hippo pathway and ER signaling in breast cancer is described in Fig. 3. On the one hand, the non-canonical roles of the components in Hippo pathway play a vital role in the management of ER signaling. YAP1 and TEAD4, co-regulators of ERα on enhancers, are augmented upon estrogen stimulation and transduction of target genes of ER signaling [101]. In addition, the expression of ERα was shown to directly be increased by YAP1 or indirectly mediated by the fork head box protein M1 (FOXM1) in the absence of the tumor suppressor Ras-association domain family 1 [102]. YAP/TAZ have also been shown to mediate the process of target gene induction by GPER [103]. On the other hand, ER can also affect the Hippo pathway. A study on mouse morula and trophoblast stem cells found that the nuclear localization of YAP was indeed regulated by ERα [104]. Moreover, the invasion and migration of TNBC were inhibited when the nuclear localization of YAP was inhibited, while sometimes the same situation did not appear in ER + breast cancer, which may have been related to the compensatory increase in the nuclear localization of YAP mediated by ER [105]. The status of ER can also affect the interaction between cellular retinoic acid binding protein 2 (CRABP2) and LATS to regulate the Hippo pathway and modulate sensitivity to endocrine therapy [106]. Moreover, GPER facilitates the progression of breast cancer by activating YAP/TAZ [103]. A study of tumor breast (226 samples) and normal (40 samples) from microarray samples found that the level of YAP expression was evidently downregulated in invasive cancer samples compared to normal tissues samples, and decreased expression of YAP was remarkably associated with ER − status [107]. This suggested that invasive breast cancer cells with reduced expression of YAP were more likely to be ER − and may have an lower threshold for becoming resistant to endocrine therapy. Feedback regulation of hormone receptors on the Hippo pathway in turn was weakened by the downregulation of ERα expression, and also was decreased by downward fluctuation of YAP. It may be the explanation for the lower YAP levels in invasive breast cancer are relative to normal breast tissue. The non-canonical Hippo pathway can in turn act on ER receptors to antagonize endocrine therapy, which can eventually leads to driving forces for tumor growth conversion to GFR signaling after estrogen-deprivation therapy. The regulation of the Hippo pathway for breast cancers dependent on different ER status and the different stage of endocrine resistance may be different [98, 106]. Thus, the study of the Hippo pathway in breast cancer cannot be generalized like other hormone-independent tumors.
Antagonism of the Hippo pathway for endocrine resistance in ER + breast cancer
Studies in the MCF7 cell line (ER-positive breast cancer) have confirmed that correcting dysregulation of the Hippo pathway is a feasible scheme to inhibit breast cancer and overcome acquired drug resistance. The roles of the Hippo pathway in ER + breast carcinoma are described in Fig. 4. In summary, YAP and TAZ surely are carcinogenic. Although the regulation of Hippo has been rarely studied in endocrine-resistant cells, the great prospect for modulation of the Hippo pathway has become an important research object. Zhou et al. [103] found that GPER’s role in inducing endocrine resistance could be regulated by the Hippo pathway and found that TAZ was overexpressed in GPERhi breast cells. GPER activated YAP/TAZ, suggesting that blockage of GPER by knockdown of YAP/TAZ was a great strategy for overcoming tamoxifen resistance in GPERhi breast cancers. Moreover, Zheng et al. [108] found that the YAP-glycolysis axis was also a target for overcoming tamoxifen resistance, based on the fact that the Hippo pathway was downstream of GPER. Li et al. [109] confirmed that downregulated YAP phosphorylation and upregulated YAP nuclear translocation directly resulted in tamoxifen resistance, which was reversed by YAP silencing.
Illustration of the roles of the Hippo pathway in ER + breast cancer. Overexpression of ZNF367 facilitates metastasis and activates Hippo/YAP signaling by inhibiting LATS [110]. Overexpression of USP9X stimulates cell proliferation by deubiquitinating and stabilizing YAP1 [111]. NE and EPI suppress breast cancer via rapid phosphorylation and cytoplasmic retention of YAP [112]. ZEB1, a transcriptional activator, interacts with YAP1 and promotes transcription [113]. The interaction of LATS1 and CRABP2 inhibits the ubiquitination of LATS1 to suppress cell invasion [106]. The STARD13-correlated ceRNA network regulates TAZ distribution, and it can inhibit the stem cell character of breast cancer through upregulation of LATS1/2 [114]
Cross talk between the Hippo pathway and BCSC-related pathways
The Hippo pathway plays a “co-correlation point” role in several networks of BCSC-related pathways, including Notch, Wnt, EGFR, PI3K/Akt, MAPK/ERK1, Hh/GLI2,TGF-β pathway. Clara et al. [85] first proposed that the Hippo pathway may be the “hub” of cancer stem cell related pathways. The cross talk between Hippo and BCSC-related pathways except ER signaling has been shown as described in Fig. 5. Hippo pathway does crosstalk these seven pathways, and there forms “bridges” between Hippo, Wnt, EGFR and PI3K/Akt pathways through WBP2. And these “bridges” do enable GFR signaling pathways and BCSC-related pathways to form crosstalk. Extensive integration and local interlinkages reveal the advantages of Hippo pathway in regulation of drug resistance through breast cancer stem cell character. In addition, Hippo, TGF-β, Hh, MAPK, Wnt, PI3K/Akt, Notch, EGFR, and ER signaling were all associated with the EMT process. It has been further revealed that these pathways facilitate the synergistic regulation of breast cancer and can cause endocrine resistance in breast cancer. It is of no doubt, then, that the dysregulation of the Hippo pathway indeed facilitated the progression of breast cancer, and exert intricate cross talk on BCSC-related pathways. BCSCs are the key to maintaining refractory survival and drug resistance for tumor cells, which is based on the coregulation of these pathways. Therefore, the “co-correlation point” role of the Hippo pathway in BCSC-related pathways may highlight a new solution for overcoming endocrine resistance.
Illustration of the crosstalk of the Hippo pathway and BCSC-related pathways. Linc-OIP5 promotes transcription via forming a positive feedback circuit between YAP and Notch signaling [115]. IMP3 indirectly promotes Wnt5B via miR145-5p and facilitates TAZ-driven gene expression [116]. YAP and TAZ can promote the Wnt/β-catenin/TCF axis and induce target genes by interaction with β-catenin, while WBP2 integrates the Hippo, Wnt, and PI3K pathway [117,118,119]. Mir-613 inhibits EGFR via directly inhibiting WBP2 and positive correlation of EGFR and WBP2 is confirmed, while EGFR promotes WBP2 phosphorylation, contributing to integration of the Wnt/β-catenin and Hippo pathways [118, 119]. YAP/TAZ mediate the synergistic function and oncogene expression induced by the PI3K and dysregulated Hippo pathways [120]. The MAPK/ERK1 pathway negatively regulates breast cancer proliferation by inhibiting YAP/TEAD [121]. YAP induces gene transcription and promotes glycolysis by wiring up the Hh/GLI2 axis [108]. The SnoN oncoprotein exerts negative feedback regulation on TGF-β signaling, while promoting TAZ signaling and enhancing gene transcription in breast cancers [122]. Zyxin forms a ternary complex with LATS and Siah, which facilitates the degradation of LATS, activation of YAP and subsequently cell proliferation [123]. The tumor suppressor Merlin can inhibit YAZ/TAZ and maintain Smad7 stability, suppressing the adaptive glycolysis facilitated by the interaction between YAP/TAZ and Smads [124]. Ski inhibits breast cancer by suppressing TAZ in a LATS-dependent manner or in a LATS-independent manner, in which NCoR1 is recruited by Ski and suppresses TAZ by binding to the TEAD-TAZ complex [125]
The interrelation between the Hippo pathway, GFR, and the Cyclin-dependent kinase 4 and 6 pathways
The rest of GFR signaling pathways and Cyclin-dependent kinase 4 and 6 pathways involved in endocrine resistance also exert cross talk on the Hippo pathway. With regards to patients with luminal B subtypes, samples with low TAZ resulted in higher pathological complete response rates after trastuzumab-based neoadjuvant therapy, suggesting that HER2 linked with TAZ expression in a consistent manner [126]. YAP/TAZ dephosphorylation and overexpression increased in trastuzumab-resistant breast cancer cells, suggesting that the dysregulated Hippo pathway further facilitated cancer cells in coordination with HER2 [127]. Inhibiting YAP and TAZ could eliminate Lapatinib resistance, suggesting that dual target therapy for HER2 and the Hippo pathway had good prospects [128]. The Hippo pathway mediated FGFR signaling, the MAPK pathway, and PI3K signaling during tumorigenesis, and YAP/TAZ were shown to be possible therapeutic targets in RTK-driven cancers [129]. The phosphorylation of MST1 depended on the activity of fibroblast growth factor receptor 4 kinase. Moreover, short-term suppression or knockdown of FGFR4 led to increased activation of MST1/2 [130]. The IGF-1R/ YAP axis has been shown to be involved in the growth of TNBC [131]. Dysregulation of the Hippo pathway also can increase resistance to CDK4/6 inhibitors through accumulation of TAZ and YAP transcription factors on the promoter of Cyclin-dependent kinase 6 [5]. These studies further demonstrated the great potential of the Hippo pathway for remedying breast cancer.
Integration of Hippo pathway in complex mechanism of endocrine resistance
These roles of Hippo pathway in the integration of ER signaling are unique to breast cancers. The regulation of Hippo pathway on cancer stem cells is also affirmed, including BCSCs. Hippo pathway can not only combat BCSCs, but also can integrate these multiple pathways involve in endocrine resistance, including GFR (PI3K/Akt/mTOR, EGFR, MAPK, HER2, IGF-1R and FGFR),BCSC-related pathways (Wnt, PI3K/Akt/mTOR, Hh, EGFR, Notch, MAPK, TGF-β, ER), and CDK4/6 pathway. The “co-correlation point” role of Hippo pathway in the multiple mechanism of endocrine resistance as described in Fig. 6. To sum up, utilizing the Hippo pathway as a therapeutic target for combating endocrine-resistant breast cancer may be a promising approach.
Agents and miRNAs for research proposals focusing on the Hippo pathway
In the process of endocrine therapy, the main driving forces for tumor growth were related to GFR signaling and even BCSC-related pathways. A dysregulated Hippo pathway did exert cross talk on these endocrine resistance-related pathways. A new therapeutic scheme that could be proposed to combat the “co-correlation point” of these pathways would focus on targeting the Hippo pathway. The combination of endocrine therapy and targeted therapy focusing on the Hippo pathway is expected to significantly improve the prognosis of patients with ER + breast cancers. There are many agents that can target the Hippo pathway, including Pevonedistat, Verteporfin, stains, and Metformin [85, 132]. Statins and Metformin are commonly used to regulate metabolism of lipids and glucoses. In recent years, there are clinical trials targeting the Hippo pathway by them, although no available results. Clinical trials are designed for targeting the Hippo pathway as illustrated in Table 3. Clinical trials of combination of endocrine therapy and agents targeting Hippo pathway for reference as illustrated in Table 4. Moreover, the regulation of miRNAs is also an alternative tool. MiRNAs are related to the Hippo pathway in breast cancers, as illustrated in Table 5 and Fig. 7. Partial miRNAs can also be involved in the regulation of EMT [94, 133,134,135] and the maintenance of stem cell character in breast cancer [94, 134, 135]. MiR-125a-5p [136], the miR-200 family [137], miR-375 [138], and miR-181b [139], have all been recommended as therapeutic agents, since all of them can regulate endocrine resistance and were confirmed to be related to the Hippo pathway in other tumors. Upregulation of miR-125a-5p in tamoxifen resistant MCF7 cells may inhibit the growth of BCSCs by suppression of TAZ, which is an effective promoter of BCSCs. It is expected that the results of preclinical and clinical data will confirm its role in the future.
Illustration of miRNAs in regulation of the Hippo pathway. MiRNAs dysregulate the Hippo pathway by inhibiting LATS and phosphorylation of YAP, or directly upregulating YAP and TAZ. MiR-520b, miR-372, miR-31, miR-93, miR-135b and miR-515-5p are tumor promotors with the dysregulation of the Hippo pathway. On the contrary, miR-199a-3p, miR-591, miR-18a, miR-506, miR-424, miR-146b, miR-574-5p, miR-205, miR-125a-5p, miR-1297 and miR-326 are tumor suppressors with the activation of the Hippo pathways
Conclusion and prospects
Targeting the Hippo pathway has been researched in a variety of tumors and has been shown to have remarkable results. The combination of targeted therapy for the Hippo pathway and chemotherapy or other targeted therapies has also achieved initial results in breast cancers. Although its dysregulation can promote the occurrence and progression of tumors, reasonable regulation of this pathway can effectively inhibit tumors and combat endocrine resistance. However, the Hippo pathway is powerful, diverse, and complex. Breast cancers differ from other tumors, in that the former is derived from more of the non-canonical roles of the Hippo pathway via hormone receptors. More studies are needed to verify the feasibility and risk of regulation of the Hippo pathway in endocrine-resistant breast cancer. Moreover, our laboratory has shown that aromatase inhibitors such as Formestane can rely on ER-independent but androgen receptor-dependent roles to suppress ER + breast cancer, suggesting that aromatase inhibitors may be highly recommended as promising agents combined with targeting the Hippo pathway to significantly overcome endocrine resistance stimulated by estrogen-deprivation therapy in postmenopausal women [157]. Targeting the Hippo pathway will create promising new tools in the fight against endocrine-resistant breast cancer.
Availability of data and materials
Not applicable.
Abbreviations
- TNBC:
-
Triple negative breast cancer
- FOXM1:
-
Fork head box protein M1
- BCSCs:
-
Breast cancer stem cells
- USP9X:
-
Ubiquitin-specific protease 9X
- ER + :
-
Estrogen receptor-positive
- CDKs:
-
Cyclin-dependent kinases
- mTOR:
-
Mammalian target of rapamycin
- WBP2:
-
WW domain-binding protein 2
- E2:
-
Estradiol
- PI3K:
-
Phosphoinositide 3-kinase
- ER:
-
Estrogen receptor
- GFR:
-
Growth factor receptor
- PFS:
-
Progression-free survival
- GPER:
-
G protein-coupled estrogen receptor
- ER − :
-
Estrogen receptor negative
- Hh:
-
Hedgehog
- TGF-β:
-
Transforming growth factor β
- IMP3:
-
Insulin-like growth factor-2 mRNA-binding protein 3
- EGFR:
-
Epidermal growth factor receptor
- ERE:
-
Estrogen response element
- MAPK:
-
Mitogen-activated protein kinase
- CRABP2:
-
Cellular retinoic acid binding protein 2
- HER2:
-
Human epidermal growth factor receptor 2
- FGFR:
-
Fibroblast growth factor receptor 1
- NCoR1:
-
Nuclear transcriptional corepressor N-CoR1
- BCAR4:
-
Breast cancer anti-estrogen resistance 4
- IGF-1R:
-
Insulin-like growth factor 1 receptor
- YAP:
-
Yes-associated protein
- Linc-OIP5:
-
Linc-Opa interacting protein 5
- TAZ:
-
Transcriptional coactivator with PDZ-binding motif
- MUC1-C:
-
The mucin 1 C-terminal subunit
- MST:
-
Mammalian sterile20-like
- LATS:
-
Large tumor suppressor
- TEAD:
-
TEA domain family members
- ZNF367:
-
Zinc finger protein 367
- EPI:
-
Epinephrine
- NE:
-
Norepinephrine
- ceRNA:
-
Competing endogenous RNA
- ERK1:
-
Extracellular signal-related kinases 1
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. The Lancet. 2005;365(9472):1687–717. https://doi.org/10.1016/s0140-6736(05)66544-0.
Pennery E. The role of endocrine therapies in reducing risk of recurrence in postmenopausal women with hormone receptor-positive breast cancer. Eur J Oncol Nurs. 2008;12(3):233–43. https://doi.org/10.1016/j.ejon.2008.01.007.
Giuliano M, Schettini F, Rognoni C, Milani M, Jerusalem G, Bachelot T, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20(10):1360–9. https://doi.org/10.1016/S1470-2045(19)30420-6.
Li Z, Razavi P, Li Q, Toy W, Liu B, Ping C, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the Hippo pathway. Cancer Cell. 2018;34(6):893-905e8. https://doi.org/10.1016/j.ccell.2018.11.006.
Pancholi S, Ribas R, Simigdala N, Schuster E, Nikitorowicz-Buniak J, Ressa A, et al. Tumour kinome re-wiring governs resistance to palbociclib in oestrogen receptor positive breast cancers, highlighting new therapeutic modalities. Oncogene. 2020;39(25):4781–97. https://doi.org/10.1038/s41388-020-1284-6.
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–9. https://doi.org/10.1056/NEJMoa1109653.
Park YH, Kim TY, Kim GM, Kang SY, Park IH, Kim JH, et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019;20(12):1750–9. https://doi.org/10.1016/S1470-2045(19)30565-0.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–48. https://doi.org/10.1056/NEJMoa1609709.
Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987–98. https://doi.org/10.1200/jco.20.02514.
Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–40. https://doi.org/10.1056/NEJMoa1813904.
Piva M, Domenici G, Iriondo O, Rabano M, Simoes BM, Comaills V, et al. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol Med. 2014;6(1):66–79. https://doi.org/10.1002/emmm.201303411.
Dubrovska A, Hartung A, Bouchez LC, Walker JR, Reddy VA, Cho CY, et al. CXCR4 activation maintains a stem cell population in tamoxifen-resistant breast cancer cells through AhR signalling. Br J Cancer. 2012;107(1):43–52. https://doi.org/10.1038/bjc.2012.105.
Liu H, Zhang HW, Sun XF, Guo XH, He YN, Cui SD, et al. Tamoxifen-resistant breast cancer cells possess cancer stem-like cell properties. Chin Med J (Engl). 2013;126(16):3030–4. https://doi.org/10.3760/cma.j.issn.0366-6999.20130227.
El-Sheemy M, Hussain I, Rea C, Arif K. The role of Nanog expression in tamoxifen-resistant breast cancer cells. OncoTargets Ther. 2015;8:1327–34. https://doi.org/10.2147/ott.S67835.
Domenici G, Aurrekoetxea-Rodriguez I, Simoes BM, Rabano M, Lee SY, Millan JS, et al. A Sox2-Sox9 signalling axis maintains human breast luminal progenitor and breast cancer stem cells. Oncogene. 2019;38(17):3151–69. https://doi.org/10.1038/s41388-018-0656-7.
Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Nat Acad Sci. 2009;106(33):13820–5. https://doi.org/10.1073/pnas.0905718106.
Simoes BM, O’Brien CS, Eyre R, Silva A, Yu L, Sarmiento-Castro A, et al. Anti-estrogen resistance in human breast tumors is driven by JAG1-NOTCH4-dependent cancer stem cell activity. Cell Rep. 2015;12(12):1968–77. https://doi.org/10.1016/j.celrep.2015.08.050.
Sansone P, Ceccarelli C, Berishaj M, Chang Q, Rajasekhar VK, Perna F, et al. Self-renewal of CD133(hi) cells by IL6/Notch3 signalling regulates endocrine resistance in metastatic breast cancer. Nat Commun. 2016;7:10442. https://doi.org/10.1038/ncomms10442.
Gelsomino L, Panza S, Giordano C, Barone I, Gu G, Spina E, et al. Mutations in the estrogen receptor alpha hormone binding domain promote stem cell phenotype through notch activation in breast cancer cell lines. Cancer Lett. 2018;428:12–20. https://doi.org/10.1016/j.canlet.2018.04.023.
Deng H, Zhang XT, Wang ML, Zheng HY, Liu LJ, Wang ZY. ER-alpha36-mediated rapid estrogen signaling positively regulates ER-positive breast cancer stem/progenitor cells. PLoS ONE. 2014;9(2):e88034. https://doi.org/10.1371/journal.pone.0088034.
Ma R, Karthik GM, Lovrot J, Haglund F, Rosin G, Katchy A, et al. Estrogen receptor beta as a therapeutic target in breast cancer stem cells. J Natl Cancer Inst. 2017;109(3):1–14. https://doi.org/10.1093/jnci/djw236.
Chan YT, Lai AC, Lin RJ, Wang YH, Wang YT, Chang WW, et al. GPER-induced signaling is essential for the survival of breast cancer stem cells. Int J Cancer. 2020;146(6):1674–85. https://doi.org/10.1002/ijc.32588.
Eden JA. Human breast cancer stem cells and sex hormones. Menopause. 2010;17(4):801–10. https://doi.org/10.1097/gme.0b013e3181d3cdd7.
Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11(3):259–73. https://doi.org/10.1016/j.ccr.2007.01.013.
Liu S, Ginestier C, Charafe-Jauffret E, Foco H, Kleer CG, Merajver SD, et al. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci USA. 2008;105(5):1680–5. https://doi.org/10.1073/pnas.0711613105.
Buckley NE, Nic An tSaoir CB, Blayney JK, Oram LC, Crawford NT, D’Costa ZC, et al. BRCA1 is a key regulator of breast differentiation through activation of Notch signalling with implications for anti-endocrine treatment of breast cancers. Nucleic Acids Res. 2013;41(18):8601–14. https://doi.org/10.1093/nar/gkt626.
Ojo D, Wei F, Liu Y, Wang E, Zhang H, Lin X, et al. Factors promoting tamoxifen resistance in breast cancer via stimulating breast cancer stem cell expansion. Curr Med Chem. 2015;22(19):2360–74. https://doi.org/10.2174/0929867322666150416095744.
Chen C, Baumann WT, Clarke R, Tyson JJ. Modeling the estrogen receptor to growth factor receptor signaling switch in human breast cancer cells. FEBS Lett. 2013;587(20):3327–34. https://doi.org/10.1016/j.febslet.2013.08.022.
Massarweh S, Schiff R. Resistance to endocrine therapy in breast cancer: exploiting estrogen receptor/growth factor signaling crosstalk. Endocr Relat Cancer. 2006;13(Suppl 1):S15-24. https://doi.org/10.1677/erc.1.01273.
Martinez-Revollar G, Garay E, Martin-Tapia D, Nava P, Huerta M, Lopez-Bayghen E, et al. Heterogeneity between triple negative breast cancer cells due to differential activation of Wnt and PI3K/AKT pathways. Exp Cell Res. 2015;339(1):67–80. https://doi.org/10.1016/j.yexcr.2015.10.006.
Schlange T, Matsuda Y, Lienhard S, Huber A, Hynes NE. Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res. 2007;9(5):R63. https://doi.org/10.1186/bcr1769.
O’Brien CS, Howell SJ, Farnie G, Clarke RB. Resistance to endocrine therapy: are breast cancer stem cells the culprits? J Mam Gland Biol Neoplasia. 2009;14(1):45–54. https://doi.org/10.1007/s10911-009-9115-y.
Manupati K, Dhoke NR, Debnath T, Yeeravalli R, Guguloth K, Saeidpour S, et al. Inhibiting epidermal growth factor receptor signalling potentiates mesenchymal–epithelial transition of breast cancer stem cells and their responsiveness to anticancer drugs. Febs J. 2017;284(12):1830–54. https://doi.org/10.1111/febs.14084.
Roop RP, Ma CX. Endocrine resistance in breast cancer: molecular pathways and rational development of targeted therapies. Future Oncol. 2012;8(3):273–92. https://doi.org/10.2217/fon.12.8.
Johnston S, Basik M, Hegg R, Lausoontornsiri W, Grzeda L, Clemons M, et al. Inhibition of EGFR, HER2, and HER3 signaling with AZD8931 in combination with anastrozole as an anticancer approach: phase II randomized study in women with endocrine-therapy-naive advanced breast cancer. Breast Cancer Res Treat. 2016;160(1):91–9. https://doi.org/10.1007/s10549-016-3979-5.
Burstein HJ, Cirrincione CT, Barry WT, Chew HK, Tolaney SM, Lake DE, et al. Endocrine therapy with or without inhibition of epidermal growth factor receptor and human epidermal growth factor receptor 2: a randomized, double-blind, placebo-controlled phase III trial of fulvestrant with or without lapatinib for postmenopausal women with hormone receptor-positive advanced breast cancer-CALGB 40302 (Alliance). J Clin Oncol. 2014;32(35):3959–66. https://doi.org/10.1200/JCO.2014.56.7941.
Smith IE, Walsh G, Skene A, Llombart A, Mayordomo JI, Detre S, et al. A phase II placebo-controlled trial of neoadjuvant anastrozole alone or with gefitinib in early breast cancer. J Clin Oncol. 2007;25(25):3816–22. https://doi.org/10.1200/JCO.2006.09.6578.
Green MD, Francis PA, Gebski V, Harvey V, Karapetis C, Chan A, et al. Gefitinib treatment in hormone-resistant and hormone receptor-negative advanced breast cancer. Ann Oncol. 2009;20(11):1813–7. https://doi.org/10.1093/annonc/mdp202.
Tryfonidis K, Basaran G, Bogaerts J, Debled M, Dirix L, Thery JC, et al. A European Organisation for Research and Treatment of Cancer randomized, double-blind, placebo-controlled, multicentre phase II trial of anastrozole in combination with gefitinib or placebo in hormone receptor-positive advanced breast cancer (NCT00066378). Eur J Cancer. 2016;53:144–54. https://doi.org/10.1016/j.ejca.2015.10.012.
Fukui F, Hayashi SI, Yamaguchi Y. Heregulin controls ERalpha and HER2 signaling in mammospheres of ERalpha-positive breast cancer cells and interferes with the efficacy of molecular targeted therapy. J Steroid Biochem Mol Biol. 2020;201:105698. https://doi.org/10.1016/j.jsbmb.2020.105698.
Hardt O, Wild S, Oerlecke I, Hofmann K, Luo S, Wiencek Y, et al. Highly sensitive profiling of CD44+/CD24− breast cancer stem cells by combining global mRNA amplification and next generation sequencing: evidence for a hyperactive PI3K pathway. Cancer Lett. 2012;325(2):165–74. https://doi.org/10.1016/j.canlet.2012.06.010.
Karthik GM, Ma R, Lövrot J, Kis LL, Lindh C, Blomquist L, et al. mTOR inhibitors counteract tamoxifen-induced activation of breast cancer stem cells. Cancer Lett. 2015;367(1):76–87. https://doi.org/10.1016/j.canlet.2015.07.017.
Lu PW, Li L, Wang F, Gu YT. Inhibitory role of large intergenic noncoding RNA-ROR on tamoxifen resistance in the endocrine therapy of breast cancer by regulating the PI3K/Akt/mTOR signaling pathway. J Cell Physiol. 2019;234(2):1904–12. https://doi.org/10.1002/jcp.27066.
Beelen K, Hoefnagel LD, Opdam M, Wesseling J, Sanders J, Vincent AD, et al. PI3K/AKT/mTOR pathway activation in primary and corresponding metastatic breast tumors after adjuvant endocrine therapy. Int J Cancer. 2014;135(5):1257–63. https://doi.org/10.1002/ijc.28769.
Yin L, Zhang XT, Bian XW, Guo YM, Wang ZY. Disruption of the ER-α36-EGFR/HER2 positive regulatory loops restores tamoxifen sensitivity in tamoxifen resistance breast cancer cells. PLoS ONE. 2014;9(9):e107369. https://doi.org/10.1371/journal.pone.0107369.
Deng H, Zhang XT, Wang ML, Zheng HY, Liu LJ, Wang ZY. ER-α36-mediated rapid estrogen signaling positively regulates ER-positive breast cancer stem/progenitor cells. PLoS ONE. 2014;9(2):e88034. https://doi.org/10.1371/journal.pone.0088034.
Kang L, Guo Y, Zhang X, Meng J, Wang ZY. A positive cross-regulation of HER2 and ER-α36 controls ALDH1 positive breast cancer cells. J Steroid Biochem Mol Biol. 2011;127(3–5):262–8. https://doi.org/10.1016/j.jsbmb.2011.08.011.
Zhao L, Qiu T, Jiang D, Xu H, Zou L, Yang Q, et al. SGCE promotes breast cancer stem cells by stabilizing EGFR. Adv Sci (Weinh). 2020;7(14):1903700. https://doi.org/10.1002/advs.201903700.
Arpino G, Green SJ, Allred DC, Lew D, Martino S, Osborne CK, et al. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clin Cancer Res. 2004;10(17):5670–6. https://doi.org/10.1158/1078-0432.Ccr-04-0110.
Jeong Y, Bae SY, You D, Jung SP, Choi HJ, Kim I, et al. EGFR is a therapeutic target in hormone receptor-positive breast cancer. Cell Physiol Biochem. 2019;53(5):805–19. https://doi.org/10.33594/000000174.
Berardi DE, Raffo D, Todaro LB, Simian M. Laminin modulates the stem cell population in LM05-E murine breast cancer cells through the activation of the MAPK/ERK pathway. Cancer Res Treat. 2017;49(4):869–79. https://doi.org/10.4143/crt.2016.378.
Xu C, Sun X, Qin S, Wang H, Zheng Z, Xu S, et al. Let-7a regulates mammosphere formation capacity through Ras/NF-κB and Ras/MAPK/ERK pathway in breast cancer stem cells. Cell Cycle. 2015;14(11):1686–97. https://doi.org/10.1080/15384101.2015.1030547.
Xu M, Ren Z, Wang X, Comer A, Frank JA, Ke ZJ, et al. ErbB2 and p38γ MAPK mediate alcohol-induced increase in breast cancer stem cells and metastasis. Mol Cancer. 2016;15(1):52. https://doi.org/10.1186/s12943-016-0532-4.
Rhodes LV, Short SP, Neel NF, Salvo VA, Zhu Y, Elliott S, et al. Cytokine receptor CXCR4 mediates estrogen-independent tumorigenesis, metastasis, and resistance to endocrine therapy in human breast cancer. Cancer Res. 2011;71(2):603–13. https://doi.org/10.1158/0008-5472.CAN-10-3185.
Jia Y, Zhou J, Luo X, Chen M, Chen Y, Wang J, et al. KLF4 overcomes tamoxifen resistance by suppressing MAPK signaling pathway and predicts good prognosis in breast cancer. Cell Signal. 2018;42:165–75. https://doi.org/10.1016/j.cellsig.2017.09.025.
Sakunrangsit N, Ketchart W. Plumbagin inhibits cancer stem-like cells, angiogenesis and suppresses cell proliferation and invasion by targeting Wnt/beta-catenin pathway in endocrine resistant breast cancer. Pharmacol Res. 2019;150:104517. https://doi.org/10.1016/j.phrs.2019.104517.
Fu Y, Wang Z, Luo C, Wang Y, Wang Y, Zhong X, et al. Downregulation of CXXC finger protein 4 leads to a tamoxifen-resistant phenotype in breast cancer cells through activation of the Wnt/beta-catenin pathway. Transl Oncol. 2020;13(2):423–40. https://doi.org/10.1016/j.tranon.2019.12.005.
Zheng W, Duan B, Zhang Q, Ouyang L, Peng W, Qian F, et al. Vitamin D-induced vitamin D receptor expression induces tamoxifen sensitivity in MCF-7 stem cells via suppression of Wnt/beta-catenin signaling. Biosci Rep. 2018;38(6). 10.1042/BSR20180595.
Zhou M, Hou Y, Yang G, Zhang H, Tu G, Du YE, et al. LncRNA-Hh strengthen cancer stem cells generation in twist-positive breast cancer via activation of hedgehog signaling pathway. Stem Cells. 2016;34(1):55–66. https://doi.org/10.1002/stem.2219.
Ramaswamy B, Lu Y, Teng Ky, Nuovo G, Li X, Shapiro CL, et al. Hedgehog signaling is a novel therapeutic target in tamoxifen-resistant breast cancer aberrantly activated by PI3K/AKT pathway. Cancer Res. 2012;72(19):5048–59. https://doi.org/10.1158/0008-5472.Can-12-1248.
Bhateja P, Cherian M, Majumder S, Ramaswamy B. The Hedgehog signaling pathway: a viable target in breast cancer? Cancers (Basel). 2019;11(8):1126. https://doi.org/10.3390/cancers11081126.
Sansone P, Berishaj M, Rajasekhar VK, Ceccarelli C, Chang Q, Strillacci A, et al. Evolution of cancer stem-like cells in endocrine-resistant metastatic breast cancers is mediated by stromal microvesicles. Cancer Res. 2017;77(8):1927–41. https://doi.org/10.1158/0008-5472.CAN-16-2129.
Lombardo Y, Faronato M, Filipovic A, Vircillo V, Magnani L, Coombes RC. Nicastrin and Notch4 drive endocrine therapy resistance and epithelial to mesenchymal transition in MCF7 breast cancer cells. Breast Cancer Res. 2014;16(3):R62. https://doi.org/10.1186/bcr3675.
Wang Y, Yu Y, Tsuyada A, Ren X, Wu X, Stubblefield K, et al. Transforming growth factor-β regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene. 2011;30(12):1470–80. https://doi.org/10.1038/onc.2010.531.
Nie Z, Wang C, Zhou Z, Chen C, Liu R, Wang D. Transforming growth factor-beta increases breast cancer stem cell population partially through upregulating PMEPA1 expression. Acta Biochim Biophys Sin (Shanghai). 2016;48(2):194–201. https://doi.org/10.1093/abbs/gmv130.
Zavadova E, Vocka M, Spacek J, Konopasek B, Fucikova T, Petruzelka L. Cellular and humoral immunodeficiency in breast cancer patients resistant to hormone therapy. Neoplasma. 2014;61(01):90–8. https://doi.org/10.4149/neo_2014_013.
Sieuwerts AM, Inda MA, Smid M, van Ooijen H, van de Stolpe A, Martens JWM, et al. ER and PI3K pathway activity in primary ER positive breast cancer is associated with progression-free survival of metastatic patients under first-line tamoxifen. Cancers (Basel). 2020;12(4):802. https://doi.org/10.3390/cancers12040802.
Knabbe C, Lippman ME, Wakefield LM, Flanders KC, Kasid A, Derynck R, et al. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987;48(3):417–28. https://doi.org/10.1016/0092-8674(87)90193-0.
Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17(23):2054–60. https://doi.org/10.1016/j.cub.2007.10.039.
Thompson BJ. YAP/TAZ: drivers of tumor growth, metastasis, and resistance to therapy. BioEssays. 2020;42(5):e1900162. https://doi.org/10.1002/bies.201900162.
Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29(6):783–803. https://doi.org/10.1016/j.ccell.2016.05.005.
Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci USA. 2010;107(4):1431–6. https://doi.org/10.1073/pnas.0911409107.
Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283(9):5496–509. https://doi.org/10.1074/jbc.M709037200.
Yang X, Li DM, Chen W, Xu T. Human homologue of Drosophila lats, LATS1, negatively regulate growth by inducing G(2)/M arrest or apoptosis. Oncogene. 2001;20(45):6516–23. https://doi.org/10.1038/sj.onc.1204817.
Ke H, Pei J, Ni Z, Xia H, Qi H, Woods T, et al. Putative tumor suppressor Lats2 induces apoptosis through downregulation of Bcl-2 and Bcl-x(L). Exp Cell Res. 2004;298(2):329–38. https://doi.org/10.1016/j.yexcr.2004.04.031.
Stewart RA, Li DM, Huang H, Xu T. A genetic screen for modifiers of the lats tumor suppressor gene identifies C-terminal Src kinase as a regulator of cell proliferation in Drosophila. Oncogene. 2003;22(41):6436–44. https://doi.org/10.1038/sj.onc.1206820.
Bao Y, Nakagawa K, Yang Z, Ikeda M, Withanage K, Ishigami-Yuasa M, et al. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J Biochem. 2011;150(2):199–208. https://doi.org/10.1093/jb/mvr063.
Zhao W, Wang M, Cai M, Zhang C, Qiu Y, Wang X, et al. Transcriptional co-activators YAP/TAZ: potential therapeutic targets for metastatic breast cancer. Biomed Pharmacother. 2021;133:110956. https://doi.org/10.1016/j.biopha.2020.110956.
Kim E, Kang JG, Kang MJ, Park JH, Kim YJ, Kweon TH, et al. O-GlcNAcylation on LATS2 disrupts the Hippo pathway by inhibiting its activity. Proc Natl Acad Sci USA. 2020;117(25):14259–69. https://doi.org/10.1073/pnas.1913469117.
Raghunathan VK, Dreier B, Morgan JT, Tuyen BC, Rose BW, Reilly CM, et al. Involvement of YAP, TAZ and HSP90 in contact guidance and intercellular junction formation in corneal epithelial cells. PLoS ONE. 2014;9(10):e109811. https://doi.org/10.1371/journal.pone.0109811.
Gill MK, Christova T, Zhang YY, Gregorieff A, Zhang L, Narimatsu M, et al. A feed forward loop enforces YAP/TAZ signaling during tumorigenesis. Nat Commun. 2018;9(1):3510. https://doi.org/10.1038/s41467-018-05939-2.
Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA. 2011;108(49):E1312–20. https://doi.org/10.1073/pnas.1110428108.
Ma Y, Yang Y, Wang F, Wei Q, Qin H. Hippo-YAP signaling pathway: a new paradigm for cancer therapy. Int J Cancer. 2015;137(10):2275–86. https://doi.org/10.1002/ijc.29073.
Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells—a clinical update. Nat Rev Clin Oncol. 2020;17(4):204–32. https://doi.org/10.1038/s41571-019-0293-2.
Fallahi E, O’Driscoll NA, Matallanas D. The MST/Hippo pathway and cell death: a non-canonical affair. Genes (Basel). 2016;7(6):28. https://doi.org/10.3390/genes7060028.
Azad T, Janse van Rensburg HJ, Lightbody ED, Neveu B, Champagne A, Ghaffari A, et al. A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat Commun. 2018;9(1):1061. https://doi.org/10.1038/s41467-018-03278-w.
Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147(4):759–72. https://doi.org/10.1016/j.cell.2011.09.048.
Duss S, Britschgi A, Bentires-Alj M. Hippo inactivation feeds tumor-initiating cells. Breast Cancer Res. 2012;14(4):318. https://doi.org/10.1186/bcr3190.
Liu J, Li J, Li P, Jiang Y, Chen H, Wang R, et al. DLG5 suppresses breast cancer stem cell-like characteristics to restore tamoxifen sensitivity by inhibiting TAZ expression. J Cell Mol Med. 2019;23(1):512–21. https://doi.org/10.1111/jcmm.13954.
Chang C, Goel HL, Gao H, Pursell B, Shultz LD, Greiner DL, et al. A laminin 511 matrix is regulated by TAZ and functions as the ligand for the alpha6Bbeta1 integrin to sustain breast cancer stem cells. Genes Dev. 2015;29(1):1–6. https://doi.org/10.1101/gad.253682.114.
Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34(6):681–90. https://doi.org/10.1038/onc.2014.5.
Li YW, Shen H, Frangou C, Yang N, Guo J, Xu B, et al. Characterization of TAZ domains important for the induction of breast cancer stem cell properties and tumorigenesis. Cell Cycle. 2015;14(1):146–56. https://doi.org/10.4161/15384101.2014.967106.
Zhang H, Lang TY, Zou DL, Zhou L, Lou M, Liu JS, et al. miR-520b promotes breast cancer stemness through Hippo/YAP signaling pathway. Onco Targets Ther. 2019;12:11691–700. https://doi.org/10.2147/OTT.S236607.
Kim T, Yang SJ, Hwang D, Song J, Kim M, Kyum Kim S, et al. A basal-like breast cancer-specific role for SRF-IL6 in YAP-induced cancer stemness. Nat Commun. 2015;6:10186. https://doi.org/10.1038/ncomms10186.
Sorrentino G, Ruggeri N, Zannini A, Ingallina E, Bertolio R, Marotta C, et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat Commun. 2017;8:14073. https://doi.org/10.1038/ncomms14073.
Yang CE, Lee WY, Cheng HW, Chung CH, Mi FL, Lin CW. The antipsychotic chlorpromazine suppresses YAP signaling, stemness properties, and drug resistance in breast cancer cells. Chem Biol Interact. 2019;302:28–35. https://doi.org/10.1016/j.cbi.2019.01.033.
Britschgi A, Duss S, Kim S, Couto JP, Brinkhaus H, Koren S, et al. The Hippo kinases LATS1 and 2 control human breast cell fate via crosstalk with ERalpha. Nature. 2017;541(7638):541–5. https://doi.org/10.1038/nature20829.
He L, Yuan L, Sun Y, Wang P, Zhang H, Feng X, et al. Glucocorticoid receptor signaling activates TEAD4 to promote breast cancer progression. Cancer Res. 2019;79(17):4399–411. https://doi.org/10.1158/0008-5472.Can-19-0012.
Wang Y, Liu J, Ying X, Lin PC, Zhou BP. Twist-mediated epithelial–mesenchymal transition promotes breast tumor cell invasion via inhibition of hippo pathway. Sci Rep. 2016;6:24606. https://doi.org/10.1038/srep24606.
Zhu C, Li L, Zhang Z, Bi M, Wang H, Su W, et al. A Non-canonical role of YAP/TEAD is required for activation of estrogen-regulated enhancers in breast cancer. Mol Cell. 2019;75(4):791-806e8. https://doi.org/10.1016/j.molcel.2019.06.010.
Rosswag S, Thiede G, Sleeman JP, Thaler S. RASSF1A suppresses estrogen-dependent breast cancer cell growth through inhibition of the yes-associated protein 1 (YAP1), inhibition of the forkhead box protein M1 (FOXM1), and activation of forkhead box transcription factor 3A (FOXO3A). Cancers (Basel). 2020;12(9):2689. https://doi.org/10.3390/cancers12092689.
Zhou X, Wang S, Wang Z, Feng X, Liu P, Lv XB, et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest. 2015;125(5):2123–35. https://doi.org/10.1172/JCI79573.
Xu S, Zhu L, Liu Y, Wang X, Zhong Y, Su Y, et al. MPP, inhibitor of estrogen receptor α, affects YAP nuclear localization during mouse blastocyst formation and in Trophoblast Stem Cells. Chin J Histochem Cytochem. 2019;28(01):6–13. https://doi.org/10.16705/j.cnki.1004-1850.2019.01.002.
Chen W, Bai Y, Patel C, Geng F. Autophagy promotes triple negative breast cancer metastasis via YAP nuclear localization. Biochem Biophys Res Commun. 2019;520(2):263–8. https://doi.org/10.1016/j.bbrc.2019.09.133.
Feng X, Zhang M, Wang B, Zhou C, Mu Y, Li J, et al. CRABP2 regulates invasion and metastasis of breast cancer through hippo pathway dependent on ER status. J Exp Clin Cancer Res. 2019;38(1). https://doi.org/10.1186/s13046-019-1345-2.
Tufail R, Jorda M, Zhao W, Reis I, Nawaz Z. Loss of Yes-associated protein (YAP) expression is associated with estrogen and progesterone receptors negativity in invasive breast carcinomas. Breast Cancer Res Treat. 2012;131(3):743–50. https://doi.org/10.1007/s10549-011-1435-0.
Zheng X, Han H, Liu GP, Ma YX, Pan RL, Sang LJ, et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 2017;36(22):3325–35. https://doi.org/10.15252/embj.201797609.
Li J, Feng X, Li C, Liu J, Li P, Wang R, et al. Downregulation of WW domain-containing oxidoreductase leads to tamoxifen-resistance by the inactivation of Hippo signaling. Exp Biol Med (Maywood). 2019;244(12):972–82. https://doi.org/10.1177/1535370219854678.
Wu X, Zhang X, Yu L, Zhang C, Ye L, Ren D, et al. Zinc finger protein 367 promotes metastasis by inhibiting the Hippo pathway in breast cancer. Oncogene. 2020;39(12):2568–82. https://doi.org/10.1038/s41388-020-1166-y.
Li L, Liu T, Li Y, Wu C, Luo K, Yin Y, et al. The deubiquitinase USP9X promotes tumor cell survival and confers chemoresistance through YAP1 stabilization. Oncogene. 2018;37(18):2422–31. https://doi.org/10.1038/s41388-018-0134-2.
Dethlefsen C, Hansen LS, Lillelund C, Andersen C, Gehl J, Christensen JF, et al. Exercise-induced catecholamines activate the Hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Res. 2017;77(18):4894–904. https://doi.org/10.1158/0008-5472.Can-16-3125.
Lehmann W, Mossmann D, Kleemann J, Mock K, Meisinger C, Brummer T, et al. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat Commun. 2016;7:10498. https://doi.org/10.1038/ncomms10498.
Zheng L, Xiang C, Li X, Guo Q, Gao L, Ni H, et al. STARD13-correlated ceRNA network-directed inhibition on YAP/TAZ activity suppresses stemness of breast cancer via co-regulating Hippo and Rho-GTPase/F-actin signaling. J Hematol Oncol. 2018;11(1):72. https://doi.org/10.1186/s13045-018-0613-5.
Zhu Q, Li J, Wu Q, Cheng Y, Zheng H, Zhan T, et al. Linc-OIP5 in the breast cancer cells regulates angiogenesis of human umbilical vein endothelial cells through YAP1/Notch/NRP1 signaling circuit at a tumor microenvironment. Biol Res. 2020;53(1):5. https://doi.org/10.1186/s40659-020-0273-0.
Samanta S, Guru S, Elaimy AL, Amante JJ, Ou J, Yu J, et al. IMP3 stabilization of WNT5B mRNA facilitates TAZ activation in breast cancer. Cell Rep. 2018;23(9):2559–67. https://doi.org/10.1016/j.celrep.2018.04.113.
Alam M, Bouillez A, Tagde A, Ahmad R, Rajabi H, Maeda T, et al. MUC1-C represses the crumbs complex polarity factor CRB3 and downregulates the Hippo pathway. Mol Cancer Res. 2016;14(12):1266–76. https://doi.org/10.1158/1541-7786.Mcr-16-0233.
Lim SK, Lu SY, Kang SA, Tan HJ, Li Z, Adrian Wee ZN, et al. Wnt signaling promotes breast cancer by blocking ITCH-mediated degradation of YAP/TAZ transcriptional coactivator WBP2. Cancer Res. 2016;76(21):6278–89. https://doi.org/10.1158/0008-5472.CAN-15-3537.
Song H, Wu T, Xie D, Li D, Hua K, Hu J, et al. WBP2 downregulation inhibits proliferation by blocking YAP transcription and the EGFR/PI3K/Akt signaling pathway in triple negative breast cancer. Cell Physiol Biochem. 2018;48(5):1968–82. https://doi.org/10.1159/000492520.
Zhao Y, Montminy T, Azad T, Lightbody E, Hao Y, SenGupta S, et al. PI3K positively regulates YAP and TAZ in mammary tumorigenesis through multiple signaling pathways. Mol Cancer Res. 2018;16(6):1046–58. https://doi.org/10.1158/1541-7786.MCR-17-0593.
Yu S, Zhang M, Huang L, Ma Z, Gong X, Liu W, et al. ERK1 indicates good prognosis and inhibits breast cancer progression by suppressing YAP1 signaling. Aging (Albany NY). 2019;11(24):12295–314. https://doi.org/10.18632/aging.102572.
Zhu Q, Le Scolan E, Jahchan N, Ji X, Xu A, Luo K. SnoN antagonizes the Hippo kinase complex to promote TAZ signaling during breast carcinogenesis. Dev Cell. 2016;37(5):399–412. https://doi.org/10.1016/j.devcel.2016.05.002.
Ma B, Cheng H, Gao R, Mu C, Chen L, Wu S, et al. Zyxin-Siah2-Lats2 axis mediates cooperation between Hippo and TGF-beta signalling pathways. Nat Commun. 2016;7:11123. https://doi.org/10.1038/ncomms11123.
Mota MSV, Jackson WP, Bailey SK, Vayalil P, Landar A, Rostas JW, et al. Deficiency of tumor suppressor Merlin facilitates metabolic adaptation by co-operative engagement of SMAD-Hippo signaling in breast cancer. Carcinogenesis. 2018;39(9):1165–75. https://doi.org/10.1093/carcin/bgy078.
Rashidian J, Le Scolan E, Ji X, Zhu Q, Mulvihill MM, Nomura D, et al. Ski regulates Hippo and TAZ signaling to suppress breast cancer progression. Sci Signal. 2015;8(363):ra14. https://doi.org/10.1126/scisignal.2005735.
Vici P, Mottolese M, Pizzuti L, Barba M, Sperati F, Terrenato I, et al. The Hippo transducer TAZ as a biomarker of pathological complete response in HER2-positive breast cancer patients treated with trastuzumab-based neoadjuvant therapy. Oncotarget. 2014;5(20):9619–25. https://doi.org/10.18632/oncotarget.2449.
González-Alonso P, Zazo S, Martín-Aparicio E, Luque M, Chamizo C, Sanz-Álvarez M, et al. The Hippo pathway transducers YAP1/TEAD induce acquired resistance to trastuzumab in HER2-positive breast cancer. Cancers (Basel). 2020;12(5):1108. https://doi.org/10.3390/cancers12051108.
Lin CH, Pelissier FA, Zhang H, Lakins J, Weaver VM, Park C, et al. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Mol Biol Cell. 2015;26(22):3946–53. https://doi.org/10.1091/mbc.E15-07-0456.
Azad T, Nouri K, Janse van Rensburg HJ, Maritan SM, Wu L, Hao Y, et al. A gain-of-functional screen identifies the Hippo pathway as a central mediator of receptor tyrosine kinases during tumorigenesis. Oncogene. 2020;39(2):334–55. https://doi.org/10.1038/s41388-019-0988-y.
Turunen SP, von Nandelstadh P, Ohman T, Gucciardo E, Seashore-Ludlow B, Martins B, et al. FGFR4 phosphorylates MST1 to confer breast cancer cells resistance to MST1/2-dependent apoptosis. Cell Death Differ. 2019;26(12):2577–93. https://doi.org/10.1038/s41418-019-0321-x.
Rigiracciolo DC, Nohata N, Lappano R, Cirillo F, Talia M, Scordamaglia D, et al. IGF-1/IGF-1R/FAK/YAP transduction signaling prompts growth effects in triple-negative breast cancer (TNBC) cells. Cells. 2020;9(4):1010. https://doi.org/10.3390/cells9041010.
Wu L, Yang X. Targeting the Hippo pathway for breast cancer therapy. Cancers (Basel). 2018;10(11):422. https://doi.org/10.3390/cancers10110422.
Huang R, Li J, Pan F, Zhang B, Yao Y. The activation of GPER inhibits cells proliferation, invasion and EMT of triple-negative breast cancer via CD151/miR-199a-3p bio-axis. Am J Transl Res. 2020;12(1):32–44.
Mayoral-Varo V, Calcabrini A, Sánchez-Bailón MP, Martín-Pérez J. miR205 inhibits stem cell renewal in SUM159PT breast cancer cells. PLoS ONE. 2017;12(11):e0188637. https://doi.org/10.1371/journal.pone.0188637.
Zhang KJ, Hu Y, Luo N, Li X, Chen FY, Yuan JQ, et al. miR-574-5p attenuates proliferation, migration and EMT in triple-negative breast cancer cells by targeting BCL11A and SOX2 to inhibit the SKIL/TAZ/CTGF axis. Int J Oncol. 2020;56(5):1240–51. https://doi.org/10.3892/ijo.2020.4995.
Liu Y, Li M, Yu H, Piao H. lncRNA CYTOR promotes tamoxifen resistance inbreast cancer cells via sponging miR-125a-5p. Int J Mol Med. 2019;45(2):497–509. https://doi.org/10.3892/ijmm.2019.4428.
Manavalan TT, Teng Y, Litchfield LM, Muluhngwi P, Al-Rayyan N, Klinge CM. Reduced expression of miR-200 family members contributes to antiestrogen resistance in LY2 human breast cancer cells. PLoS ONE. 2013;8(4):e62334. https://doi.org/10.1371/journal.pone.0062334.
Liu L, Shen W, Zhu Z, Lin J, Fang Q, Ruan Y, et al. Combined inhibition of EGFR and c-ABL suppresses the growth of fulvestrant-resistant breast cancer cells through miR-375-autophagy axis. Biochem Biophys Res Commun. 2018;498(3):559–65. https://doi.org/10.1016/j.bbrc.2018.03.019.
Lu Y, Roy S, Nuovo G, Ramaswamy B, Miller T, Shapiro C, et al. Anti-microRNA-222 (anti-miR-222) and -181B suppress growth of tamoxifen-resistant xenografts in mouse by targeting TIMP3 protein and modulating mitogenic signal. J Biol Chem. 2011;286(49):42292–302. https://doi.org/10.1074/jbc.M111.270926.
Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16(4):357–66. https://doi.org/10.1038/ncb2936.
Liu J, Li J, Chen H, Wang R, Li P, Miao Y, et al. Metformin suppresses proliferation and invasion of drug-resistant breast cancer cells by activation of the Hippo pathway. J Cell Mol Med. 2020;24(10):5786–96. https://doi.org/10.1111/jcmm.15241.
Wu D, Jia H, Zhang Z, Li S. Circ_0000511 accelerates the proliferation, migration and invasion, and restrains the apoptosis of breast cancer cells through the miR-326/TAZ axis. Int J Oncol. 2021;58(4):1. https://doi.org/10.3892/ijo.2021.5181.
Liu Y, Zhang Q, Wu J, Zhang H, Li X, Zheng Z, et al. Long non-coding RNA A2M-AS1 promotes breast cancer progression by sponging microRNA-146b to Upregulate MUC19. Int J Gen Med. 2020;13:1305–16. https://doi.org/10.2147/ijgm.S278564.
Hu J, Ji C, Hua K, Wang X, Deng X, Li J, et al. Hsa_circ_0091074 regulates TAZ expression via microRNA-1297 in triple negative breast cancer cells. Int J Oncol. 2020;56(5):1314–26. https://doi.org/10.3892/ijo.2020.5000.
Geng Z, Wang W, Chen H, Mao J, Li Z, Zhou J. Circ_0001667 promotes breast cancer cell proliferation and survival via Hippo signal pathway by regulating TAZ. Cell Biosci. 2019;9:104. https://doi.org/10.1186/s13578-019-0359-y.
Liu Y, Li M, Yu H, Piao H. lncRNA CYTOR promotes tamoxifen resistance in breast cancer cells via sponging miR-125a-5p. Int J Mol Med. 2020;45(2):497–509. https://doi.org/10.3892/ijmm.2019.4428.
Qiao K, Ning S, Wan L, Wu H, Wang Q, Zhang X, et al. LINC00673 is activated by YY1 and promotes the proliferation of breast cancer cells via the miR-515–5p/MARK4/Hippo signaling pathway. J Exp Clin Cancer Res. 2019;38(1):418. https://doi.org/10.1186/s13046-019-1421-7.
Huang X, Tang F, Weng Z, Zhou M, Zhang Q. MiR-591 functions as tumor suppressor in breast cancer by targeting TCF4 and inhibits Hippo-YAP/TAZ signaling pathway. Cancer Cell Int. 2019;19:108. https://doi.org/10.1186/s12935-019-0818-x.
Cheng X, Chen J, Huang Z. miR-372 promotes breast cancer cell proliferation by directly targeting LATS2. Exp Ther Med. 2018;15(3):2812–7. https://doi.org/10.3892/etm.2018.5761.
Zhu HY, Bai WD, Ye XM, Yang AG, Jia LT. Long non-coding RNA UCA1 desensitizes breast cancer cells to trastuzumab by impeding miR-18a repression of Yes-associated protein 1. Biochem Biophys Res Commun. 2018;496(4):1308–13. https://doi.org/10.1016/j.bbrc.2018.02.006.
Xie D, Song H, Wu T, Li D, Hua K, Xu H, et al. MicroRNA-424 serves an anti-oncogenic role by targeting cyclin-dependent kinase 1 in breast cancer cells. Oncol Rep. 2018;40(6):3416–26. https://doi.org/10.3892/or.2018.6741.
Hua K, Jin J, Zhao J, Song J, Song H, Li D, et al. miR-135b, upregulated in breast cancer, promotes cell growth and disrupts the cell cycle by regulating LATS2. Int J Oncol. 2016;48(5):1997–2006. https://doi.org/10.3892/ijo.2016.3405.
Hua K, Yang W, Song H, Song J, Wei C, Li D, et al. Up-regulation of miR-506 inhibits cell growth and disrupt the cell cycle by targeting YAP in breast cancer cells. Int J Clin Exp Med. 2015;8(8):12018–27.
Beillard E, Ong SC, Giannakakis A, Guccione E, Vardy LA, Voorhoeve PM. miR-Sens—a retroviral dual-luciferase reporter to detect microRNA activity in primary cells. RNA. 2012;18(5):1091–100. https://doi.org/10.1261/rna.031831.111.
Fang L, Du WW, Yang W, Rutnam ZJ, Peng C, Li H, et al. MiR-93 enhances angiogenesis and metastasis by targeting LATS2. Cell Cycle. 2012;11(23):4352–65. https://doi.org/10.4161/cc.22670.
Yu SJ, Hu JY, Kuang XY, Luo JM, Hou YF, Di GH, et al. MicroRNA-200a promotes anoikis resistance and metastasis by targeting YAP1 in human breast cancer. Clin Cancer Res. 2013;19(6):1389–99. https://doi.org/10.1158/1078-0432.CCR-12-1959.
Gao L, Bao Z, Deng H, Li X, Li J, Rong Z, et al. The beneficial androgenic action of steroidal aromatase inactivators in estrogen-dependent breast cancer after failure of nonsteroidal drugs. Cell Death Dis. 2019;10(7):494. https://doi.org/10.1038/s41419-019-1724-9.
Acknowledgements
We thank all the members of Sichuan Provincial Center for Gynaecology and Breast Disease for the technical assistance and research support.
Funding
This research was funded by the Fund for High-level Talents in Luzhou City (No. 02-00040055).
Author information
Authors and Affiliations
Contributions
ATT was in charge of conception, design, investigation, data analyses, funding acquisition, editing, review and formal analysis of the findings being published. JC wrote original draft and drew figures. JC gave assistance to investigation, data analyses, translation. RLW was responsible for supervising the process. QQL, ZHR, YLW and LZ gave assistance to translation and language. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, J., Wan, R., Li, Q. et al. Utilizing the Hippo pathway as a therapeutic target for combating endocrine-resistant breast cancer. Cancer Cell Int 21, 306 (2021). https://doi.org/10.1186/s12935-021-01999-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-01999-5