Abstract
Cholangiocarcinoma (CCA) is a rare tumor that arises from cholangiocytes, the epithelial cells of the bile duct. The tumor is characterized by insidious onset, high degree of malignancy, poor prognosis and high recurrence rate. Due to the lack of specific biomarkers, it is difficult to diagnose CCA early and evaluate prognosis. Extracellular vesicles (EVs), which include apoptotic bodies, microvesicles and exosomes, have emerged as having important roles in cell-to-cell communication in both normal physiology and pathological conditions. Some research has found that EVs play a crucial role in the occurrence and development of CCA. EVs can carry specific molecular substances such as nucleic acids and proteins, which have potential for the diagnosis and therapy of CCA. This article reviews the current knowledge on the role of EVs in CCA. We highlight EVs and their functions in the physiology and pathophysiology of CCA, and discuss their therapeutic potential and their role as biomarkers.
Similar content being viewed by others
Introduction
Cholangiocarcinoma (CCA) is the most common malignancy of the biliary tree, accounting for approximately 3% of all gastrointestinal tumors and is the second most common primary liver tumor after hepatocellular carcinoma (HCC) [1, 2]. The incidence of CCA varies geographically and demographically, and the overall incidence is still on the rise worldwide [3]. Although the 1-year survival has improved over time, the 5-year survival is less than 10% [4]. Most patients with CCA do not possess exact risk factors and the clinical manifestation may be nonspecific, even in the late-stage of the disease [5]. As such, early detection could improve survival, and this highlights the requirements for novel methods to diagnose and treat CCA.
Recently, the emerging role of extracellular vesicles (EVs) in cholangiocarcinoma progression has attracted extensive attention. To date, the role of EVs has changed from being nonfunctional discards of cellular components to the current research focus [6, 7]. EVs are nanosized, membrane-bound vesicles released from cells that can transport cargo, including DNA, RNA, and proteins, between cells as a form of intercellular communication [8, 9]. With so many contents, the nascent field of EVs has evolved to have a sharper focus, especially in oncology [10]. Therefore, EVs and their derived cargos have emerged as new biomarkers for tumor diagnosis. Tumor-derived EVs play a key role in modulating intercellular communication between tumor and stromal cells in local and distant microenvironments [11, 12]. In addition, EVs can still be used for therapeutic purposes as targets, immunomodulators and delivery vehicles [13]. In this review, we highlight and discuss about the relationship between EVs and CCA, with a special focus on their roles and potential clinical application values as biomarkers and therapeutic targets in CCA.
Epidemiology, risk factors, diagnosis and treatment of CCA
Cholangiocarcinoma is a devastating tumor, currently classified as intrahepatic (iCCA), perihilar (pCCA), or distal (dCCA), characterized by varying degrees of desmoplastic reaction and increasing morbidity and mortality worldwide [14, 15]. While it is more common in Asia, its incidence has risen significantly in Europe and North America in recent decades [16]. Approximately 35,660 patients with iCCA are diagnosed each year in the United States, and the 5-year survival rate is about 10% [17, 18]. The incidence of CCA is highest in Thailand, with 113 per 100,000 men and 50 per 100,000 women per year [19]. In most case of CCA, the etiology is unknown, but chronic inflammation and cell injury in the bile duct shown a high risk of occurrence of CCA [14]. Pathologically, the release of inflammatory cytokines, increased cell death and proliferation, as well as changes in the liver in fibrosis contribute to the occurrence of tumor [20]. Primary sclerosing cholangitis is considered as the main risk factor for CCA [21]. The diagnostic basis mainly includes imaging methods (ultrasound, computed tomography, magnetic resonance imaging and fluorodeoxyglucose positron emission tomography), histological analysis of a tumor biopsy and serum nonspecific tumor biomarkers, such as carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) [22,23,24]. However, the sensitivity of these tests in the diagnosis of CCA is limited, especially in the early stages of the disease [25]. Management strategies include multispecialty treatments, with consideration of surgical resection, targeted radiation therapy, and systemic chemotherapy [24, 26, 27]. Surgical resection is the only potentially curative treatment, but the majority of patients present with advanced cancer and recurrence after resection is common [16]. Lymph node metastasis is a prominent feature of CCA [28]. Diagnosed CCA is usually advanced and often inoperable, leading to a poor prognosis [29].
Details about EVs
Classification of EVs
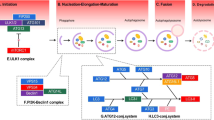
According to the current knowledge of their biogenesis, EVs can be broadly divided into two main categories: microvesicles and exosomes [30, 31]. Microvesicles (MVs, 100–1000 nm in diameter) are secreted by the shedding or outward budding of the plasma membrane [32]. The formation of microvesicles is the result of the dynamic interaction of phospholipid redistribution and cytoskeletal protein contraction [33]. Since little information about biogenesis and MV release is understood, we focused on the function of exosomes in CCA.
Characteristics of exosomes
In 1983, Johnstone et al. first isolated exosomes from the supernatant of sheep reticulocyte culture medium [34]. However, due to the role of exosomes being unclear at that time, it is thought to be a nonfunctional particle in the cell. Whereas exosomes are 40–150 nm, endosome-derived, small EVs which are secreted by most cells [35]. RNA, DNA and proteins are reported to be actively and selectively incorporated into intraluminal vesicles, which reside within multivesicular endosomes and are the precursor of exosomes [36, 37]. The release of exosomes into the extracellular space is facilitated by the fusion of multivesicular bodies (MVBs) limiting the membrane with the plasma membrane [35, 38]. Then, exosomes can be carried away by extracellular fluid, such as saliva, urine, blood, semen, amniotic fluid, ascites, alveolar lavage fluid, milk, synovial fluid and cerebrospinal fluid, and taken up by other cells [39,40,41]. Besides, bile included [42]. The function of exosomes depends on the type and contents of their parent cells. Exosomes from normal cells play a role in maintaining stability in vivo, while tumor cell-derived exosomes are associated with tumor progression [40, 43].
In addition, we need to identify exosomes that can distinguish them from other EVs by their size and proteins markers. For instance, MVB-associated proteins (tumor susceptibility gene 101 (TSG101) and ALIX) [44, 45], fusion proteins, membrane transport proteins (flotillin-1) [46], tetraspanins (CD63, CD81and CD9) [36], and heat shock proteins (HSP70.1 and HSP20) [42, 47] are often used as protein markers to recognize exosomes in scientific research. Currently, with the identification of a large number of cargo molecules in exosomes, their functions, including regulating immune function, enhancing metastasis, and modulating intercellular communication, have also been explored in tumor cells [48,49,50]. Some research shows that exosomes from highly metastatic breast cancer cells (4T1 and EO771 cells) can modulate the favorable microenvironment for lung and liver metastasis colonization [51]. In addition, various immune cell-derived exosomes, such as natural killer cells, dendritic cells, macrophages, neutrophils, mast cells and myeloid-derived suppressors, can act on tumor cells, modulating the growth, metastasis and response to chemotherapy [48, 52]. Next, we mainly elucidate the role of exosomes in CCA.
Characterization of EVs associated with CCA
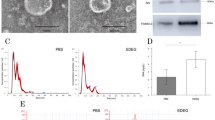
As mentioned above, EVs can be produced in both cells and body fluids, and their characteristics are similar to each other. Although EVs can be extracted by many methods, differential centrifugation is the most widely used method. Accumulated evidence has reported that EVs can be extracted from the serum, bile and CCA cells of patients with bile duct carcinoma and identified by specific markers, as shown in Table 1. The morphological and molecular characteristics of these EVs indicate that they are mainly exosomes. However, the contents of EVs are numerous, and their functions are different, and these need to be further studied.
EVs regulate the progression of CCA
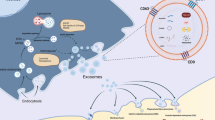
With the further development of EVs, increasing evidence has been presented to demonstrate the role of EVs in the progression of CCA (Fig. 1). Bile duct carcinoma usually has a dense stroma that contains immune cells (including neutrophils, tumor associated macrophages, natural killer cells, and T and B lymphocytes) and an extracellular matrix that promotes connective tissue proliferation [53, 54]. In particular, cancer-associated fibroblasts (CAFs), which communicate not only with tumor cells, but also with stromal cells, play a central role in the progression of CCA [55, 56]. CAFs influence the behavior of CCA by releasing various metabolites and soluble factors, such as vascular endothelial growth factor (VEGF)-A and VEGF-C, which may lead to dilation of the lymphatic vasculature and tumor cell intravasation [57]. Therefore, it also has the property of easy transfer [18]. In this process, EVs play a crucial role in facilitating the communication of various signals (Table 2).
Roles of tumor cells derived exosomes in the progression of CCA. Exosomes are critically involved in CCA progression including tumorigenesis, development, immune escape and metastasis by transferring functional biomolecules. CCA cell derived exosomes induced the expression of β-catenin and decreased the expression of E-cadherin to increase motility of normal cells. CCA cell derived exosomes interact with marrow mesenchymal stem cells (MSCs) to modulate the microenvironment and promote CCA growth. Moreover, CCA cell derived exosomes participate in immune escape by inhibiting the cytokine-induced killer (CIK) cells
The function of EVs in tumor microenvironment
Cholangiocytes can be actively involved in the development of bile duct disease by stimulating the recruitment and activation of inflammatory cells in the bile duct microenvironment [58]. Katsumi et al. found that activated bile duct cells are involved in activating the proinflammatory polarization of damage-associated molecular patterns (DAMPs) through the receptor for advanced glycation end products (RAGE) signaling pathway by releasing DAMPs as EV cargo [59]. Prior to this, Masyyuk et al. verified exosomes in rat bile [60]. Meanwhile, in the process of studying the cilium interaction of bile duct cells, exosomes were found to induce intracellular signals and functional responses, verifying that bile exosomes participate in intercellular signal communication [60]. This laid a foundation for the influence of bile duct carcinoma exosomes on tumor progression.
In the study of the tumor microenvironment of CCA, Haga et al. exposed marrow mesenchymal stem cells (MSCs) to CCA cell-derived EVs, which enhanced expression of alpha-smooth muscle actin mRNA and release of cytokines/chemokines such as IL-6, thus regulating the tumor microenvironment and promoting the growth of CCA. In addition, CCA cell-derived EVs can contribute to the formation of the tumor stroma through the fibroblast differentiation of MSCs [61]. This further revealed the effect of tumor cell-derived EVs on the local microenvironment. However, they did not identify the specific contents of the EVs. Moreover, proteins in exosomes are essential agents for tumor growth [62, 63]. Another study demonstrated the protein spectrum of CCA-derived exosomes and their potential roles. These researchers isolated exosomes from CCA cell lines (KKU-M213 and KKU-100) and incubated them with normal bile duct cells (H69). After proteomics analysis, exosomes were found to be internalized into H69 cells, resulting in migration and invasion of H69 cells, but failing to induce proliferation. In addition, the exosomes of KKU-M213 cells induced the expression of β-catenin and decreased the expression of E-cadherin, suggesting that exosomes might induce the migration and invasion of bile duct cells through the direct transfer of oncogene proteins between cells, thus affecting the specific intracellular mechanism related to CCA carcinogenesis [64]. The proteomics analysis of normal bile duct cells and CCA cells confirmed the differences between these two cell types, and these differences need to be further studied. Moreover, exosomes from another CCA cell line (RBE) could inhibit the antitumor activity of cytokine-induced killer (CIK) cells by downregulating the populations of CD3+, CD8+, NK (CD56+) and CD3+CD56+ cells and secreting TNF-α and perforin [65]. According to some research, mutations in the IDH1 gene is common in a variety of tumors, including iCCA [66, 67]. Recent studies have shown that the R132C mutation is the most common type of IDH1 mutation in iCCA. The IDH1R132C mutation leads to the downregulation of P2RX7 expression, which further affects the exosome secretion of tumor cells, ultimately affecting the progression of CCA [68]. However, more functional experiments on P2RX7 are needed.
Exosomal noncoding RNAs in CCA
In addition, noncoding RNA also play a crucial role in tumor development [69]. As a functional regulatory molecule, noncoding RNAs, such as microRNAs (miRNAs) and circular RNAs (circRNAs), could mediate cellular processes, including chromatin, transcription, posttranscriptional modification and signal transduction, and, of course, predict prognosis [69,70,71].
Exosomal microRNAs in CCA
MiRNAs are small noncoding RNAs composed of 19–24 nucleotides [72]. Kitdumrongthum et al. found different miRNA expression profiles in the exosomes released by CCA cells and cholangiocytes, and many miRNAs with abnormal regulation had functions related to a variety of oncogenes [73]. For example, miR-205-5p, the most upregulated miRNA, down-regulation of miR-205-5p can inhibit invasion and migration of CCA cells [73]. Moreover, miR-205-5p has also been reported in other cancers, such as breast cancer and gastric cancer [74, 75]. However, in contrast to CCA, the expression of miR-205-5p is downregulated in breast cancer, and miR-205-5p has an antitumor effect in breast cancer [76]. Li and colleagues reported that EVs could transport miRNA species between human CCA cells and CAFs. They used LX2-derived EVs carrying miR-195 to inhibit CCA growth and improve survival in a mouse model of CCA, demonstrating the communication between the tumor and microenvironment [77]. Epithelial–mesenchymal transition (EMT) is a biological process in which epithelial cells gradually change and lose epithelial characteristics and differentiate into mesenchymal phenotypes, and it is closely related to the invasiveness and motility of tumor cells [78,79,80]. CCA-derived EVs could transfer miR-30e and inhibit EMT by directly targeting the Snail in receptor cells, thus inhibiting the invasion and migration of bile duct cancer cells [81]. In addition, miR-200a/c-3p in serum exosomes was significantly positively correlated with the CCA stage, and mainly involved in lymphatic metastasis of tumors [82].
Exosomal circRNAs in CCA
Recent research has demonstrated that circRNAs also have a biological role in CCA [83, 84]. Wang et al. found that the level of circ-0000284 was increased in CCA cell lines, tumor tissues and plasma exosomes, thus enhancing the migration, invasion and proliferation of CCA cells in vivo and in vitro. The circ-0000284/miR-637/LY6E regulatory axis was involved in this process [85, 86]. In addition, exosome-mediated circ-0000284 could stimulate the malignant behavior of surrounding normal cells and ultimately promote the progression of CCA [86].
EVs as novel biomarkers for CCA
A major reason for the poor prognosis of CCA is the lack of early detection. Late diagnosis delays optimal treatment and leads to lower survival. Therefore, it is necessary to develop new methods for the diagnosis of CCA. In recent years, studies have shown that EVs have huge potential in the diagnosis of diseases due to their unique properties and great progress has been made in studying of EVs as tumor diagnostic markers [41, 87, 88]. EVs in bile and blood have opened up new ideas for the early diagnosis of CCA. Compared with traditional CA-199 and CEA, EVs have higher diagnostic value in CCA, which is summarized in Fig. 2.
The isolation of EVs
With the development of technology, many exosome separation and purification techniques, including ultracentrifugation, ultrafiltration, immunoaffinity capture, size-exclusion chromatography, microfluidic techniques and charge neutralization-based polymer precipitation, have been exploded [89,90,91]. Among them, the most common application is ultracentrifugation, which is the gold standard for exosome separation, even though it has some limitations, such as having a low efficiency and being time consuming [92,93,94]. In the CCA research process, the EVs in the culture media of CCA were separated mainly by ultracentrifugation [61, 64, 65]. Similarly, EVs are extracted from the serum by ultracentrifugation, and polymer-based precipitation kit [95, 96]. However, there is a study that supports some commercial kits, such as ExoQuick and miR-CURY, as being better than ultracentrifugation, even with a limited quantity of EVs [92]. Bile is a lipid-rich fluid, that is secreted primarily by hepatocytes, and contains almost all body components: lipids, proteins, carbohydrates, vitamins, mineral salts and trace elements [96, 97]. Because of the characteristics and complexity of bile extraction, we mainly summarize the isolation of bile here. Bile samples of CCA are usually collected by endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic biliary drainage (PTBD) and surgery, and then bile is immediately centrifuged at 4 ℃ to remove the cell debris and filtered through a 200 nm filter. Finally, the supernatant is collected and added to an ultracentrifuge tube, diluted with PBS for further purification, mainly including ultracentrifugation, PEG precipitation, membrane filtration and affinity purification [98,99,100]. Some research, however, initially collected bile and then rapidly diluted with PBS for further centrifuge [42]. The isolation of EVs were used immediately or stored at − 80 °C until use (Fig. 3). However, research in this area is limited, and there is still much room for improvement in the separation and purification of exosomes.
Bile EVs
MiRNA in EVs is protected the membrane from degrading enzymes, and this determines its highly stable characteristics in the extracellular environment, thus, miRNA is used for the diagnosis of a variety of cancers, including CCA [101, 102]. Li et al. identified and characterized EVs in human bile for the first time, and miRNA-laden EVs in human bile could be used for the diagnosis of CCA. They defined a new biliary vesicle miR-based panel (miR-191, miR-486-3p, miR-1274b, miR-16 and miR-484) for the diagnosis of CCA with a sensitivity of 67% and specificity of 96% [98]. This research initiates studying EVs in bile. Han and coworkers found that the expression levels of miR-30d-5p and miR-92a-3p in the bile of CCA patients were specifically upregulated compared with those in the bile of patients with benign biliary disease [103]. Compared with CEA and CA19-9, miR-30d-5p had the best effect in differentiating CCA and common bile duct disease, with a sensitivity of 81.1%, specificity of 60.5% and area under the curve (AUC) value of 0.730. In addition, the identification of CCA using the combination of the two bile miRNAs and serum CA19-9 levels was weaker than that of miR-30d-5p alone [103].
Long noncoding RNAs (lncRNAs) in bile also play the same role in CCA as miRNAs. Ge et al. conducted a series of experiments on bile exosomes, and showed that the expression of the lncRNAs ENST00000588480.1 and ENST00000517758.1 in the CCA group was significantly increased compared with that of the control group. The AUC of the combined detection of the two lncRNAs was 0.709, and the sensitivity and specificity were 82.9% and 58.9%, respectively [99]. Moreover, with the increase in the tumor TNM stage, the expression levels of ENST00000588480.1 and ENST00000517758.1 were significantly increased, and they could be potential diagnostic markers [99]. The content of EVs in bile can be used as a diagnostic marker, and the concentration of EVs also has potential diagnostic value. Severino et al. found that the accuracy of the bile EV count in the diagnosis of CCA was 100% with a threshold value of 9.46 × 1014 nanoparticles, with an AUC of 1, and this could correctly distinguish malignant and nonmalignant common bile duct stenoses in this research [42].
Serum EVs
There are also some diagnostic markers of CCA in serum EVs. The new proteomic features found by Ander Arbelaiz in serum EVs of primary sclerosing cholangitis (PSC), CCA and HCC patients have potential diagnostic value [104]. For example, fibrinogen gamma chain (FIBG), alpha-1-acid glycoprotein 1 (A1AG1) and S100A8 (S10A8) proteins have the strongest differential diagnoses of CCA and PSC, with AUC values of 0.796, 0.794 and 0.759, respectively [104]. Similarly, using proteomic methods, Weeraphan et al. studied the exosomal phosphoproteome of M213 and M213D5 in CCA cells, and showed that Ser255 of HSP90B was highly phosphorylated in tissues of CCA patients with a low TNM stage (I and II) compared to those with a TNM stage of III or IV. ROC analysis showed that HSP90B-S255 was a new potential biomarker for metastatic CCA with an AUC value of 0.936 (sensitivity 87.27%, specificity 97.62%) [105]. Moreover, Shen et al. studied exosomal miRNA in peripheral blood samples from CCA patients and healthy controls. The results showed that the serum extracellular miR-200 family, especially miR-200c-3p, had the strongest diagnostic ability for CCA than that in serum CA19-9, with an AUC of 0.93, which is worth further study [82]. Meanwhile, positive of Annexin V, epithelial cell adhesion molecule (EpCAM), asialoglycoprotein receptor 1 (ASGPR1) and tumor-associated microparticles found in serum EVs were shown to have the potential to differentiate HCC and CCA from tumor-free individuals; after tumor resection, the number of these microparticles decreased, which proved a correlation with the presence of the tumor [95].
Summary of the role of EVs in diagnosing CCA
The studies above indicate that miRNAs, lncRNAs and proteins in blood and bile could be used as diagnostic indicators of CCA (summarized in Table 3). It can be concluded that although there are many substances in EVs, exosomes are the main diagnostic agents. Exosomes coated with lipid bilayers are more stable and more suitable as diagnostic markers [8]. The concentration of EVs in bile may increase as a result of bile flow disorder caused by bile duct stenosis or obstruction in CCA patients [106]. In addition, potential tumor-derived biomarkers may be secreted directly into bile by adjacent CCA cells, and local sampling may be more likely to detect candidate biomarkers directly related to the tumor [106]. Therefore, compared with circulating serum sampling, bile sampling can improve diagnostic performance [103]. However, the study of the EVs in CCA is still not complete. For example, the mechanism of EVs in bile duct cancer is not clear and how to efficiently extract and detect EVs in body fluids remains unclear.
Application of EVs in CCA therapy
To date, due to the difficulty in the early diagnosis of CCA, there are few treatment options, and radical surgical resection is the only effective treatment method [107, 108]. Postoperative adjuvant chemotherapy can improve the survival and cure rates, but the effect of chemotherapy is not enough [18]. With the development of research on EVs, the treatment of CCA has a new direction. The characteristics of tumor-secreted EVs in regulating the immune microenvironment illustrate their clinical potential in immunotherapy, therapeutic targeting and drug delivery [11, 109].
Therapeutic targets
Since the tumor microenvironment promotes the progression and invasion of CCA, targeting the microenvironment and related cells is a strategy for the treatment of CCA [110, 111]. Chen et al. found that RBE-derived exosomes could inhibit the antitumor activity of CIK cells [65]. This suggests that the effect of CIK cell-based immunotherapy is related to EVs, which may be a potential therapeutic target. In addition, circ-0000284 may be a therapeutic target for CCA. Wang et al. proved that the knockdown of exosomal circ-0000284 inhibited CCA growth and metastasis in vivo through animal experiments [86]. Besides, according to Zhang’s research, mutations in the IDH1 gene alter the function of IDH1 and affect the development of CCA by promoting exosome release [68]. Interestingly, IDH1 inhibitors have been reported. For example, the safety and clinical efficacy of mutant IDH1 inhibitor ivosidenib in the recurrence or refractory IDH1-mutated acute myeloid leukemia were demonstrated [112]. At present, inhibitors of IDH1 (AG120 and IDH305) are being tested in iCCA patients [113]. This provides a potential treatment of CCA. Furthermore, one study reported that inhibition of miR-205-5p in the exosomes of CCA could reduce the invasion and migration of CCA, and the miR-200 family was associated with drug resistance [73, 114].
Drug delivery
EVs are natural membrane vesicles involved in intercellular communication; accumulating evidence has revealed that EVs have the characteristics of stability and low immune reactivity, and exosomes in EVs can effectively transport a variety of different types of cargo to target cells [109, 115]. As a result, EVs can also be selected as a therapeutic tool to modulate the function of CCA cells by delivering cargo media [35, 116]. Stromal derived EVs are suitable for the delivery of materials to CCA cells, and this property can be exploited for delivering antitumor therapy to CCA cells [117]. Li et al. proved that EVs could transport miR-195 from fibroblasts to cancer cells. In addition, fibroblasts-derived EVs loaded with miR-195, play a key role in the CCA rat model, by reducing the size of tumors and improving survival in the treated rats [77]. Moreover, after incubating the miR-30e-enriched EVs with CCA cells, Zhang found that the expression of miR-30e in receptor CCA cells increased, which ultimately regulated the invasion and migration of cells. This demonstrates that miR-30e-enriched EVs can be ingested by recipient cells as a means of transferring miR-30e [81]. A recent study showed that methotrexate-loaded tumor-cell-derived microvesicles were injected into the bile duct lumen of patients with extrahepatic CCA, which mobilized and activated neutrophils and alleviated biliary obstruction [118]. These studies suggest that EVs carrying designed cargo can be used as media carriers to manage the progression of CCA.
Conclusion and future prospects
EVs are present through the occurrence, development and metastasis of tumors, providing new clues for the diagnosis and treatment of CCA. Many substances in EVs, such as miRNAs, circRNAs, proteins and even EV concentrations, can be used as new biomarkers. It is also important to improve the early diagnosis of CCA. Moreover, according to the role of EVs in the tumor microenvironment and immunity, corresponding targeted drugs and immunotherapy can be used for the treatment of CCA. Exosomal programmed-death ligand (PD-L1) has been found to contribute to immunosuppression and has been associated with the anti-PD-1 response, although it is mainly involved in melanoma [119]. Moreover, PD-L1expression in iCCA and perihilar cholangiocarcinoma has also been reported, and is mainly expressed in tumors with a high density of tumor-infiltrating lymphocytes [120]. Immunotherapy for PD-L1 in CCA has entered clinical research, but the efficacy is not clear [121]. EVs have shown great potential in the immunotherapy of tumors, although there is not much data on the immunotherapy of EVs in CCA and therefore, further research is needed. In summary, this review focuses on the current research status of EVs in CCA. First, the characteristics of EVs in CCA were described. Second, the mechanism of EVs in tumor growth and metastasis was discussed. Finally, we demonstrated that the contents of EVs could be used for the clinical diagnosis and treatment of CCA. Although the existing studies have partially uncovered the mechanism of EVs in CCA, there are still a few challenging problems to solve. Firstly, the detailed mechanism to describe the role of EVs in CCA needs further clarification. In addition, standardized methods for the separation, purification and analysis of EVs in body fluids are needed. Last but not least, most of the pathophysiological studies are conducted through in vitro analysis, and there are few in vivo experiments based on EVs in animal models. Follow-up studies should be conducted to better apply EVs to the clinical diagnosis and treatment of CCA in the future. Therefore, more efforts are needed to study the role of EVs in CCA.
Availability of data and materials
The materials supporting the conclusions of this review are included in the article.
Abbreviations
- CCA:
-
Cholangiocarcinoma
- HCC:
-
Hepatocellular carcinoma
- EVs:
-
Extracellular vesicles
- MVs:
-
Microvesicles
- MVBs:
-
Multivesicular bodies
- TSG101:
-
Tumor susceptibility gene 101
- HSP70.1:
-
Heat shock proteins 70.1
- HSP20:
-
Heat shock proteins 20
- TEM:
-
Transmission electron microscopy
- NTA:
-
Nanoparticle tracking analysis
- CAFs:
-
Cancer-associated fibroblasts
- VEGF-A:
-
Vascular endothelial growth factor-A
- VEGF-C:
-
Vascular endothelial growth factor-C
- RAGE:
-
Receptor for advanced glycation end products
- DAMPs:
-
Damage-associated molecular patterns
- MSCs:
-
Marrow mesenchymal stem cells
- iCCA:
-
Intrahepatic cholangiocarcinoma
- miRNAs:
-
MicroRNAs
- circRNAs:
-
Circular RNAs
- EMT:
-
Epithelial–mesenchymal transition
- CA19-9:
-
Carbohydrate antigen 19-9
- CEA:
-
Carcinoembryonic antigen
- pCCA:
-
Perihilar cholangiocarcinoma
- dCCA:
-
Distal cholangiocarcinoma
- FIBG:
-
Fibrinogen gamma chain
- A1AG1:
-
Alpha-1-acid glycoprotein 1
- S10A8:
-
S100A8
- AMPN:
-
Aminopeptidase N
- VNN1:
-
Pantetheinase
- PIGR:
-
Polymeric immunoglobulin receptor
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- PTBD:
-
Percutaneous transhepatic biliary drainage
- AUC:
-
Area under the curve
- lncRNAs:
-
Long noncoding RNAs
- PSC:
-
Primary sclerosing cholangitis
- EpCAM:
-
Epithelial cell adhesion molecule
- ASGPR1:
-
Asialoglycoprotein receptor 1
- BBO:
-
Benign biliary obstruction
- BBD:
-
Benign biliary disease
- MCBDS:
-
Malignant common bile duct stenoses
- NMCBDS:
-
Nonmalignant common bile duct stenoses
- HC:
-
Healthy controls
- PD-L1:
-
Programmed-death ligand
References
Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–29.
Pellino A, Loupakis F, Cadamuro M, Dadduzio V, Fassan M, Guido M, et al. Precision medicine in cholangiocarcinoma. Transl Gastroenterol Hepatol. 2018;3:40.
Montomoli J, Erichsen R, Norgaard M, Hoyer M, Hansen JB, Jacobsen JB. Survival of patients with primary liver cancer in central and northern Denmark, 1998–2009. Clin Epidemiol. 2011;3(Suppl 1):3–10.
Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29(2):221–32.
Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54(1):173–84.
Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–88.
Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51.
Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 2015;65(8):783–97.
Roma-Rodrigues C, Raposo LR, Cabral R, Paradinha F, Baptista PV, Fernandes AR. Tumor microenvironment modulation via gold nanoparticles targeting malicious exosomes: implications for cancer diagnostics and therapy. Int J Mol Sci. 2017;18(1):162.
Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1–11.
Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30(6):836–48.
Sheehan C, D’Souza-Schorey C. Tumor-derived extracellular vesicles: molecular parcels that enable regulation of the immune response in cancer. J Cell Sci. 2019. https://doi.org/10.1242/jcs.235085.
Walker S, Busatto S, Pham A, Tian M, Suh A, Carson K, et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9(26):8001–17.
Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41(1):5–15.
Alsaleh M, Leftley Z, Barbera TA, Sithithaworn P, Khuntikeo N, Loilome W, et al. Cholangiocarcinoma: a guide for the nonspecialist. Int J Gen Med. 2019;12:13–23.
Blechacz B, Cholangiocarcinoma. Current knowledge and new developments. Gut Liver. 2017;11(1):13–26.
Zhang H, Yang T, Wu M, Shen F. Intrahepatic cholangiocarcinoma: epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 2016;379(2):198–205.
Sato K, Glaser S, Alvaro D, Meng F, Francis H, Alpini G. Cholangiocarcinoma: novel therapeutic targets. Expert Opin Ther Targets. 2020;24(4):345–57.
Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma---evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111.
Razumilava N, Gores GJ. Cholangiocarcinoma. The Lancet. 2014;383(9935):2168–79.
Kirstein MM, Vogel A. Epidemiology and risk factors of cholangiocarcinoma. Visc Med. 2016;32(6):395–400.
Tshering G, Dorji PW, Chaijaroenkul W, Na-Bangchang K. Biomarkers for the diagnosis of cholangiocarcinoma: a systematic review. Am J Trop Med Hyg. 2018;98(6):1788–97.
Tsen A, Barbara M, Rosenkranz L. Dilemma of elevated CA 19-9 in biliary pathology. Pancreatology. 2018;18(8):862–7.
Doherty B, Nambudiri VE, Palmer WC. Update on the diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep. 2017;19(1):2.
Tang Z, Yang Y, Zhang J, Fu W, Lin Y, Su G, et al. Quantitative proteomic analysis and evaluation of the potential prognostic biomarkers in cholangiocarcinoma. J Cancer. 2019;10(17):3985–99.
Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol. 2019;37(8):658–67.
Dover LL, Oster RA, McDonald AM, DuBay DA, Wang TN, Jacob R. Impact of adjuvant chemoradiation on survival in patients with resectable cholangiocarcinoma. HPB (Oxford). 2016;18(10):843–50.
Ji GW, Zhu FP, Zhang YD, Liu XS, Wu FY, Wang K, et al. A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur Radiol. 2019;29(7):3725–35.
Turgeon MK, Maithel SK. Cholangiocarcinoma: a site-specific update on the current state of surgical management and multi-modality therapy. Chin Clin Oncol. 2020;9(1):4.
Stahl PD, Raposo G. Exosomes and extracellular vesicles: the path forward. Essays Biochem. 2018;62(2):119–24.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28.
Xie F, Zhou XX, Fang MY, Li HY, Tu YF, Su P, et al. Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv Sci. 2019;6(24):1901779.
Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113(1):1–11.
Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–78.
Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of exosome composition. Cell. 2019;177(2):428-445.e18 (.).
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113(8):E968-77.
Wan Z, Gao X, Dong Y, Zhao Y, Chen X, Yang G, et al. Exosome-mediated cell-cell communication in tumor progression. Am J Cancer Res. 2018;8(9):1661–73.
Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–12.
Street JM, Koritzinsky EH, Glispie DM, Star RA, Yuen PS. Urine exosomes: an emerging trove of biomarkers. Adv Clin Chem. 2017;78:103–22.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977.
Li Y, Zhao J, Yu S, Wang Z, He X, Su Y, et al. Extracellular vesicles long RNA sequencing reveals abundant mRNA, circRNA, and lncRNA in human blood as potential biomarkers for cancer diagnosis. Clin Chem. 2019;65(6):798–808.
Severino V, Dumonceau JM, Delhaye M, Moll S, Annessi-Ramseyer I, Robin X, et al. Extracellular vesicles in bile as markers of malignant biliary stenoses. Gastroenterology. 2017;153(2):495–504 (e8.).
Makler A, Asghar W. Exosomal biomarkers for cancer diagnosis and patient monitoring. Expert Rev Mol Diagn. 2020;20(4):387–400.
Jadli AS, Ballasy N, Edalat P, Patel VB. Inside(sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol Cell Biochem. 2020;467(1–2):77–94.
Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27.
Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–25.
Zhang X, Wang X, Zhu H, Kranias EG, Tang Y, Peng T, et al. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS ONE. 2012;7(3):e32765.
Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72–81.
Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49(3):347–60.
Seo N, Akiyoshi K, Shiku H. Exosome-mediated regulation of tumor immunology. Cancer Sci. 2018;109(10):2998–3004.
Wen SW, Sceneay J, Lima LG, Wong CS, Becker M, Krumeich S, et al. The biodistribution and immune suppressive effects of breast cancer-derived exosomes. Cancer Res. 2016;76(23):6816–27.
Huang Y, Liu K, Li Q, Yao Y, Wang Y. Exosomes function in tumor immune microenvironment. Adv Exp Med Biol. 2018;1056:109–22.
Sirica AE, Gores GJ. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology. 2014;59(6):2397–402.
Rimassa L, Personeni N, Aghemo A, Lleo A. The immune milieu of cholangiocarcinoma: from molecular pathogenesis to precision medicine. J Autoimmun. 2019;100:17–26.
Chuaysri C, Thuwajit P, Paupairoj A, Chau-In S, Suthiphongchai T, Thuwajit C. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep. 2009;21(4):957–69.
Vaquero J, Aoudjehane L, Fouassier L. Cancer-associated fibroblasts in cholangiocarcinoma. Curr Opin Gastroenterol. 2020;36(2):63–9.
Cadamuro M, Brivio S, Mertens J, Vismara M, Moncsek A, Milani C, et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. J Hepatol. 2019;70(4):700–9.
Pinto C, Giordano DM, Maroni L, Marzioni M. Role of inflammation and proinflammatory cytokines in cholangiocyte pathophysiology. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt B):1270–8.
Katsumi T, Guicciardi ME, Azad A, Bronk SF, Krishnan A, Gores GJ. Activated cholangiocytes release macrophage-polarizing extracellular vesicles bearing the DAMP S100A11. Am J Physiol Cell Physiol. 2019;317(4):C788-C99.
Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol. 2010;299(4):G990-9.
Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles. 2015;4:24900.
Blomme A, Fahmy K, Peulen O, Costanza B, Fontaine M, Struman I, et al. Myoferlin is a novel exosomal protein and functional regulator of cancer-derived exosomes. Oncotarget. 2016;7(50):83669–83.
Kim H, Kim DW, Cho JY. Exploring the key communicator role of exosomes in cancer microenvironment through proteomics. Proteome Sci. 2019;17:5.
Dutta S, Reamtong O, Panvongsa W, Kitdumrongthum S, Janpipatkul K, Sangvanich P, et al. Proteomics profiling of cholangiocarcinoma exosomes: a potential role of oncogenic protein transferring in cancer progression. Biochim Biophys Acta. 2015;1852(9):1989–99.
Chen JH, Xiang JY, Ding GP, Cao LP. Cholangiocarcinoma-derived exosomes inhibit the antitumor activity of cytokine-induced killer cells by down-regulating the secretion of tumor necrosis factor-alpha and perforin. J Zhejiang Univ Sci B. 2016;17(7):537–44.
Waitkus MS, Diplas BH, Yan H. Biological role and therapeutic potential of IDH mutations in cancer. Cancer Cell. 2018;34(2):186–95.
Farshidfar F, Zheng S, Gingras MC, Newton Y, Shih J, Robertson AG, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 2017;18(11):2780–94.
Zhang X, Miao R, Liu T, Xiang X, Gu J, Jia Y, et al. IDH1 as a frequently mutated gene has potential effect on exosomes releasement by epigenetically regulating P2RX7 in intrahepatic cholangiocarcinoma. Biomed Pharmacother. 2019;113:108774.
Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18(1):5–18.
Chang W, Wang Y, Li W, Geng Z. Long non-coding RNA myocardial infarction associated transcript promotes the proliferation of cholangiocarcinoma cells by targeting miR-551b-3p/CCND1 axis. Clin Exp Pharmacol Physiol. 2020;47(6):1067–75.
Yu J, Zhang B, Zhang H, Qi Y, Wang Y, Wang W, et al. E2F1-induced upregulation of long non-coding RNA LMCD1-AS1 facilitates cholangiocarcinoma cell progression by regulating miR-345-5p/COL6A3 pathway. Biochem Biophys Res Commun. 2019;512(2):150–5.
Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–74.
Kitdumrongthum S, Metheetrairut C, Charoensawan V, Ounjai P, Janpipatkul K, Panvongsa W, et al. Dysregulated microRNA expression profiles in cholangiocarcinoma cell-derived exosomes. Life Sci. 2018;210:65–75.
Ma C, Shi X, Guo W, Feng F, Wang G. miR-205-5p downregulation decreases gemcitabine sensitivity of breast cancer cells via ERp29 upregulation. Exp Ther Med. 2019;18(5):3525–33.
Yao L, Shi W, Gu J. Micro-RNA 205-5p is involved in the progression of gastric cancer and targets phosphatase and tensin homolog (PTEN) in SGC-7901 human gastric cancer cells. Med Sci Monit. 2019;25:6367–77.
Xiao Y, Humphries B, Yang C, Wang Z. MiR-205 dysregulations in breast cancer: the complexity and opportunities. Noncoding RNA. 2019;5(4):53.
Li L, Piontek K, Ishida M, Fausther M, Dranoff JA, Fu R, et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology. 2017;65(2):501–14.
Blackwell RH, Foreman KE, Gupta GN. The role of cancer-derived exosomes in tumorigenicity & epithelial-to-mesenchymal transition. Cancers. 2017;9(8):105.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8.
Ota Y, Takahashi K, Otake S, Tamaki Y, Okada M, Aso K, et al. Extracellular vesicle-encapsulated miR-30e suppresses cholangiocarcinoma cell invasion and migration via inhibiting epithelial-mesenchymal transition. Oncotarget. 2018;9(23):16400–17.
Shen L, Chen GP, Xia QF, Shao SJ, Fang HX. Exosomal miR-200 family as serum biomarkers for early detection and prognostic prediction of cholangiocarcinoma. Int J Clin Exp Pathol. 2019;12(10):3870.
Xu Y, Yao Y, Zhong X, Leng K, Qin W, Qu L, et al. Downregulated circular RNA hsa_circ_0001649 regulates proliferation, migration and invasion in cholangiocarcinoma cells. Biochem Biophys Res Commun. 2018;496(2):455–61.
Jiang XM, Li ZL, Li JL, Xu Y, Leng KM, Cui YF, et al. A novel prognostic biomarker for cholangiocarcinoma: circRNA Cdr1as. Eur Rev Med Pharmacol Sci. 2018;22(2):365–71.
Louis C, Desoteux M, Coulouarn C. Exosomal circRNAs: new players in the field of cholangiocarcinoma. Clin Sci. 2019;133(21):2239–44.
Wang S, Hu Y, Lv X, Li B, Gu D, Li Y, et al. Circ-0000284 arouses malignant phenotype of cholangiocarcinoma cells and regulates the biological functions of peripheral cells through cellular communication. Clin Sci. 2019;133(18):1935–53.
Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl. 2015;9(3–4):358–67.
Rahbarghazi R, Jabbari N, Sani NA, Asghari R, Salimi L, Kalashani SA, et al. Tumor-derived extracellular vesicles: reliable tools for Cancer diagnosis and clinical applications. Cell Commun Signal. 2019;17(1):73.
Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804.
Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684–707.
Kluszczynska K, Czernek L, Cypryk W, Peczek L, Duchler M. Methods for the determination of the purity of exosomes. Curr Pharm Des. 2019;25(42):4464–85.
Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS ONE. 2017;12(1):e0170628.
Tang YT, Huang YY, Zheng L, Qin SH, Xu XP, An TX, et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. 2017;40(3):834–44.
Peng Q, Zhang J, Zhou G. Comparison of plasma exosomes by differential ultracentrifugation and solvent precipitation methods. Clin Lab. 2018;64(6):991–8.
Julich-Haertel H, Urban SK, Krawczyk M, Willms A, Jankowski K, Patkowski W, et al. Cancer-associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J Hepatol. 2017;67(2):282–92.
Bruggenwirth IMA, Porte RJ, Martins PN. Bile composition as a diagnostic and prognostic tool in liver transplantation. Liver Transplant. 2020. https://doi.org/10.1002/lt.25771.
Reshetnyak VI. Physiological and molecular biochemical mechanisms of bile formation. World J Gastroenterol. 2013;19(42):7341–60.
Li L, Masica D, Ishida M, Tomuleasa C, Umegaki S, Kalloo AN, et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology. 2014;60(3):896–907.
Ge X, Wang Y, Nie J, Li Q, Tang L, Deng X, et al. The diagnostic/prognostic potential and molecular functions of long non-coding RNAs in the exosomes derived from the bile of human cholangiocarcinoma. Oncotarget. 2017;8(41):69995–70005.
Yan IK, Berdah VX, Patel T. Isolation of extracellular RNA from bile. Methods Mol Biol. 2018;1740:59–67.
Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids–the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8(8):467–77.
Olaizola P, Lee-Law PY, Arbelaiz A, Lapitz A, Perugorria MJ, Bujanda L, et al. MicroRNAs and extracellular vesicles in cholangiopathies. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt B):1293–307.
Han HS, Kim MJ, Han JH, Yun J, Kim HK, Yang Y, et al. Bile-derived circulating extracellular miR-30d-5p and miR-92a-3p as potential biomarkers for cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2020;19(1):41–50.
Arbelaiz A, Azkargorta M, Krawczyk M, Santos-Laso A, Lapitz A, Perugorria MJ, et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2017;66(4):1125–43.
Weeraphan C, Phongdara A, Chaiyawat P, Diskul-Na-Ayudthaya P, Chokchaichamnankit D, Verathamjamras C, et al. Phosphoproteome profiling of isogenic cancer cell-derived exosome reveals HSP90 as a potential marker for human cholangiocarcinoma. Proteomics. 2019;19(12):e1800159.
Mayr C, Beyreis M, Wagner A, Pichler M, Neureiter D, Kiesslich T. Deregulated microRNAs in biliary tract cancer: functional targets and potential biomarkers. Biomed Res Int. 2016;2016:4805270.
Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, et al. Surgery for cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):143–55.
Rahnemai-Azar AA, Weisbrod AB, Dillhoff M, Schmidt C, Pawlik TM. Intrahepatic cholangiocarcinoma: current management and emerging therapies. Expert Rev Gastroenterol Hepatol. 2017;11(5):439–49.
Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–56.
Zhou G, Sprengers D, Mancham S, Erkens R, Boor PPC, van Beek AA, et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J Hepatol. 2019;71(4):753–62.
Gentilini A, Pastore M, Marra F, Raggi C. The role of stroma in cholangiocarcinoma: the intriguing interplay between fibroblastic component, immune cell subsets and tumor epithelium. Int J Mol Sci. 2018;19(10):2885.
Golub D, Iyengar N, Dogra S, Wong T, Bready D, Tang K, et al. Mutant isocitrate dehydrogenase inhibitors as targeted cancer therapeutics. Front Oncol. 2019;9:417.
Massironi S, Pilla L, Elvevi A, Longarini R, Rossi RE, Bidoli P, et al. New and emerging systemic therapeutic options for advanced cholangiocarcinoma. Cells. 2020;9(3):688.
Okamoto K, Miyoshi K, Murawaki Y. miR-29b, miR-205 and miR-221 enhance chemosensitivity to gemcitabine in HuH28 human cholangiocarcinoma cells. PLoS ONE. 2013;8(10):e77623.
Barile L, Vassalli G. Exosomes. Therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78.
Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. 2018;16(1):81.
Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54(S2):789–92.
Gao Y, Zhang H, Zhou N, Xu P, Wang J, Gao Y, et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng. 2020;4(7):743–53.
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–6.
Fontugne J, Augustin J, Pujals A, Compagnon P, Rousseau B, Luciani A, et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget. 2017;8(15):24644–51.
Jakubowski CD, Azad NS. Immune checkpoint inhibitor therapy in biliary tract cancer (cholangiocarcinoma). Chin Clin Oncol. 2020;9(1):2.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural Science Foundation (Grant Number 81872036), and Lanzhou Science and Technology Bureau (Grant Number 2019-4-43), and Science and Technology Project of Chengguan District of Lanzhou City (Grant Number 2019JSCX0092 and 2019RCCX0038), and Lanzhou talent innovation project (Grant Number 2018-RC-13).
Author information
Authors and Affiliations
Contributions
MB, WF, WM and YL designed the research. MB drafted the manuscript. XL, WM, YL, GS, WF critically revised the manuscript. MB, WF, LG, JC, CH, HM, JZ, PY and BB provided intellectual contribution. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bai, M., Fu, W., Su, G. et al. The role of extracellular vesicles in cholangiocarcinoma. Cancer Cell Int 20, 435 (2020). https://doi.org/10.1186/s12935-020-01526-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-020-01526-y