Abstract
Objectives
Undifferentiated embryonic cell transcription factor-1 (UTF1) plays a critical role in the developmental timing during embryonic development. However, there is little paper dealing with UTF1 expressed in adult tissues. In the present study, we evaluate the expression of UTF1 in breast cancer and its correlation with clinicopathological parameters.
Methods
Real-time polymerase chain reaction (real-time PCR) was applied to detect the expression of UTF1 mRNA in the 55 pairs of samples of breast cancer tissues and match normal tissues. △△CT method was used to evaluate the relative quantity of target mRNA expression.
Results
Among the 55 pairs of samples of breast cancer tissues and match normal tissues adjacent to the tumor, the UTF1 mRNA levels in normal tissues were significantly higher than those observed in breast cancer tissues (p < 0.001). UTF1 mRNA levels expression correlated with lymph node metastasis (p = 0.002) and tumor size (p < 0.001).
Conclusions
Expression of UTF1 in breast cancer tissues were confirmed in this study. Decreased expression of UTF1 mRNA in breast cancer tissues was maybe one of the factors impact on tumorigenes in breast cancer patients.
Similar content being viewed by others
Introduction
Currently breast cancer remains one of the most common cancers worldwide, and one of the leading causes of cancer related death in the word. Identification of biological markers ability to predict risk of prognosis in breast cancer is limited. To improve the clinical outcome of patients with advanced breast carcinoma, it is necessary to target novel biomarker genes, which appear to be involved in carcinoma development, as we had described previously [1],[2].
Previous reports have indicated that there was a possible correlation of undifferentiated embryonic cell transcription factor-1 (UTF1) with the biological of cancer cell [3]. And the low expression of UTF1 may connect with poorer prognosis among patients with cervical cancer [4].
Undifferentiated embryonic cell transcription factor-1 (UTF1), a transcriptional co-activator, was first isolated and identified from mouse F9 embryonic carcinoma cells [5]. The human UTF1 gene is located on chromosome 10q26 and consists of two exons and one short intron, which plays a critical role in the developmental timing during embryonic development [6],[7]. UTF1 is readily expressed in embryos and embryonic stem cells, but it is either undetectable or its expression remains at low levels in normal adult tissues, suggesting that UTF1 may play a critical role in cell proliferation and/or differentiation during embryonic development.
Recently, UTF1 confirmed the ability to facilitate the reprogramming of human somatic cells to induced pluripotent stem (iPS) cells [8]. Thus, UTF1 plays an important role in stem cells, and the expression of UTF1 cancer cells may have stem cell-like features. This prompted us to evaluate UTF1 status in human beast cancer. In addition, UTF1 has been reported to bind to a specific subset of mRNAs and modulate many gene translations, including those for p27 and Oct-3/4 [9].
The expression of UTF1 was subsequently detected in germ cell neoplasms [10], and cervical cancer [4]. Although UTF1 has been extensive studied in other cancers, no investigation has been conducted in breast cancer. We hypothesize that UTF1 may play a role in the oncogenesis of breast cancer. The present study was undertaken to examine the expression of UTF1 in breast cancer tissues by using a validated specific and sensitive real-time quantitative PCR assay. Here, we examined fifty-five cases of breast cancer tissues for the potential difference of UTF1 expression in breast cancer tissues and their matched normal tissues, and to assess the relationship between UTF1 expression and clinicopathological parameters in breast cancer patients.
Materials and methods
Case selection
Specimens were obtained from 55 patients who underwent surgery of breast cancer at the Department of Breast and Thyroid Surgery of Shaoxing Hospital of Zhejiang University, between July 2012 and November 2013. Informed consent was obtained from all patients, and the study was conducted according to the guidelines of the Hospital Ethics Committee. The patients aged 35 to 73 years (mean 58.7 years). The correlation between the expression of UTF1 and clinicopathologic parameters including age, differentiation statue and pTNM pathological classification according to the International Union against Cancer (UICC) were evaluated. The clinicopathologic feature of the 55 cases were summarized in Table 1.
RNA extraction and cDNA synthesis
Total RNA was extracted from fleshly frozen gastric tissues using the Trizol reagent (Invitrogen life Technologies, USA). Total RNA was reverse reverse-transcribed into single-strand complementary DNA (cDNA) using moloney-murine leukemia (M-MLV) reverse transcriptase (Promega, Madison,WI, USA). Briefly, the RNAwas denatured by heating for 5 min at 70°C, cooled on ice, and then used for reverse transcription (2 μg of total RNA, 25U of RNAse inhibitor, 0.5 mM each of dNTPs, 1.5 μM reverse primer and 200U of M-MLV reverse transcriptase in a total volume of 25 μl). For reverse transcription, tubes were incubated at 42°C for 60 min, followed by rapid cooling.
Real-time quantitative PCR
Real-time RT-PCR analyses were performed with the ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA). 25 μl reaction mixture containing 2 μl of cDNA template, 1 μl each of sense and anti-sense primers, 1 × SYBR Green Universal PCR Mix was amplified as follow: denaturation at 95°C for 10 min and 40 cycles at 95°C for 30 s, 55°C for 30 s and 72°C for 40 s. Real-time quantitative PCR reaction was performed in triplicate for each sample and a mean value of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) used to calculate mRNA levels. Quantitative analysis was performed using the comparative CT method [11],[12]. The UTF-1 mRNA copy numbers in normal and tumor tissues were normalized to mRNA copy numbers of the house keeping gene, GAPDH to give a value △CT. This final value was to determine changes in expression of UTF-1 in each sample. The primer sequences for UTF-1 were as follows: forward primer 5'- ATGGGGCTGCTGGGCGACAACG-3', reverse primer 5'- GGGGAGGCGTCCGCAGACTTCG-3'. The primers for GAPDH were taken from a previously published assay [13]. Fluorescent data were converted into RQ measurements, which stand for relative expression automatically by the SDS system software and exported to Microsoft Excel. Thermal dissociation plots were examined for biphasic melting curves, indicative of whether primerdimers or other nonspecific products could be contributing to the amplification signal.
Statistical analysis
Statistical analysis was conducted using the statistical program SPSS 15.0 for Windows (SPSS, Chicago, IL, USA). Pre-treatment characteristics were analyzed using the 2-tailed χ2 test. The 2-tailed t-test was used to evaluate the correlation between UTF-1 expression and clinicopathologic parameters.
Results
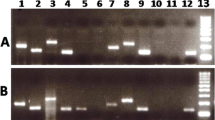
Expressions of UTF-mRNA in breast cancer tissues by real-time PCR
As shown in Table 2, of the 55 samples of breast cancer tissues and matched normal tissues adjacent to the tumor, expression of UTF-1 was detected in total samples. In normal tissues, the UTF-1 mRNA levels ranged from 0.039 to 41.96 with a median of 20.16. In breast cancer tissues, the UTF-1 mRNA levels ranged from 0.008 to 6.263 with a median of 1.517. The UTF-1 mRNA levels in normal tissues were significantly higher than those observed in breast cancer tissues (p < 0.001).
Correlation between UTF-and clinicopathological parameter
According to median expression level of UTF-1, 55 cases of breast cancer patients were divided into two groups, high UTF-1 expression group (UTF-1 expression level >1.517) and low UTF-1 expression group (UTF-1 expression level > 1.517). The mean number of metastasis lymph nodes in high UTF-1 group is lower than low UTF-1 expression group (0.33 vs. 1.75), the differences among them had statistically significant (p = 0.002) (Table 3). Moreover, tumor size was different between high expression UTF-1 versus low expression UTF-1 (1.34 vs. 2.46), the difference also have statistically significant (p < 0.001) (Table 3).
Discussion
In the present study, we found the relative quantity of UTF-1 mRNA mRNA in 55 samples of breast cancer tissues as determined by real-time PCR. UTF-1 mRNA was expressed more predominantly in normal tissues adjacent to the tumor than it was expressed in breast cancer tissues. We showed that UTF-1 was lower expressed in breast cancer tissues than corresponding normal tissues. Previous studies have reported that UTF-1 is expressed in various human epithelial-type neoplasias, such as cervical cancer [4]. At present, Mouallif et al. evaluated UTF1 expression levels immunohistochemically in eight normal epithelia including breast (prostate, endometrium, bladder, colon, oesophagous, lung and kidney) and their corresponding tumors in a recent paper [14].
In the current study, real-time quantitative PCR was used to compare the expressions of UTF-1 mRNAs in 55 breast cancers and their matched normal tissues, which allows detecting UTF-1expression in breast tissues with very low level. Furthermore, we correlated these findings with the clinicopathological parameter of the breast cancers. The result showed that the low expression level of UTF-1 mRNAs in breast cancer patients was significantly associated with metastasis lymph nodes and tumor size. Our present study clarify that UTF-1 protein could act as a potential biomarker for prognosis assessment of breast cancer. Related mechanism is worthy of further investigation.
Previous studies have shown the expression of UTF-1 cells was identified in a subpopulation of stem cell-like cells [15] and the expression of UTF-1 may influence the susceptibility of cancer cells to chemotherapeutic agents and radiotherapy. These data suggested that positive expression of UTF-1 impact on cancer patients’ survival not only influence the susceptibility of cancer cells to chemotherapeutic agents, but also other mechanism such as cell proliferation [16], stem cell-like features [17],[18].
Recently, it was reported that the UTF1-overexpressing cells transactived p27Kip1 resulting in G1/S arrest, which may provide an explanation for UTF1 functions in cervical carcinogenesis. The similar results may present in breast cancer patients. In summary, our study demonstrated that the expression level of UTF1 may play a role in the oncogenesis and may serve as a biomarker for prognosis. Based on our findings, UTF1 gene could be a potential target for the treatment of breast cancer. The findings of this study present a novel knowledge of UTF1 and a potential future prospect for breast cancer treatment.
Authors’ contributions
CX conceived and designed the experiments. CX and YZ performed the experiments. WC performed statistical analysis of all data. CX wrote the paper. All authors are in agreement with the content of the manuscript and this submission. All authors read and approved the final manuscript.
References
Xie SD, Xu CY, Shen JG, Jiang ZN, Wang LB: HER 2/neu protein expression in gastric cancer is associated with poor survival. Mol Med Rep. 2009, 2: 943-946. 10.3892/mmr_00000180.
Xu CY, Guo JL, Jiang ZN, Xie SD, Shen JG, Shen JY, Wang LB: Prognostic role of estrogen receptor alpha and estrogen receptor beta in gastric cancer. Ann Surg Oncol. 2010, 17: 2503-2509. 10.1245/s10434-010-1031-2.
Kristensen DM, Nielsen JE, Skakkebaek NE, Graem N, Jacobsen GK, Rajpert-De Meyts E, Leffers H: Presumed pluripotency markers UTF-1 and REX-1 are expressed in human adult testes and germ cell neoplasms. Hum Reprod. 2008, 23: 775-782. 10.1093/humrep/den010.
Wu XL, Zheng PS: Undifferentiated embryonic cell transcription factor-1 (UTF1) inhibits the growth of cervical cancer cells by transactivating p27Kip1. Carcinogenesis. 2013, 34: 1660-1668. 10.1093/carcin/bgt102.
Okuda A, Fukushima A, Nishimoto M, Orimo A, Yamagishi T, Nabeshima Y, Kuro-o M, Nabeshima Y, Boon K, Keaveney M, Stunnenberg HG, Muramatsu M: UTF1, a novel transcriptional coactivator expressed in pluripotent embryonic stem cells and extra-embryonic cells. EMBO J. 1998, 17: 2019-2032. 10.1093/emboj/17.7.2019.
Fukushima A, Okuda A, Nishimoto M, Seki N, Hori TA, Muramatsu M: Characterization of functional domains of an embryonic stem cell coactivator UTF1 which are conserved and essential for potentiation of ATF-2 activity. J Biol Chem. 1998, 73: 25840-25849. 10.1074/jbc.273.40.25840.
Nishimoto M, Fukushima A, Okuda A, Muramatsu M: The gene for the embryonic stem cell coactivator UTF1 carries a regulatory element which selectively interacts with a complex composed of Oct-3/4 and Sox-2. Mol Cell Biol. 1999, 19: 5453-5465.
Pfannkuche K, Fatima A, Gupta MK, Dieterich R, Hescheler J: Initial colony morphology-based selection for iPS cells derived from adult fibroblasts is substantially improved by temporary UTF1-based selection. PLoS One. 2010, 5: e9580-10.1371/journal.pone.0009580.
Liu A, Cheng L, Du J, Peng Y, Allan RW, Wei L, Li J, Cao D: Diagnostic utility of novel stem cell markers SALL4, OCT4, NANOG, SOX2, UTF1, and TCL1 in primary mediastinal germ cell tumors. Am J Surg Pathol. 2010, 34: 697-706.
Wang P, Li J, Allan RW, Guo CC, Peng Y, Cao D: Expression of UTF1 in primary and metastatic testicular germ cell tumors. Am J Clin Pathol. 2010, 134: 604-612. 10.1309/AJCPB44HBKINJNYU.
Schmittgen TD, Livak KJ: Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008, 3: 1101-1108. 10.1038/nprot.2008.73.
Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001, 25: 402-408. 10.1006/meth.2001.1262.
Xu C, Shen J, Xie S, Jiang Z, Huang L, Wang L: Positive expression of Lin28 is correlated with poor survival in gastric carcinoma. Med Oncol. 2013, 30: 382-10.1007/s12032-012-0382-x.
Mouallif M, Albert A, Zeddou M, Ennaji MM, Delvenne P, Guenin S: Expression profile of undifferentiated cell transcription factor 1 in normal and cancerous human epithelia. Int J Exp Pathol. 2014, 95: 251-259. 10.1111/iep.12077.
Kues WA, Herrmann D, Barg-Kues B, Haridoss S, Nowak-Imialek M, Buchholz T, Streeck M, Grebe A, Grabundzija I, Merkert S, Martin U, Hall VJ, Rasmussen MA, Ivics Z, Hyttel P, Niemann H: Derivation and characterization of sleeping beauty transposon-mediated porcine induced pluripotent stem cells. Stem Cells Dev. 2013, 22: 124-135. 10.1089/scd.2012.0382.
Lin CH, Yang CH, Chen YR: UTF1 deficiency promotes retinoic acid-induced neuronal differentiation in P19 embryonal carcinoma cells. Int J Biochem Cell Biol. 2012, 44: 350-357. 10.1016/j.biocel.2011.11.008.
Brown S, Teo A, Pauklin S, Hannan N, Cho CH, Lim B, Vardy L, Dunn NR, Trotter M, Pedersen R, Vallier L: Activin/Nodal signaling controls divergent transcriptional networks in human embryonic stem cells and in endoderm progenitors. Stem Cells. 2011, 29: 1176-1185. 10.1002/stem.666.
Kooistra SM, van den Boom V, Thummer RP, Johannes F, Wardenaar R, Tesson BM, Veenhoff LM, Fusetti F, O'Neill LP, Turner BM, De Haan G, Eggen BJ: Undifferentiated embryonic cell transcription factor 1 regulates ESC chromatin organization and gene expression. Stem Cells. 2010, 28: 1703-1714. 10.1002/stem.497.
Grant support
Zhejiang Nature Science Foundation of China Q13H160045 (Chaoyang Xu), Science and Health Care Foundation grant Zhejiang, China 201345046, 201468770 (Chaoyang Xu), Shaoxing nonprofit technology applied research projects of China 2012B70055 (Chaoyang Xu), and Medical Association research Clinical funds of China 2012ZYC-A75 (Liming Huang).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Xu, C., Zhou, Y. & Chen, W. Expression of undifferentiated embryonic cell transcription factor-1 (UTF1) in breast cancers and their matched normal tissues. Cancer Cell Int 14, 116 (2014). https://doi.org/10.1186/s12935-014-0116-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-014-0116-6