Abstract
Background
Nerol (C10H18O), an acyclic monoterpene, naturally presents in plant essential oils, and is used widely in food, cosmetics and pharmaceuticals as the valuable fragrance. Meanwhile, chemical synthesis is the only strategy for large-scale production of nerol, and the disadvantages of chemical synthesis greatly limit the production and its application. These defects drive the interests of researchers shift to the production of nerol by eco-friendly methods known as biosynthesis methods. However, the main technical bottleneck restricting the biosynthesis of nerol is the lacking of corresponding natural aroma-producing microorganisms.
Results
In this study, a novel multi-stress-tolerant probiotics Meyerozyma guilliermondii GXDK6 with aroma-producing properties was identified by whole genome sequencing and metabolomics technology. GXDK6 showed a broad pH tolerance in the range of 2.5–10.0. The species also showed salt tolerance with up to 12% NaCl and up to 18% of KCl or MgCl2. GXDK6 exhibited heavy-metal Mn2+ tolerance of up to 5494 ppm. GXDK6 could also ferment with a total of 21 kinds of single organic matter as the carbon source, and produce abundant aromatic metabolites. Results from the gas chromatography–mass spectrometry indicated the production of 8–14 types of aromatic metabolites (isopentanol, nerol, geraniol, phenylethanol, isobutanol, etc.) when GXDK6 was fermented up to 72 h with glucose, sucrose, fructose, or xylose as the single carbon source. Among them, nerol was found to be a novel aromatic metabolite from GXDK6 fermentation, and its biosynthesis mechanism had also been further revealed.
Conclusion
A novel aroma-producing M. guilliermondii GXDK6 was identified successfully by whole genome sequencing and metabolomics technology. GXDK6 showed high multi-stress-tolerant properties with acid–base, salty, and heavy-metal environments. The aroma-producing mechanism of nerol in GXDK6 had also been revealed. These findings indicated the aroma-producing M. guilliermondii GXDK6 with multi-stress-tolerant properties has great potential value in the fermentation industry.

Similar content being viewed by others
Background
Nerol (C10H18O), an acyclic monoterpene, naturally presents in plant essential oils and is used widely in food, cosmetics and pharmaceuticals as the valuable fragrance [1, 2]. Meanwhile, nerol possesses antifungal activity against Aspergillus niger [3] and Aspergillus flavus [4] and it can be used as a potential antifungal agent for food preservation. The global annual demand for nerol is more than 5,000 tons per year, but the global production capacity of nerol is only about 3,000 tons, which is still in greater demand for improvement. At present, chemical synthesis is the only way for large-scale production of nerol, but the disadvantages of chemical synthesis such as high cost, complicated process, serious pollution, low yield, and more by-products greatly limit the production and its application. These defects drive the interests of researchers shift to the production of nerol by eco-friendly methods, which are often known as biosynthesis methods. However, the main technical bottleneck restricting the biosynthesis of nerol is the lacking of corresponding natural aroma-producing microorganisms, especially the aroma-producing probiotics.
Aroma-producing probiotics has been demonstrated to be greatly beneficial to hosts and ecological balance. In the past few decades, well-known aroma-producing probiotics had been identified with yeasts, Lactobacillus, and Bacillus subtilis [5]. These species can produce abundant beneficial metabolites through their own metabolic regulations, which showed extremely high practical application. However, most probiotics exist in specific environments, resulting in the interspecies variations among the aroma substances often produced by different probiotics. Most probiotics cannot be fermented for a long time because of the unfavorable conditions, such as the acidification of carbohydrates [6].
To date, known aroma-producing probiotics are generally condition-dependent microorganisms [7]. Few probiotics can ferment different nutrient substrates and generate beneficial aromatic metabolites under special environments (e.g., high salt, extremely strong alkaline, or strong acidic condition), and sustain prolonged aroma production [8, 9]. The screening of aroma-producing probiotics with multistress tolerance has become increasingly urgent [10].
M. guilliermondii is known for the production of flavour compounds in fermented food products, has been widely used in food fermentation in recent years [11, 12]. For example, Coda et al. [11] used M. guilliermondii to ferment wheat bread, results showed that many active compounds could be synthesized by M. guilliermondii, and the shelf life of wheat bread was effectively extended by 14 days. M. guilliiermondii had also been demonstrated to belong to a kind of probiotic with great potential, which can produce various health bioactive compound, such as the efficient production of isoflavone aglycone [13, 14], the overproduction of vitamin B2 (riboflavin) [15]. Herein, a novel aroma-producing M. guilliermondii GXDK6 with multistress-tolerant properties was isolated and identified from subtropical mangrove sediments mainly on the basis of whole genome sequencing. Metabolomics technology based on gas chromatography–mass spectrometry (GC–MS) was also performed to investigate the production of aroma. Among the aromatic productions, nerol was found as a novel aromatic metabolite from GXDK6 fermentation, and its biosynthesis mechanism had also been further revealed. To the authors’ knowledge, M. guilliermondii GXDK6 is the first multi-stress-tolerant probiotics from subtropical marine mangrove sediments. This research shows the novel strategies of natural biosynthesis of nerol and also provides new materials and theoretical references for the industrial production of aroma-producing species.
Results and discussion
Physicochemical characterization of GXDK6
As shown in Fig. 1a, the colony of GXDK6 was milky white, round, convex, and smooth. The SEM results showed that GXDK6 was similar to the typical yeast species of Pichia anomala Y197-13 [16]. In addition, the cell size was between 2–12 μm, and the cell morphology was oval or semi-oval with a smooth surface (Fig. 1b).
Physicochemical characteristics of M. guilliermondii GXDK6. a Colony characteristics; b Morphology of GXDK6 as observed under a scanning electron microscope; c The relative biomass of GXDK6 in the pH range of 2.5–10.0; d The relative biomass of GXDK6 under gradually increasing salt concentration; e The relative biomass of GXDK6 under different heavy metals; f The relative biomass of GXDK6 in the temperature range of 25–50 °C
Multi-stress-tolerant properties of GXDK6
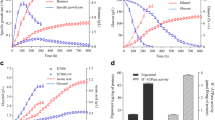
As shown in Fig. 1c, GXDK6 could be incubated continuously in the pH range from 2.5 to 10.0. This result indicated that GXDK6 had a strong resistance to acids and alkalis. However, when GXDK6 was incubated in an acidic condition at pH 2.5 or pH 3.0, the relative biomass GXDK6 was higher than that at pH 9.5 or 10.0, respectively. This result suggested that GXDK6 showed better resistance to an acidic environment.
The salt-tolerance results of GXDK6 indicated that the species was a probiotic with tolerance to high-salt concentrations (Fig. 1d). GXDK6 could be incubated continuously up to 12% NaCl, with the relative biomass of approximately 10% with that in 1% NaCl, and up to 18% KCl or MgCl2, with the relative biomass of near 90% with that with no salt condition. These findings indicated that the growth of GXDK6 decreased drastically with increasing concentration of NaCl up to 12% but showed no significant impact with increasing concentration of KCl or MgCl2 up to 18%. As shown in Fig. 1e, GXDK6 also showed good tolerance to the seven heavy metals (i.e., Cd2+, Cu2+, Mn2+, Co2+, Ni2+, Cr2+, and Zn2+). The maximum tolerated concentration for Ni2+ was 5.8 ppm, while Mn2+ was 5494 ppm. The tolerance for Cu2+ was also more than 1000 ppm, but the maximum tolerance for Co2+, Cr2+, and Zn2+ was more than 250 ppm. This characteristic shows the potential of the species for the deodorization and bioremediation in heavy metal environments. Similar results were reported by Fernández et al. [17] and Bhakta et al. [18]. The temperature sensitivity of GXDK6 was also investigated (Fig. 1f). GXDK6 could be incubated at 25–45 ℃, and the optimal incubation temperature was 30–37 ℃. The relative biomass decreased drastically to 25.22% at 45 ℃ compared with that at 30 ℃. In addition, when the incubation temperature increased to 50 ℃, GXDK6 stopped to reproduce. A similar result was reported by Ndubuisi et al. [19], who found that the growth of heat-resistant Pichia was significantly inhibited by increasing temperature, and the ethanol production efficiency of Pichia was lowered.
Whole genome sequencing analysis of GXDK6
The whole genomic sequencing of GXDK6 was performed using the whole genome shotgun method reported by Marcel et al. [20]. The number of reads of GXDK6 was 8, 319, 572 items, the total bases were 2, 477, 073, 673 bps, of which the GC content accounted for 38.88% (Additional file 1). The fuzzy bases were only 0.001%, Q20% was 94.60%, and Q30% was 86.73%. Genome of GXDK6 was ~ 15,000 bps. The results showed that the extracted DNA was a clear single band, indicating that the extracted DNA was suitable for subsequent analysis (Fig. 2a). Then, a single-base mass distribution map of the sequencing results was constructed (Fig. 2b). The abscissa is the base position of the reads (5ʹ–3ʹ), while the ordinate is the base Q value statistics of all reads. The red and blue lines represent the median and average value of the reads. The yellow line shows that the reads are in the 25% –75% interval, and the tentacles represent that the reads are in the 10–90% interval. The results showed that the bases in the middle had higher base quality. The average quality of the data filtered was also reliable (Fig. 2c).
Identification and whole genome sequencing analysis of M. guilliermondii GXDK6. a The genomic DNA verification map of the Pichia strain; b Quality scores of the Pichia strain across all bases; c The sequence length distribution of the third-generation sequencing data; d The spliced genome information of the Pichia strain; e The phylogenetic tree of the Pichia strain
The sequence length distribution of the third-generation sequencing data is also shown in Fig. 2d, which was mainly used to reflect the average mass distribution of the sequencing data [21]. From Fig. 1e, the whole genome sequence length was good, while the ratio of the uncertain bases to the length of the splicing sequence was 0. This result indicated that the sequence could be used for subsequent gene splicing.
Sequencing and analysis of the ITS domain indicated that the strain with higher consistency basically belong to Meyerozyma sp. The evolutionary distance between GXDK6 and other species has been showed in the species evolutionary tree clearly (Fig. 2e). GXDK6 has a high affinity with 88% confidence level with M. guilliermondii KAML05, M. guilliermondii IFM6377, etc., instead of P. guilliermondii ATCC6260. Therefore, GXDK6 was classified as yeast Meyerozyma guilliermondii.
A complete sequence comparison of the genome sequences was conducted by using the online software BUSCO (http://busco.ezlab.org, v3.0.2) to obtain the percentage of single-copy genes in the total single-copy genes [22]. As shown in Fig. 2e, the spliced genome data of the species were relatively complete. According to the alignment results, GXDK6 belonged to the Pichia genus of the Saccharomyces family and showed the closest relation to M. guilliermondii. Therefore, the yeast Pichia was further confirmed as M. guilliermondii GXDK6.
Ability for single-organic matter fermentation
As shown in Fig. 3a, 21 organic matters (i.e., glucose, sucrose, fructose, xylose, xylan, sorbitol, raffinose, mannose, trehalose, cellulose, maltose, arabic candy, inulin, mannitol, sorbose, D-galactose, cellobiose, wheat bran, ethanol, succinic, and L-rhamnose) as the sole carbon source could be fermented by GXDK6. This result indicated that GXDK6 showed a strong ability to utilize organic matter, such as pentose and hexose.
Ability of GXDK6 for organic matter fermentation. a Relative biomass of M. guilliermondii GXDK6 under the different single-carbon sources; (b) Growth curve of GXDK6 with xylose, L-arabinose, glucose and sucrose as the single-carbon source, respectively; c The peak spectrum of GXDK6 fermented with glucose; d The peak spectrum of GXDK6 fermented with sucrose; e The peak spectrum of GXDK6 fermented with fructose; f The peak spectrum of GXDK6 fermented with xylose
Figure 3b showed that the GXDK6 grew best when used with sorbitol as the sole carbon source, with a dry cell weight of ~ 1.499 g/L, but grew slowest with L-rhamnose, with a dry cell weight of ~ 0.437 g/L. Therefore, the growth rate results showed significant differences in the rate by which GXDK6 utilized diverse organic matters. The order of utilization could be summarized as follows: sorbitol > xylan > raffinose > mannose > sucrose > fructose > trehalose > inulin > maltose > arabic candy > mannitol > glucose > sorbose > D-galactose > cellobiose > xylose > cellulose > ethanol > wheat bran > succinic > L-rhamnose.
Many types of aroma-producing yeasts, such as Hansenula, Candida, S. cerevisiae, Pichia pastoris, and Sphaeropsis sphaeroides, had been reported [23]. The different yeasts also presented diverse aroma-producing characteristics, such as floral fragrance, fruity, delicateness, sweetness, and wine aroma. However, an aroma-producing yeast that can ferment various organic matters, produce abundant aromatic beneficial metabolites, and possess a strong multi-stress tolerance to various environments has not been reported yet. Therefore, M. guilliermondii GXDK6 will be a potential probiotic with important application value.
The metabolites produced by GXDK6 with glucose, sucrose, fructose, or xylose as sole carbon sources was detected with GC–MS method, results showed that 14, 20, 16, and 26 peaks were detected in the samples (Fig. 3a–d). Among them, when GXDK6 was fermented with six carbon sugars (glucose, sucrose) as a single carbon source for 72 h, the top five substances with peak areas were 3,5-ditert-butylphenol, sec-butyl cyclohexyl sulfide, isoamyl alcohol, 3,5-dimethylbenzaldehyde, and ethanol, respectively. However, when GXDK6 was fermented for 72 h with pentose (fructose, xylose) as a single carbon source, the top five substances in peak area were 3,5- ditert-butylphenol, sec-butyl cyclohexyl sulfide, 3,5-dimethylbenzaldehyde, nerol, and isoamyl alcohol (or 2-ethyl hexanol), respectively. Thus, GXDK6 could ferment with different kinds of organic matter as the sole carbon source to produce aromatic metabolites. However, the distinct metabolic regulation networks and mechanisms remain unknown [24, 25], which still need further study.
Metabolomic analysis of GXDK6
As shown in Fig. 4, the metabolites of GXDK6 produced from the selected carbon sources can be classified as alcohols, esters, acids, hydrocarbons, aldehydes, ketones, sulfide, phenolics, and other organic substance (Fig. 4a). However, alcohols, lipids and organic acids are usually defined as the main aromatic metabolites. Based on this, the main aromatic metabolites produced by GXDK6 fermentation of glucose and sucrose are 9 and 13 types, respectively (Table 1), and the aromatic metabolites produced by fermentation of fructose and xylose are 8 and 14 types, respectively (Table 1).
Metabolomic analysis of GXDK6 in fermenting different carbon sources. a Classification of the metabolites; b Wayne diagram analysis of the aromatic metabolites; c Top 5 aromatic metabolites of GXDK6 fermented with glucose; d Top5 aromatic metabolites of GXDK6 fermented with sucrose; e Top 5 aromatic metabolites of GXDK6 fermented with fructose; f Top 5 aromatic metabolites of GXDK6 fermented with xylose
Among these aromatic metabolites, no matter which carbon source is used for fermentation, there are five aromatic metabolites which are the same (Fig. 4b), namely isoamyl alcohol, nerol, phenethyl alcohol, formic acid, and propionic acid, respectively. These results indicate that GXDK6 has the same metabolic pathway when fermenting five-carbon sugar and six-carbon sugar. In order to further studying the difference of aroma production from inconsistent carbon sources of GXDK6 fermentation, the top five aromatic metabolites were selected according to the percentage of content. Among them, isopentanol (15.24, 17.60%), ethanol (9.83, 9.13%), nerol (6.60, 6.46%), isobutanol (9.83, 9.13%), and 2- ethylhexanol (9.83, 9.13%) are the top five aromatic metabolites of six-carbon sugar (glucose and sucrose) fermented by GXDK6. Among the aromatic metabolites of five-carbon sugars (fructose and xylose) fermented by GXDK6, nerol (9.17, 10.15%), isopentanol (2.29, 5.39%), phenethyl alcohol (1.77, 1.62%), formic acid (1.09%, 1.23%), 2- ethylhexanol (1.09%, Fig. 4e) or propionic acid (1.49%) ranked in the top five (Fig. 4f). These results suggested that GXDK6 showed distinct fermentation abilities with different carbon sources as substrates. However, this species could also produce partial similar beneficial aromatic metabolites, which could be due to its diverse mechanisms in fermenting organic matters [26, 27].
Metabolic pathways of nerol and its biosynthesis mechanism
In order to further study the molecular mechanism of aroma production by GXDK6 fermentation, nerol (Additional file 2) was taken as a representative novel aromatic metabolite from M. guilliermondii, which was further compared and analyzed with the whole genome data of GXDK6 (Additional file 3), and the metabolic pathway was further elucidated. As shown in Fig. 5, the upstream sources of glycolysis or citric acid cycle 1-deoxy-d-xylulose5-phosphate geranyl diphosphate or linalool nerol, was firstly converted into final geranic acid or 8-Oxogeranial. In this process, the proteins involved in nerol synthesis were geranyldi phosphotase, monoterphenyl diphosphatase, and geraniol isomerase. The proteins involved in nerol metabolic transformation were geraniol 8-hydroxyase, alcohol dehydrogenase, and geraniol dehydrogenase. As reported by Zong et al. [10], nerol was biosynthesized in the metabolic engineered Escherichia coli from glucose with an accumulation of 0.053 ± 0.015 mg/L, and the biosynthesis mechanism had also been revealed. The truncated neryl diphosphate synthase gene tNDPS1 was expressed that catalyzed isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) form neryl diphosphate (NPP), and then the nerol synthase gene GmNES was co-expressed to synthesize the final product nerol from NPP. However, the accumulation of nerol of GXDK6 was ~ 2.740 mg/L (p < 0.05) when fermented with glucose as the substrate, which is higher than it was produced by E.coli fermentation [10], and the biosynthesis mechanism of nerol in native Meyerozyma guilliermondii has not been reported yet.
The structure and function of the six proteins were further investigated (Fig. 5), results showed that geranyl diphosphatase was a dimer protein with ~ 65 kDa, and its corresponding ligand was found as Mn2+ (Fig. 5b), which suggested that geranyl diphosphatase could be bound and interacted with Mn2+, and promoted the catalytic reaction to produce more nerol [28]. Monoterpenyl diphosphatase was also a dimer protein with a molecular weight ~ 68 kDa, but its corresponding ligand had not been found yet (Fig. 5c), this indicated it should be a non-allosteric enzyme with auxiliary catalysis [29]. Geraniol isomerase was a pentameric protein with ~ 44 kDa (Fig. 5d), and its corresponding ligand was found as geraniol (ligand for nonmetallic ion not shown), indicating that the existence of geraniol was beneficial to the catalytic reaction [30]. In summary, these proteins were indispensable and directly participate in the regulation and biosynthesis of nerol.
The subsequent metabolism or transformation of nerol was catalyzed by geraniol 8-hydroxyase, alcohol dehydrogenase, and geraniol dehydrogenase. Among them, geraniol 8-hydroxyase was a monomeric protein of ~ 55 kDa [31], and its corresponding ligand had not been found yet (Fig. 5e). Alcohol dehydrogenase was also a monomeric protein with a molecular weight of ~ 39 kDa [32], its corresponding ligand was found as Zn2+ (Fig. 5f), indicating alcohol dehydrogenase could be bound and interacted with Zn2+, it would contribute to the formation of nerol. Geraniol dehydrogenase was a dimer protein with ~ 41 kDa [33], and its corresponding ligand had not been found (Fig. 5g), suggesting that it could be a non-allosteric enzyme with auxiliary catalysis. These evidences indicated that the generation of nerol was mainly attributed to the existence of corresponding enzymatic system and metabolic pathway in GXDK6. Furthermore, nerol was classified as a typical example of aromatic metabolites in GXDK6, why GXDK6 can maintain a long-term aroma production should be the contribution of various aromatic metabolites. Therefore, the biosynthesis mechanism of nerol will contribute to better understand of the aroma-producing mechanism of GXDK6.
Conclusions
A novel aroma-producing M. guilliermondii GXDK6 was identified successfully by whole genome sequencing and metabolomics technology. GXDK6 showed high multi-stress-tolerant properties with acid–base, salty, and heavy-metal environments. Furthermore, GXDK6 could be fermented with 21 organic matters (including pentose and hexose) as the sole carbon sources and could produce abundant aromatic metabolites, such as alcohols, esters, acids, etc. The aroma-producing mechanism of nerol in GXDK6 had also been revealed. These findings indicated the M. guilliermondii GXDK6 has great potential value in the fermentation industry.
Materials and methods
Species and reagents
GXDK6 was isolated from the subtropical mangrove sediments in Beibu Gulf of South China Sea (21°29′25.74″N, 109°45′49.43″E) and deposited in the China General Microbiological Culture Collection Center (CGMCC) under CGMCC No. 16007.
The chemical reagents were analytical grade. Glucose, fructose, maltose, sorbitol, lactose, sucrose, mannitol, soluble starch, bran, cassava flour, and bagasse were purchased from Sigma-Aldrich, Inc. (Darmstadt, Germany). Yeast powder, agar powder, peptone, HCl, NaOH, NaCl, and KCl were purchased from Novagen (Darmstadt, Germany). Magnesium chloride, copper chloride, manganese chloride, and cobalt chloride were purchased from Sangon Biotech (Shanghai) Co., Ltd., (Shanghai, China).
Physicochemical characterization of GXDK6
Colony characteristics and cell morphology
GXDK6 was incubated on the GBM agar plate at 30 ℃ for 48 h. The single colony was observed under the Olympus BX53M optical microscope (Olympus, Japan). The single colony without any chemical treatment was also scanned using a scanning electron microscope (TM3000; Olympus, Japan), with a scanning voltage set to 10 kV and magnification of 10, 000 folds.
Multi-stress-tolerant properties of GXDK6
The acid–base stress tolerance of GXDK6 was investigated in the pH range of 2.5–10.0. Salt tolerance was investigated by using NaCl, KCl, and MgCl2 in the concentration range of 12%–18%. Heavy-metal tolerance was investigated using seven heavy metals (i.e., Cd2+, Cu2+, Mn2+, Co2+, Ni2+, Cr2+, and Zn2+) in the concentration range of 0–5494 ppm. Temperature sensitivity was also investigated at 25–50 ℃. Three parallel experiments were performed, and the inoculation rate of GXDK6 was 2% (v:v). The growth of GXDK6 was evaluated using the turbidity method as reported by Irache et al. [34], with slight modifications.
Whole-genome sequencing analysis
GXDK6 was incubated by using an enrichment technique and GBM liquid medium (0.2% yeast extract, 0.2% beef extract, 0.5% polypeptone, 0.6% sucrose, pH 7.0). Both enrichment processes were carried out at 200 rpm and 30 ℃ for 8 h. The bacterial cells were then centrifuged at 8, 000 rpm and 4 °C for 10 min. Then, the cells were washed repeatedly with 0.1 mol/L PBS buffer.
The whole genomic DNA of GXDK6 was extracted using the CTAB method as reported by Van et al. [35], with slight modifications. The purity of the extracted DNA was verified by PCR (Polymerase Chain Reaction) and agarose gel electrophoresis [36]. The ITS gene was amplified by PCR using ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) /ITS4 universal primers (5′- TCCTCCGCTTATTGATATGC-3′) [37]. The ITS sequencing data of GXDK6 were deposited to the National Microbiology Data Center database (http://nmdc.cn) under the Accession Number of NMDCN000022O.
The whole-genome sequencing and analysis of GXDK6 were performed by the BGI Genomics Co., Ltd. (Shenzhen, China). An online software FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc) was used for the quality control of the second-generation sequencing downtime data. To determine the percentage of single-copy genes in the total single-copy genes, an online software BUSCO (http://busco.ezlab.org, v3.0.2) was conducted to complete the sequence comparison of the genome sequences. Hmmscan software [38] was adopted to predict the presence of Carbohydrate-Active enzymes (http://www.cazy.org) [39] genes in the genome sequence. The whole genome sequencing data of GXDK6 were deposited to the National Microbiology Data Center database (http://nmdc.cn) under the Accession Number of NMDC60014229.
Aroma-producing properties of GXDK6
Fermentation of organic matter substrates
A total of 21 kinds of single organic matter (i.e., glucose, sucrose, fructose, xylose, xylan, sorbitol, raffinose, mannose, trehalose, cellulose, maltose, arabic candy, inulin, mannitol, sorbose, D-galactose, cellobiose, wheat bran, ethanol, succinic, and L-rhamnose; 2%) were added to the carbon-free medium [0.05% NaCl, 0.2% (NH4)2SO4, 0.05% K2HPO4, 0.05% KH2PO4, 0.02% MgSO4‧7H2O]. Among these compounds, ethanol was filtered through a 0.22-μm microporous membrane and then added to the sterilized (115 ℃ for 15 min) carbon-free medium. Then, 2% (v:v) of GXDK6 was inoculated in the above medium containing a single organic matter. To explore the ability of GXDK6 to ferment the single organic matters, experiments were performed on a rotary shaker at 200 rpm and 30 ℃ for 48 h.
Metabolomic analysis of GXDK6
GXDK6 was incubated in the medium with glucose, sucrose, fructose, or xylose as the sole carbon source for 72 h. Then, the fermentations were separated by centrifugation at 12, 000 × g for 10 min. The supernatants were then freeze-dried to powder by a refrigerated centrifugal concentrator (Shanghai, China). The powder samples were then derivatized for 120 min by using a 0.1 mg/mL methoxyamine hydrochloride-pyridine solution (Reagents for GC level) and alkylated for 120 min by using trifluoroacetamide (Reagent for GC level). When completed, the samples were centrifuged at 12, 000 × g for 10 min. The aromatic metabolites were detected and analyzed using GC–MS according to Pautova et al. [40], with slight modifications.
Annotation analysis of genes related to aroma production in GXDK6
According to the above metabolomics results of GXDK6, the genes related to aromatics metabolites were retrieved in the KEGG database (https://www.kegg.jp/kegg/genes.html). Sequence of the retrieved genes was then compared and annotated with the genomic data of GXDK6, which is used to screen the key genes of GXDK6 in regulating the expression of aromatic metabolites.
Data analysis of GXDK6
A phylogenetic tree was constructed using the MEGA7.0 software [41]. The whole genome sequencing results were analyzed using the FastQC software [21]. Data fitting and mapping analysis were performed using the Origin 9.0 software. Statistical analysis of other experimental data was performed using SPSS 17.0, and P values < 0.05 indicated significant differences.
Availability of data and materials
All the data generated or analyzed during this study are included in the manuscript and its additional file.
Abbreviations
- GC–MS:
-
Gas chromatography–mass spectrometry
- M. guilliermondii :
-
Meyerozyma guilliermondii
- PCR:
-
Polymerase chain reaction
- ITS:
-
Internal transcribed spacer
- IPP:
-
Isopentenyl diphosphate
- DMAPP:
-
Dimethylallyl diphosphate
- NPP:
-
Neryl diphosphate
References
Feng S, Huang M, Crane JH, Wang Y. Characterization of key aroma-active compounds in lychee (Litchi chinensis Sonn.). J Food Drug Anal. 2018;26(2):497–503. https://doi.org/10.1016/j.jfda.2017.07.013.
Saeed Y, Faezeh D, Ali MB, Saeid H, Tumach Y, Khosro M. Morphological, essential oil and biochemical variation of Dracocephalum moldavica L. populations. J Appl Res Med Aroma. 2018;10:S332772936. https://doi.org/10.1016/j.jarmap.2018.06.005.
Wang Y, Zeng X, Zhou Z, Xing K, Tessema A, Zeng H, Tian J. Inhibitory effect of nerol against Aspergillus niger on grapes through a membrane lesion mechanism. Food Control. 2015;55:54–61. https://doi.org/10.1016/j.foodcont.2015.02.029.
Tian J, Gan Y, Pan C, Zhang M, Wang X, Tang X, Peng X. Nerol-induced apoptosis associated with the generation of ROS and Ca (2+) overload in saprotrophic fungus Aspergillus flavus. Appl Microbiol Biotechnol. 2018;102(15):6659–72. https://doi.org/10.1007/s00253-018-9125-z.
Nedovic V, Gibson B, Mantzouridou TF, Bugarski B, Djordjevic V, Kalusevic A, Paraskevopoulou A, Sandell M, Smogrovicova D, Yilmaztekin M. Aroma formation by immobilized yeast cells in fermentation processes. Yeast. 2015;32(1):173–216. https://doi.org/10.1002/yea.3042.
Du Toit E, Vesterlund S, Gueimonde M, Salminen S. Assessment of the effect of stress-tolerance acquisition on some basic characteristics of specific probiotics. Int J Food Microbiol. 2013;165(1):51–6. https://doi.org/10.1016/j.ijfoodmicro.2013.04.022.
Bletz MC, Loudon AH, Becker MH, Bell SC, Woodhams DC, Minbiole KPC, Harris RN. Mitigating amphibian chytridiomycosis with bioaugmentation: characteristics of effective probiotics and strategies for their selection and use. Ecol Lett. 2013;16(6):807–20. https://doi.org/10.1111/ele.12099.
Srisukchayakul P, Charalampopoulos D, Karatzas KA. Study on the effect of citric acid adaptation toward the subsequent survival of Lactobacillus plantarum NCIMB 8826 in low pH fruit juices during refrigerated storage. Food Res Int. 2018;111:198–204. https://doi.org/10.1016/j.foodres.2018.05.018.
Martínez Cruz P, Ibáñez AL, Monroy Hermosillo OA, Ramírez Saad HC. Use of probiotics in aquaculture. ISRN Microbiology. 2012;2012:1–13. https://doi.org/10.5402/2012/916845.
Zong Z, Hua Q, Tong X, Li D, Wang C, Guo D, Liu Z. Biosynthesis of nerol from glucose in the metabolic engineered Escherichia coli. Biores Technol. 2019;287:121410. https://doi.org/10.1016/j.biortech.2019.121410.
Coda R, Rizzello CG, Cagno RD, Trani A, Cardinali G, Gobbetti M. Antifungal activity of Meyerozyma guilliermondii: Identification of active compounds synthesized during dough fermentation and their effect on long-term storage of wheat bread. Food Microbiol. 2013;33(2):243–51. https://doi.org/10.1016/j.fm.2012.09.023.
Wah TT, Walaisri S, Assavanig A, Niamsiri N, Lertsiri S. Co-culturing of Pichia guilliermondii enhanced volatile flavor compound formation by Zygosaccharomyces rouxii in the model system of Thai soy sauce fermentation. Int J Food Microbiol. 2013;160(3):282–9. https://doi.org/10.1016/j.ijfoodmicro.2012.10.022.
Tayel AA, El-Tras WF, Moussa SH, El-Agamy MA. Antifungal action of Pichia anomala against aflatoxigenic Aspergillus flavus and its application as a feed supplement. J Sci Food Agric. 2013;93(13):3259–63. https://doi.org/10.1002/jsfa.6169.
Kim WCKN. Isolation, identification, and characterization of Pichia guilliermondii K123–1 and Candida fermentati SI, producing isoflavone β-glycosidase to hydrolyze isoflavone glycoside efficiently, from the Korean Traditional Soybean Paste. J Appl Biol Chem. 2009;52:163–9. https://doi.org/10.3839/jabc.2009.028.
Boretsky YR, Pynyaha YV, Boretsky VY, Fedorovych DV, Fayura LR, Protchenko O, Philpott CC, Sibirny AA. Identification of the genes affecting the regulation of riboflavin synthesis in the flavinogenic yeast Pichia guilliermondii using insertion mutagenesis. FEMS Yeast Res. 2011;11(3):307–14. https://doi.org/10.1111/j.1567-1364.2011.00720.x.
Kim HR, Kim JH, Bai DH, Ahn BH. Microbiological characteristics of wild yeast strain Pichia anomala Y197–13 for Brewing Makgeolli. Mycobiology. 2013;41(3):139–44. https://doi.org/10.5941/MYCO.2013.41.3.139.
Fernández PM, Martorell MM, Blaser MG, Ruberto LAM, de Figueroa LIC, Mac Cormack WP. Phenol degradation and heavy metal tolerance of Antarctic yeasts. Extremophiles. 2017;21(3):445–57. https://doi.org/10.1007/s00792-017-0915-5.
Bhakta JN, Ohnishi K, Munekage Y, Iwasaki K, Wei MQ. Characterization of lactic acid bacteria-based probiotics as potential heavy metal sorbents. J Appl Microbiol. 2012;112(6):1193–206. https://doi.org/10.1111/j.1365-2672.2012.05284.x.
Ndubuisi IA, Qin Q, Liao G, Wang B, Moneke AN, Ogbonna JC, Jin C, Fang W. Effects of various inhibitory substances and immobilization on ethanol production efficiency of a thermotolerant Pichia kudriavzevii. Biotechnol Biofuels. 2020. https://doi.org/10.1186/s13068-020-01729-5.
Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen Y, Chen Z, et al. Genome sequencing in open microfabricated high density picoliter reactors. Nature. 2005;437(7057):376–80. https://doi.org/10.1038/nature03959.
Kim T, Seo HD, Hennighausen L, Lee D, Kang K. Octopus-toolkit: a workflow to automate mining of public epigenomic and transcriptomic next-generation sequencing data. Nucleic Acids Res. 2018;46(9):e53. https://doi.org/10.1093/nar/gky083.
Hunt M, Kikuchi T, Sanders M, Newbold C, Berriman M, Otto TD. REAPR: a universal tool for genome assembly evaluation. Genome Biol. 2013;14(5):R47. https://doi.org/10.1186/gb-2013-14-5-r47.
Lai YT, Cheng KC, Lai CN, Lai YJ. Isolation and identification of aroma producing strain with esterification capacity from yellow water. PLoS ONE. 2019;14(2):e211356. https://doi.org/10.1371/journal.pone.0211356.
Irma Isnafia A, Dyah Nurul A, Zakiah W, Cahyo B. Physicochemical properties, fatty acid prfiles, and sensory characteristics of fermented beef sausage by probiotics Lactobacillus plantarum IIA-2C12 or Lactobacillus acidophilus IIA-2B4. J Food Sci. 2016;81(11):M2761–9. https://doi.org/10.1111/1750-3841.13509.
Liu Y, Cheng H, Liu H, Ma R, Ma J, Fang H. Fermentation by multiple bacterial strains improves the production of bioactive compounds and antioxidant activity of goji juice. Molecules. 2019. https://doi.org/10.3390/molecules24193519.
Singh A, Vishwakarma V, Singhal B. Metabiotics: the functional metabolic signatures of probiotics: current state-of-art and future research priorities-metabiotics: probiotics effector molecules. Adv Biosci Biotechnol. 2018;09(04):147–89. https://doi.org/10.4236/abb.2018.94012.
Wang M, Zhang X, Wang Y, Li Y, Chen Y, Zheng H, Ma F, Ma CW, Lu B, Xie Z, et al. Metabonomic strategy for the detection of metabolic effects of probiotics combined with prebiotic supplementation in weaned rats. RSC Adv . 2018;8(9):5042–57. https://doi.org/10.1039/c7ra12067b.
Nah J, Song SJ, Back K. Partial characterization of farnesyl and geranylgeranyl diphosphatases induced in rice seedlings by UV-C irradiation. Plant Cell Physiol. 2001;42(8):864–7. https://doi.org/10.1093/pcp/pce102.
Rai A, Smita SS, Singh AK, Shanker K, Nagegowda DA. Heteromeric and homomeric geranyl diphosphate synthases from catharanthus roseus and their role in monoterpene indole alkaloid biosynthesis. Mol Plant. 2013;6(005):1531–49. https://doi.org/10.1093/mp/sst058.
Marmulla R, Safaric B, Markert S, Schweder T, Harder J. Linalool isomerase, a membrane-anchored enzyme in the anaerobic monoterpene degradation in Thauera linaloolentis 47Lol. BMC Biochem. 2016;17:6. https://doi.org/10.1186/s12858-016-0062-0.
Sintupachee S, Promden W, Ngamrojanavanich N, Sitthithaworn W, De-Eknamkul W. Functional expression of a putative geraniol 8-hydroxylase by reconstitution of bacterially expressed plant CYP76F45 and NADPH-cytochrome P450 reductase CPR I from Croton stellatopilosus Ohba. Phytochemistry. 2015;118:204–15. https://doi.org/10.1016/j.phytochem.2015.08.005.
Magnusson AO, Szekrenyi A, Joosten HJ, Finnigan J, Charnock S, Fessner WD. nanoDSF as screening tool for enzyme libraries and biotechnology development. The FEBS Journal. 2019;286(1):184–204. https://doi.org/10.1111/febs.14696.
Noge K, Kato M, Mori N, Kataoka M, Tanaka C, Yamasue Y, Nishida R, Kuwahara Y. Geraniol dehydrogenase, the key enzyme in biosynthesis of the alarm pheromone, from the astigmatid mite iCarpoglyphuslactis/i (Acari: Carpoglyphidae). The FEBS Journal. 2010;275(11):2807–17. https://doi.org/10.1111/j.1742-4658.2008.06421.x.
Irache JM, Durrer C, Ponchel G, Duchêne D. Determination of particle concentration in latexes by turbidimetry. Int J Pharm. 1993;90(3):R9–12. https://doi.org/10.1016/0378-5173(93)90203-R.
van Burik JA, Schreckhise RW, White TC, Bowden RA, Myerson D. Comparison of six extraction techniques for isolation of DNA from filamentous fungi. Med Mycol. 1998;36(5):299–303.
Ehrhardt DW, Frommer WB. New technologies for 21st century plant science. Plant Cell. 2012;24(2):374–94. https://doi.org/10.1105/tpc.111.093302.
Dupont D, Gaucherand P, Wallon M. Fortuitous diagnosis of Trichomoniasis by PCR using panfungal primers. Int J Infect Dis . 2020;90:234–6. https://doi.org/10.1016/j.ijid.2019.11.008.
Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35(9):3100–8. https://doi.org/10.1093/nar/gkm160.
Lombard V, Golaconda RH, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014. https://doi.org/10.1093/nar/gkt1178.
Pautova AK, Bedova AY, Sarshor YN, Beloborodova NV. Determination of aromatic microbial metabolites in blood serum by gas chromatography-mass spectrometry. J Anal Chem. 2018;73(2):160–6. https://doi.org/10.1134/s106193481802008.
Xia W, Zhao P, Yi Z, Cui Y. Phylogenetic and specific sequence analysis of four paralogs in insect Aquaporins. Mol Med Rep. 2017;16(4):4903–8. https://doi.org/10.3892/mmr.2017.7148.
Acknowledgements
Not applicable.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 31760437), the Science and Technology Basic Resources Investigation Program of China (Grant No. 2017FY100704), the Natural Science Fund for Distinguished Young Scholars of Guangxi Zhuang Autonomous Region of China (Grant No. 2019GXNSFFA245011), the Natural Science Foundation of Guangxi Zhuang Autonomous Region of China (Grant No. 2018GXNSFAA050090, 2017GXNSFBA198101).
Author information
Authors and Affiliations
Contributions
XM: Formal analysis, Methodology, Investigation, Data curation, Software, Roles/Writing—original draft. XC: Data curation, Formal analysis, Software, Roles/Writing—original draft. QH Methodology, Formal analysis, Software. HS: Methodology, Data curation, Formal analysis. RY: Data curation, Formal analysis. RB: Validation, Visualization. BY: Funding acquisition, Resources. QO: Formal analysis, Funding acquisition. QL: Formal analysis. SH: Conceptualization, Supervision, Validation. CJ: Conceptualization, Funding acquisition, Supervision, Writing—review & editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have agreed to submit this manuscript to microbial cell factories.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Statistics of the sequencing data of GXDK6.
Additional file 2.
Mass spectrum analysis of nerol when fermented with GXDK6 using glucose as the substrate.
Additional file 3.
Gene annotation results related to the biosynthesis of nerol in GXDK6 (Identity ≥ 30%).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mo, X., Cai, X., Hui, Q. et al. Whole genome sequencing and metabolomics analyses reveal the biosynthesis of nerol in a multi-stress-tolerant Meyerozyma guilliermondii GXDK6. Microb Cell Fact 20, 4 (2021). https://doi.org/10.1186/s12934-020-01490-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-020-01490-2