Abstract
Background
The triglyceride-glucose (TyG) index is regarded as a dependable alternative for assessing insulin resistance (IR), given its simplicity, cost-effectiveness, and strong correlation with IR. The relationship between the TyG index and adverse outcomes in patients with coronary heart disease (CHD) is not well established. This study examines the association of the TyG index with long-term adverse outcomes in hospitalized CHD patients.
Methods
In this single-center prospective cohort study, 3321 patients hospitalized with CHD were included. Multivariate Cox regression models were employed to assess the associations between the TyG index and the incidence of all-cause mortality and major adverse cardiovascular events (MACEs). To examine potential nonlinear associations, restricted cubic splines and threshold analysis were utilized.
Results
During a follow-up period of 9.4 years, 759 patients (22.9%) succumbed to mortality, while 1291 (38.9%) experienced MACEs. Threshold analysis demonstrated a significant “U”-shaped nonlinear relationship with MACEs, with different hazard ratios observed below and above a TyG index of 8.62 (below: HR 0.71, 95% CI 0.50–0.99; above: HR 1.28, 95% CI 1.10–1.48). Notably, an increased risk of all-cause mortality was observed only when the TyG index exceeded 8.77 (HR 1.53, 95% CI 1.19–1.96).
Conclusions
This study reveals a nonlinear association between the TyG index and both all-cause mortality and MACEs in hospitalized CHD patients with CHD. Assessing the TyG index, particularly focusing on individuals with extremely low or high TyG index values, may enhance risk stratification for adverse outcomes in this patient population.
Similar content being viewed by others
Introduction
Coronary heart disease (CHD) represents a prevalent form of cardiovascular disease and a leading cause of mortality globally, exerting a considerable burden on public health worldwide [1]. Exploring predictive markers for risk stratification in patients with CHD is crucial to mitigate adverse outcomes.
Insulin resistance (IR), often occurring years before the onset of type 2 diabetes (T2DM), is characterized by a reduced response to insulin, diminishing its effectiveness [2]. IR plays a central role in the early stages of T2DM and is closely associated with the onset and progression of CHD [3]. Insulin resistance, as an abnormal metabolic status, can affect the levels of various metabolic markers in the body, including triglycerides and glucose. Simental et al. [4] first discovered a strong correlation between the triglyceride-glucose (TyG) index, obtained by normalizing the product of fasting glucose and triglycerides, and insulin resistance, surpassing the correlation observed with the homeostasis model assessment-estimated insulin resistance index. The TyG index also exhibits better predictive performance in identifying diabetic patients than when using fasting glucose and triglycerides separately [5]. Subsequent extensive research has confirmed TyG index as a cost-effective and convenient alternative marker of insulin resistance [6].
In the past decade, numerous studies have been conducted to ascertain if the TyG index can act as an alternative indicator of IR in stratifying the risk for adverse outcomes. The TyG index is not only associated with the occurrence of cardiovascular disease (CVD) [7,8,9,10,11,12,13,14,15] but also with adverse outcomes in patients with CVD [16,17,18,19,20,21,22,23]. Numerous studies have independently confirmed the association of the TyG index with adverse outcomes in CHD patients, both with and without T2DM [18, 24, 25]. However, many studies have yielded conflicting results [26, 27]. Recent research has also suggested a potential “U”-shaped nonlinear association between the TyG index and mortality in patients with CVD [28, 29], as well as the incidence of CVD in older individuals [30].
The inconsistent conclusions from these studies highlight the need for further research to confirm the relationship between the TyG index and adverse cardiovascular events. Currently, there is a lack of comprehensive cohort studies supporting the predictive value of the TyG index for adverse outcomes over a long period in hospitalized patients with CHD, especially concerning the potential nonlinear association between the TyG index and these outcomes. This study aims to examine the association between the TyG index and adverse outcomes over an extended period in hospitalized patients with CHD and to explore potential nonlinear associations.
Methods
Study design and participants
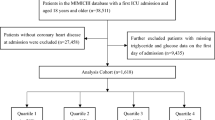
This was a prospective cohort study conducted from October 2010 to September 2014. A total of 3,670 hospitalized patients aged 20 to 90 years, diagnosed with CHD and hemodynamically stable, were consecutively recruited from the Department of Cardiology at the Chinese PLA General Hospital [31, 32]. Eligibility for recruitment required a diagnosis of coronary heart disease confirmed through coronary angiography, showing at least one stenosis greater than 50% [33]. We excluded patients who: (1) had conditions such as cardiogenic shock, refractory hypertension, hypertrophic cardiomyopathy, severe valvular heart disease, malignant tumors, severe anemia, myocarditis, and active infections; (2) had a life expectancy of less than one year; (3) were missing key variables. After excluding three heart transplant patients, 175 patients with missing key variables, and 171 lost to follow-up, a total of 3,321 patients were included in the study. The rate of loss to follow-up was 4.9%. The ethics committee of Chinese PLA General Hospital approved this study, and all participants provided written informed consent at the baseline visit.
TyG index
We extracted fasting triglyceride and blood glucose values, measured at admission, from the electronic medical records. The TyG index was calculated as follows [4]: \(TyG\,index=ln \left(\frac{fasting\,triglyceride\,(mg/dL) \times fasting\, glucose\,(mg/dL)}{2} \right)\). We stratified all patients into five groups based on their TyG index quintiles, with each group comprising 664 individuals, except for the first quintile group.
Covariates
We collected clinical data of the patients, including cardiovascular risk factors, medication usage, and baseline details such as age, gender, vital signs, physical examination, and biochemical parameters. The estimated glomerular filtration rate (eGFR) was calculated using the modified Modification of Diet in Renal Disease (MDRD) equation [34]: \(eGFR\,(ml/min/1.73\,m^{2})=175 \times Scr\,(mg/dL) - 1.234 \times Age - 0.179 \times 0.79\,(if\,female).\). The body mass index (BMI) was calculated as follows: \(BMI\,(kg/m^{2})=weight\,(kg)\,height^{2}\,(m).\).
Outcomes and follow-up
The primary outcome of the study was all-cause mortality, while the secondary outcome encompassed major adverse cardiovascular events (MACEs). MACEs were defined as a composite of cardiovascular mortality, myocardial infarction (MI), stroke or transient ischemic attack (TIA), and heart failure (HF) or hospitalization for heart failure (HHF). Telephone follow-ups with all recruited patients occur every two years, with the most recent follow-up deadline in March 2023. Patients without recorded events by this date were considered as right-censored in the analysis.
Statistical analysis
Continuous variables were expressed as either mean with standard deviation (SD) or median with interquartile range (IQR); categorical variables were presented as count and percentage. Descriptive analyses were conducted using Student’s t-test, Mann-Whitney U test, and χ2 test, as appropriate.
For the survival analysis, multivariate Cox regression models were utilized to investigate the association between the TyG index and long-term outcomes, including all-cause mortality and MACEs. The TyG index was evaluated as a categorical variable in the multivariable Cox regression model. This model adjusted for age, gender, BMI, smoking, acute coronary syndrome (ACS), previous MI, stroke, hypertension, T2DM, statin, beta blocker, angiotensin-converting enzyme inhibitor (ACE-I)/angiotensin II receptor blocker (ARB), left ventricular ejection fraction (LVEF), eGFR, total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C). LVEF, TC, and LDL-C underwent natural logarithm transformation prior to inclusion in the models. Nonlinear associations were evaluated using restricted cubic splines to fit smooth curves. Threshold analyses for each model were conducted. The threshold value was determined by exhaustively exploring all possible values, identifying the optimal threshold point based on maximizing the likelihood, and subsequently applying a segmented fit of the Cox regression model.
All statistical analyses were performed using Stata version 17.0 (StataCorp LLC, College Station, TX, USA) and R version 4.1.2 (The R Project for Statistical Computing, Vienna, Austria). A two-tailed p-value of < 0.05 was deemed statistically significant.
Results
Baseline characteristics
Table 1 presents the baseline characteristics of the 3,321 participants included in the study, stratified by their survival status. The mean age of the study population was 61.7 ± 11.7 years; 72.4% were male; the TyG index ranged from 6.10 to 12.03 with a mean (SD) of 8.9 (0.7). Compared to survivors, deceased patients exhibited higher levels of age, systolic blood pressure (BP), creatinine, FBG, and prevalence of ACS, T2DM, hypertension, previous MI, and stroke. Conversely, deceased patients had lower levels of BMI, diastolic BP, LVEF, eGFR, and fasting TG, along with a decreased prevalence of statin use and current smoking. Baseline characteristics of study participants, stratified by diabetes status, are presented in the additional file (Additional file 1: Table S1).
Association between TyG index and adverse outcomes
The total follow-up time for 3321 patients with CHD was 29,007.8 person-years (PYs). The median follow-up duration stood at 9.39 (8.70–10.52) years. Throughout the follow-up period, 759 patients encountered all-cause mortality, yielding an incidence rate of 26.17 per 1000 PYs. In addition, 1291 patients experienced MACEs, with an incidence rate of 49.17 per 1000 PYs (Table 2).
The results of the multivariable Cox regression model suggest a potential nonlinear relationship between the TyG index, when included as a categorical variable in the model, and the all-cause mortality and MACEs (Table 2).
Nonlinear association of TyG index with adverse outcomes
To examine the nonlinear association between the TyG index and adverse outcomes, we employed restricted cubic splines. This was followed by threshold analyses for each model and a segmented fit of the Cox regression model.
In the crude model, a significant “U”-shaped nonlinear association was observed between the TyG index and both all-cause mortality and MACEs (both p for nonlinearity < 0.05; Fig. 1). Threshold analysis identified distinct inflection points for all-cause mortality (TyG index = 8.93) and MACEs (TyG index = 8.95; both p for log-likelihood ratio < 0.05). Table 3 presents the segmented fitting results of the Cox regression model.
In the fully adjusted model, the TyG index maintained its significant “U”-shaped nonlinear association with MACEs (TyG index < 8.62: per unit increase, HR 0.71, 95% CI 0.50–0.99; TyG index > 8.62: per unit increase, HR 1.28, 95% CI 1.10–1.48; p for log-likelihood ratio = 0.009; Fig. 2; Table 3). Additionally, the TyG index continued to exhibit a significant nonlinear association with all-cause mortality (p for nonlinearity = 0.015). However, threshold analysis revealed significance only when the TyG index exceeded 8.77, indicating a higher risk of all-cause mortality (per unit increase, HR 1.53, 95% CI 1.19–1.96). Below this threshold, the TyG index was not significantly associated with all-cause mortality (per unit increase, HR 1.01, 95% CI 0.73–1.38) (Table 3).
The nonlinear association of TyG index with adverse outcomes in the fully adjusted model. The nonlinear association of TyG index with (a) all-cause mortality and (b) MACEs. Spline curves were adjusted for age, gender, BMI, smoking, ACS, previous myocardial infarction, stroke, hypertension, diabetes mellitus, statin, beta blocker, ACE-I/ARB, LVEF, eGFR, TC, LDL-C
We also investigated the nonlinear relationship between the TyG index and adverse outcomes among diabetic and non-diabetic patient subgroups in the multivariate model. In both subgroups, a “U”-shaped association was noted between the TyG index and MACEs (nonlinear p < 0.05; Fig. 3b, d). In non-diabetic patients, a nonlinear association was observed between the TyG index and all-cause mortality (nonlinear p = 0.035; Fig. 3a), with an increased risk of mortality at a TyG index above the inflection point. Conversely, in diabetic patients, there was a linear relationship between the increase in the TyG index and the risk of all-cause mortality (nonlinear p = 0.301; Fig. 3c).
The nonlinear association of the TyG index with adverse outcomes in the fully adjusted model for both diabetic and non-diabetic patients. The nonlinear association of TyG index with (a) all-cause mortality and (b) MACEs in non-diabetic patients. The nonlinear association of TyG index with (c) all-cause mortality and (d) MACEs in diabetic patients. Spline curves were adjusted for age, gender, BMI, smoking, ACS, previous myocardial infarction, stroke, hypertension, diabetes mellitus, statin, beta blocker, ACE-I/ARB, LVEF, eGFR, TC, LDL-C. When the nonlinear association is significant, the reference point is the inflection point; otherwise, it is the median of the TyG index
Additionally, we have presented the relationships between triglycerides and glucose with all-cause mortality and MACEs (Additional file 1: Fig. S1), as well as the relationship between TyG index and the components of MACEs (Additional file 1: Fig. S2) in Figures in the appendix.
Discussion
In this study, we have for the first time identified a significant nonlinear association between the baseline TyG index and increased risk of all-cause mortality and MACEs over an extended period in hospitalized patients with CHD. The results from the threshold analysis revealed a distinct inflection point in the association between TyG index and all-cause mortality and MACEs, where TyG index demonstrated a U-shaped association with MACEs. Beyond the inflection point, an elevated TyG index correlated with an increased risk of all-cause mortality.
Previous clinical studies have linked the TyG index with CVD in the general population without a history of CVD [7,8,9]. This correlation has been observed in various groups, including middle-aged and elderly individuals, adolescents, individuals with and without T2DM, populations at high risk for CVD, and postmenopausal women [10,11,12,13,14,15]. Among CVD patients, an elevated TyG index has been associated with an increased risk of major adverse cardiovascular and cerebrovascular events in individuals with stable CHD combined with T2DM [16]. Numerous studies have independently confirmed the association of the TyG index with MACEs in patients with ACS, irrespective of T2DM status [17,18,19,20,21,22,23]. In our study, we also noted that an elevated TyG index beyond 8.77 and 8.62 was significantly associated with increased risks of both all-cause mortality and MACEs. Further examination of the nonlinear association between the TyG index and adverse outcomes in both diabetic and non-diabetic patients showed a transition to a linear association in diabetic patients, where an increase in the TyG index correlated with an increased risk of all-cause mortality. The observed change in the association may arise from the smaller number of patients with CHD combined with T2DM included in this study.
The precise biological mechanism connecting the TyG index to adverse cardiovascular events in patients with CHD remains elusive. As a reliable surrogate for IR, the primary mechanism behind the negative outcomes associated with a heightened TyG index could be related to IR. Patients with IR typically exhibit a heightened risk of metabolic disorders, such as hyperglycemia, dyslipidemia, and hypertension, all closely linked to negative CVD outcomes [35,36,37]. Additionally, IR can cause increased platelet activity and elevated adhesion-induced expression of thromboxane A2-dependent tissue factors in platelets, leading to thrombosis and inflammation [38]. This may partly explain the occurrence of ischemic events observed in CHD patients, including MI and stroke. Furthermore, prolonged IR can lead to enhanced activity of the sympathetic nervous system and renal sodium retention, resulting in higher blood pressure and increased cardiac afterload [39, 40]. Chronic hyperglycemia and dyslipidemia caused by IR can induce oxidative stress, exacerbate inflammatory responses, impair endothelial function, and promote the proliferation of smooth muscle cells and collagen deposition. These factors might contribute to cardiac fibrosis and eventually heart failure [22, 41].
Previous research has indicated that extremely low levels of TG or FBG are linked to adverse health outcomes and could precipitate disease [42]. The results of this study also demonstrate a similar trend (Additional file, Figure S1). Hypoglycemia has been associated with an increased risk of cardiovascular events or cerebrovascular stroke [43]. Similarly, low TG levels have been recognized as a predictive factor for cardiac mortality in patients with heart failure [44]. Prior studies have demonstrated a significant relationship between a reduction in the TyG index within a specific range and a heightened risk of adverse events [28,29,30]. In our study, a significant association was found between a reduction in the TyG index and an increased long-term risk of MACEs in CHD patients when the TyG index fell below 8.62. A similar trend was noted in the association between the TyG index and all-cause mortality, although the statistical significance of this trend might be limited by the sample size and residual confounders.
Previous research indicates that an elevated TyG index is associated with an increased risk of major adverse cardiovascular and cerebrovascular events in individuals with stable CHD combined with T2DM [16]. Numerous studies have independently confirmed the association of the TyG index with MACEs in diabetic patients with CHD [18, 24, 45,46,47]. As for non-diabetic patients with CHD, Zhao et al. showed that the TyG index may serve as a useful predictive marker for MACEs in the non-diabetic population with ACS patients [25]. Our study results reveal that the TyG index is not only associated with MACEs in CHD patients but also exhibit a “U”-shaped nonlinear relationship. However, some researchers present different perspectives. A cohort study involving 5,489 non-diabetic patients who underwent percutaneous coronary intervention (PCI) indicated that the TyG index is not an effective predictive factor for adverse cardiovascular prognosis in non-diabetic patients undergoing PCI [48]. A similar conclusion was also published in another cohort study, suggesting that the TyG index should not be used as a predictor of MACEs and all-cause mortality among non-diabetic patients with MI at a 1-year follow-up [49]. Currently, there are no other research reports the nonlinear relationship between TyG index and MACEs. However, other researchers have identified a “U”-shaped nonlinear association between the TyG index and all-cause mortality and cardiovascular mortality through threshold analysis [28,29,30]. This finding suggests that the potential protective or harmful effects of different TyG index levels on either side of the inflection points might counterbalance within the integrated model. This counterbalance could explain why some researchers have inferred a lack of significant correlation between the TyG index and adverse outcomes [26, 27, 48, 49].
This study also explored the correlation between the TyG index and each component of MACEs in CHD patients (Additional file, Figure S2). We observed that cardiovascular mortality in patients with CHD showed an elevated risk when the TyG index exceeded the inflection point, mirroring the trend observed in all-cause mortality. This differs from the “U”-shaped nonlinear correlation of the TyG index with cardiovascular mortality and all-cause mortality found by Zhang et al. in CVD patients [28]. Additionally, we identified a “U”-shaped nonlinear correlation between the TyG index and long-term HF/HHF in patients with CHD.
Previous studies have not explored the predictive value of the TyG index for long-term adverse outcomes in hospitalized patients with CHD. The unique contribution of this study is its identification of a nonlinear relationship between the TyG index and long-term adverse outcomes in a substantial cohort of hospitalized patients with CHD, particularly noting the “U”-shaped relationship between the TyG index and subsequent MACEs. Nevertheless, this study is not without limitations. Firstly, as a single-center study involving adults in China, its findings might not extend to other demographic groups. Secondly, despite efforts to control confounding variables, eliminating all residual confounding factors affecting prognosis, such as nutritional and socioeconomic status, is unattainable due to the lack of relevant variables. Thirdly, the study did not account for the dynamic changes in the TyG index of patients. Future research should focus on the dynamic changes in patients’ TyG index and their association with adverse outcomes, as well as interventions targeting the TyG index to improve clinical outcomes.
Our study presented evidence of a nonlinear association between the TyG index and long-term adverse outcomes in both diabetic and non-diabetic populations with CHD. Upon further recognition of the clinical utility of the TyG index in the future, it could be automatically calculated by a computer based on the patient’s measured fasting glucose and triglycerides, similar to eGFR (calculated by the computer based on creatinine), providing clinicians with greater convenience in guiding clinical decisions.
Conclusion
This study revealed a nonlinear relationship between the TyG index and both all-cause mortality and MACEs among hospitalized patients with CHD. Assessing the TyG index, particularly focusing on individuals with extremely low or high TyG index values, could improve risk assessment for adverse outcomes in this group. Future studies are warranted to investigate interventions that target the TyG index to enhance clinical outcomes.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- ACE-I:
-
Angiotensin-converting enzyme inhibitor
- ACS:
-
Acute coronary syndrome
- ARB:
-
Angiotensin II receptor blocker
- BMI:
-
Body Mass Index
- BP:
-
Blood pressure
- CHD:
-
Coronary heart disease
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- eGFR:
-
Estimated glomerular filtration rate
- FBG:
-
Fasting blood glucose
- HF:
-
Heart failure
- HHF:
-
Hospitalization for heart failure
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- IR:
-
Insulin resistance
- LDL-C:
-
Low-density lipoprotein cholesterol
- LVEF:
-
Left ventricular ejection fraction
- MACEs:
-
Major adverse cardiovascular events
- MDRD:
-
Modification of diet in renal disease
- MI:
-
Myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- PYs:
-
Person-years
- SD:
-
Standard deviation
- T2DM:
-
Type 2 diabetes mellitus
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- TIA:
-
Transient ischemic attack
- TyG:
-
Triglyceride-glucose
References
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2020 update: a Report from the American Heart Association. Circulation. 2020;141(9):e139–e596.
Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–95.
Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953.
Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Navarro-Gonzalez D, Sanchez-Inigo L, Pastrana-Delgado J, Fernandez-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the vascular-metabolic CUN cohort. Prev Med. 2016;86:99–105.
Gastaldelli A. Measuring and estimating insulin resistance in clinical and research settings. Obesity (Silver Spring). 2022;30(8):1549–63.
Cui H, Liu Q, Wu Y, Cao L. Cumulative triglyceride-glucose index is a risk for CVD: a prospective cohort study. Cardiovasc Diabetol. 2022;21(1):22.
Sanchez-Inigo L, Navarro-Gonzalez D, Fernandez-Montero A, Pastrana-Delgado J, Martinez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–97.
Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran lipid and glucose study. Cardiovasc Diabetol. 2020;19(1):155.
Feng G, Yang M, Xu L, Liu Y, Yu J, Zang Y, et al. Combined effects of high sensitivity C-reactive protein and triglyceride-glucose index on risk of cardiovascular disease among middle-aged and older Chinese: evidence from the China Health and Retirement Longitudinal Study. Nutr Metab Cardiovasc Dis. 2023;33(6):1245–53.
Xu X, Huang R, Lin Y, Guo Y, Xiong Z, Zhong X, et al. High triglyceride-glucose index in young adulthood is associated with incident cardiovascular disease and mortality in later life: insight from the CARDIA study. Cardiovasc Diabetol. 2022;21(1):155.
Liu L, Wu Z, Zhuang Y, Zhang Y, Cui H, Lu F, et al. Association of triglyceride-glucose index and traditional risk factors with cardiovascular disease among non-diabetic population: a 10-year prospective cohort study. Cardiovasc Diabetol. 2022;21(1):256.
Zhao M, Xiao M, Tan Q, Lu F. Triglyceride glucose index as a predictor of mortality in middle-aged and elderly patients with type 2 diabetes in the US. Sci Rep. 2023;13(1):16478.
Cai XL, Xiang YF, Chen XF, Lin XQ, Lin BT, Zhou GY, et al. Prognostic value of triglyceride glucose index in population at high cardiovascular disease risk. Cardiovasc Diabetol. 2023;22(1):198.
Liu Q, Si F, Liu Z, Wu Y, Yu J. Association between triglyceride-glucose index and risk of cardiovascular disease among postmenopausal women. Cardiovasc Diabetol. 2023;22(1):21.
Jin JL, Sun D, Cao YX, Guo YL, Wu NQ, Zhu CG, et al. Triglyceride glucose and haemoglobin glycation index for predicting outcomes in diabetes patients with new-onset, stable coronary artery disease: a nested case-control study. Ann Med. 2018;50(7):576–86.
Xie E, Ye Z, Wu Y, Zhao X, Li Y, Shen N, et al. The triglyceride-glucose index predicts 1-year major adverse cardiovascular events in end-stage renal disease patients with coronary artery disease. Cardiovasc Diabetol. 2023;22(1):292.
Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80.
Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetol. 2019;18(1):150.
Mao Q, Zhou D, Li Y, Wang Y, Xu SC, Zhao XH. The triglyceride-glucose index predicts coronary artery disease severity and cardiovascular outcomes in patients with non-ST-Segment elevation acute coronary syndrome. Dis Markers. 2019;2019:6891537.
Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, et al. High triglyceride-glucose index is Associated with Poor Cardiovascular outcomes in nondiabetic patients with ACS with LDL-C below 1.8 mmol/L. J Atheroscler Thromb. 2022;29(2):268–81.
Gao S, Ma W, Huang S, Lin X, Yu M. Impact of triglyceride-glucose index on long-term cardiovascular outcomes in patients with myocardial infarction with nonobstructive coronary arteries. Nutr Metab Cardiovasc Dis. 2021;31(11):3184–92.
Wu Z, Guo D, Chen S, Sun X, Zhang Y, Liu X, et al. Combination of the triglyceride-glucose index and EuroSCORE II improves the prediction of long-term adverse outcomes in patients undergoing coronary artery bypass grafting. Diabetes Metab Res Rev. 2023;39(8):e3710.
Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, et al. Predictive effect of triglyceride–glucose index on clinical events in patients with type 2 diabetes mellitus and acute myocardial infarction: results from an observational cohort study in China. Cardiovasc Diabetol. 2021;20(1):43.
Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Triglyceride-glucose index as a surrogate marker of insulin resistance for Predicting Cardiovascular outcomes in nondiabetic patients with Non-ST-Segment elevation Acute Coronary Syndrome undergoing percutaneous coronary intervention. J Atheroscler Thromb. 2021;28(11):1175–94.
Cho YR, Ann SH, Won KB, Park GM, Kim YG, Yang DH, et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci Rep. 2019;9(1):6129.
Drwila-Stec D, Rostoff P, Gajos G, Nessler J, Konduracka E. Predictive value of metabolic score for insulin resistance and triglyceride glucose-BMI among patients with acute myocardial infarction in 1-year follow-up. Coron Artery Dis. 2023;34(5):314–9.
Zhang Q, Xiao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2023;22(1):279.
Li H, Jiang Y, Su X, Meng Z. The triglyceride glucose index was U-shape associated with all-cause mortality in population with cardiovascular diseases. Diabetol Metab Syndr. 2023;15(1):181.
Ye Z, Xie E, Gao Y, Li P, Tu Y, Guo Z, et al. The triglyceride glucose index is associated with future cardiovascular disease nonlinearly in middle-aged and elderly Chinese adults. BMC Endocr Disord. 2022;22(1):242.
Wang J, Liu J, Guo W, Bai Y, Li H, Chen H, et al. Multiple biomarkers in the context of conventional risk factors in patients with coronary artery disease. J Am Coll Cardiol. 2017;69(22):2769–70.
Wang J. Prognostic performance of multiple biomarkers in the context of conventional risk factors in patients with coronary artery disease, M.D. dissertation, Medical School of Chinese PLA, 2018. (In Chinese). https://kns.cnki.net/kcms2/article/abstract?v=yO8aEz8cbf0fgq101ys7813jDKwh-c93-lnJm3-lyHiW3c0KfWD-bguG_mbnzUPUwzO3FUm38NcN3MkoKbiwo2CxPGBKUD9Iz2whKaOY1yepbYVskQjI4A2gbLh82LnRYc8RJ87Ik8sgNuezeQQxMg==&uniplatform=NZKPT&language=CHS.
Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation. 2022;145(3):e18–14.
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–44.
Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68.
Yang Q, Vijayakumar A, Kahn BB. Metabolites as regulators of insulin sensitivity and metabolism. Nat Rev Mol Cell Biol. 2018;19(10):654–72.
Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126(1):12–22.
Gerrits AJ, Koekman CA, van Haeften TW, Akkerman JW. Platelet tissue factor synthesis in type 2 diabetic patients is resistant to inhibition by insulin. Diabetes. 2010;59(6):1487–95.
da Silva AA, do Carmo JM, Li X, Wang Z, Mouton AJ, Hall JE. Role of Hyperinsulinemia and Insulin Resistance in hypertension: metabolic syndrome revisited. Can J Cardiol. 2020;36(5):671–82.
Zhou MS, Schulman IH, Zeng Q. Link between the renin-angiotensin system and insulin resistance: implications for cardiovascular disease. Vasc Med. 2012;17(5):330–41.
Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766.
Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides x glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism. 2011;60(12):1673–6.
Galassetti P, Davis SN. Effects of insulin per se on neuroendocrine and metabolic counter-regulatory responses to hypoglycaemia. Clin Sci (Lond). 2000;99(5):351–62.
Kozdag G, Ertas G, Emre E, Akay Y, Celikyurt U, Sahin T, et al. Low serum triglyceride levels as predictors of cardiac death in heart failure patients. Tex Heart Inst J. 2013;40(5):521–8.
Chen L, Ding XH, Fan KJ, Gao MX, Yu WY, Liu HL, et al. Association between triglyceride-glucose index and 2-Year adverse Cardiovascular and cerebrovascular events in patients with type 2 diabetes Mellitus who underwent off-pump coronary artery bypass grafting. Diabetes Metab Syndr Obes. 2022;15:439–50.
Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):31.
Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19(1):108.
Yang J, Tang YD, Zheng Y, Li C, Zhou Q, Gao J, et al. The impact of the triglyceride-glucose index on poor prognosis in NonDiabetic patients undergoing percutaneous coronary intervention. Front Endocrinol (Lausanne). 2021;12:710240.
Drwila D, Rostoff P, Gajos G, Nessler J, Konduracka E. Prognostic value of the triglyceride-glucose index among non-diabetic patients with acute myocardial infarction at one-year follow-up. Kardiol Pol. 2021;79(10):1116–23.
Acknowledgements
None.
Funding
Not available.
Author information
Authors and Affiliations
Contributions
Study concept and design: HBL, YHY and BCH; Acquisition of data: JX, CX, YPD, JQ, WYG, TKH and ZYZ;Analysis and interpretation of data: BCH, LL and XQZ; Drafting of the manuscript: BCH, LL and JX; Critical revision of the manuscript for important intellectual content: HBL, YHY, BCH, LL, JX, CX, JQ, YWG, TKH, YPD and ZYZ.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The experimental protocol adhered to the ethical guidelines of the Helsinki Declaration and received approval from the Human Ethics Committee of Chinese PLA General Hospital. Written informed consent was obtained from each participant or their guardian.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Baseline characteristics of study participants stratified by diabetes status. Figure S1. The association of triglycerides and glucose with long-term adverse outcomes in hospitalized patients with CHD. Figure S2. The association between TyG index and each component of long-term MACEs in hospitalized patients with CHD.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hao, B., Lyu, L., Xu, J. et al. The relationship between triglyceride-glucose index and prospective key clinical outcomes in patients hospitalised for coronary artery disease. Cardiovasc Diabetol 23, 40 (2024). https://doi.org/10.1186/s12933-024-02132-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02132-2