Abstract
Background
Hyperglycaemia is frequent in acute ischemic stroke and denotes a bad prognosis, even in the absence of pre-existing diabetes. However, in clinical trials treatment of elevated glucose levels with insulin did not improve stroke outcome, suggesting that collateral effects rather than hyperglycaemia itself aggravate ischemic brain damage. As reactive glucose metabolites, glyoxal and methylglyoxal are candidates for mediating the deleterious effects of hyperglycaemia in acute stroke.
Methods
In 135 patients with acute stroke, we used liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) to measure glyoxal, methylglyoxal and several of their glycated amino acid derivatives in serum. Results were verified in a second cohort of 61 stroke patients. The association of serum concentrations with standard stroke outcome scales (NIHSS, mRS) was tested.
Results
Glucose, glyoxal, methylglyoxal, and the glyoxal-derived glycated amino acid Nδ-(5-hydro-4-imidazolon-2-yl)ornithine (G-H1) were positively correlated with a bad stroke outcome at 3 months as measured by mRS90, at least in one of the two cohorts. However, the glycated amino acids Nε-carboxyethyllysine (CEL) and in one cohort pyrraline showed an inverse correlation with stroke outcome probably reflecting lower food intake in severe stroke. Patients with a poor outcome had higher serum concentrations of glyoxal and methylglyoxal.
Conclusions
The glucose-derived α-dicarbonyl glyoxal and glycated amino acids arising from a reaction with glyoxal are associated with a poor outcome in ischemic stroke. Thus, lowering α-dicarbonyls or counteracting their action could be a therapeutic strategy for hyperglycaemic stroke.
Similar content being viewed by others
Background
Research has focused on the harmful effects of hyperglycaemia on some organs, including kidney, retina or heart. Less is known about the impact of hyperglycaemia on the brain, despite its frequent association with stroke. Being a major risk factor for stroke, diabetes mellitus partially explains why hyperglycaemia is diagnosed in up to 75% of patients with acute stroke [1]. However, even without pre-existing diabetes mellitus, hyperglycaemia is frequent in stroke patients during the acute phase. In ischemic stroke, hyperglycaemia on admission is associated with a poor neurological outcome, an increased risk of symptomatic intracranial haemorrhage, and enhanced mortality [2,3,4]. Importantly, several studies have shown that in nondiabetic stroke patients hyperglycaemia revealed an even stronger predictive value [5,6,7]. Together with preclinical data, this provides evidence that acute hyperglycaemia is detrimental for functional recovery, but the mechanisms and molecular mediators by which acute hyperglycaemia influences the neurological outcome are still unclear [8]. Candidates to worsen the outcome in hyperglycaemia are α-dicarbonyls, a group of glucose metabolites, prominent representatives being glyoxal and methylglyoxal (Fig. 1). Glyoxal is mainly generated via glucose autoxidation while methylglyoxal is a by-product of glycolysis [9, 10]. Another source for α-dicarbonyls is the glycation of proteins. Condensation reactions of free amino groups of proteins with reducing sugars, like glucose, lead to the formation of Schiff bases and subsequent Amadori rearrangements. Both Schiff bases and Amadori products undergo further rearrangements, resulting in α-dicarbonyls, like glyoxal and methylglyoxal [11]. Glyoxal and methylglyoxal share a highly reactive chemical structure and are able to modify proteins, DNA and lipids, thereby impeding the function of various macromolecules [12]. The products of their reaction with protein residues like lysine and arginine are referred to as advanced glycation end products (AGEs), which may also be derived from Amadori products [13, 14].
Schematic illustration of the connection of glucose metabolism, α-dicarbonyl production, and formation of related glycated amino acids [60]. While glyoxal and pyrraline can be generated directly from glucose by autooxidation, glycolysis is necessary to provide the methylglyoxal precursor molecules glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. α-Dicarbonyls modify arginine or lysine residues resulting in the formation of G-H1 and CML or MG-H1, CEL and argpyrimidine through the reaction with glyoxal and methylglyoxal, respectively. Furthermore, glycated amino acids can be absorbed after digestion of food
Modification of proteins by α-dicarbonyls and the interaction of AGEs with the receptor for AGEs (RAGE) increase oxidative stress and create a proinflammatory environment [15, 16]. In rodents, several studies have revealed detrimental effects of α-dicarbonyls, AGEs, or RAGE signalling on the outcome after experimental stroke [17,18,19,20,21,22].
In human hyperglycaemic stroke, however, evidence for the involvement of α-dicarbonyls and AGEs is less clear. Glycation of proteins in human samples can be investigated by tryptic hydrolysis of proteins and enrichment of glycated peptides, followed by mass spectrometry [23,24,25,26]. Another way of monitoring AGE production is to measure blood concentrations of glycated amino acids that are endogenously formed by proteolysis of AGEs or by the reaction of α-dicarbonyls with free amino acids (Fig. 1) [27]. However, glycated amino acids are also ingested with the diet [28,29,30,31]. As patients often remain fasting during the acute phase of stroke, it is unclear whether specific glycated amino acids rise due to hyperglycaemia or fall due to fasting. Several methods are available for the detection of α-dicarbonyls and glycated amino acids. Immunoassays and the measurement of skin autofluorescence, which reflects the presence of glycation adducts, are subject to numerous technical errors [27]. The best sensitivity and the highest specificity is achieved by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) [27]. So far, the few clinical studies that have investigated AGEs or individual glycated amino acids in acute stroke patients have used immunoassays or the measurement of skin autofluorescence [32,33,34]. Thus, concentrations of α-dicarbonyls or glycated amino acids and their association with neurological outcome after hyperglycaemic stroke is still unclear.
Therefore, we performed a quantitative analysis of the α-dicarbonyls glyoxal and methylglyoxal and the corresponding glycated amino acids Nδ-(5-hydro-4-imidazolon-2-yl)ornithine (G-H1), Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)ornithine (MG-H1), Nε-carboxymethyllysine (CML), Nε-carboxyethyllysine (CEL), argpyrimidine, and pyrraline in serum samples of acute stroke patients. The primary objective was to determine whether these reactive glucose metabolites accumulate in stroke patients with an adverse outcome, indicating that they may cause the known toxic effects associated with hyperglycaemia.
Methods
Patients/Subjects/Cohorts
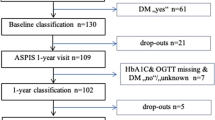
Acute ischemic stroke within the prior 4 days was the inclusion criterion. We collected blood samples from patients on admission (cohort 1 and 2) and on the three consecutive days until discharge (cohort 1). Due to early discharge or other reasons, some patients were lost to follow-up (Additional File 1 Table S1, Additional File 1 Table S2). The time between the last meal and blood sampling was documented according to the information provided by the patients or accompanying persons. The diagnosis of diabetes or other risk factors was based on the history.
Detection of α-dicarbonyls, glycated amino acids and glucose
Samples were stored at -80° C before analysis. Samples from cohort 1 and cohort 2 were measured in separate experiments by the same laboratory. α-Dicarbonyls were extracted as described previously with some modifications [20]. For protein precipitation, 40 µl of ice-cold trichloroacetic acid (20%, w/v, Sigma-Aldrich) was added to 100 µl of serum. After adding 80 µl water and 20 µl of the isotopically labelled internal standard deuterated methylglyoxal (d4-MG, 400 nM in H2O), the sample was incubated on ice for 10 min followed by centrifugation (20,000 x g, 4° C, 10 min). The supernatant was transferred to a glass vial, and the α-dicarbonyls were derivatized to the respective quinoxaline compounds with isotopically labelled d8-o-phenylenediamine (CDN Isotopes, 10 µl, 0.5 mM in 200 mM HCl/500 µM diethylenetriaminepentaacetic acid (DETAPAC, Sigma-Aldrich) for 4 h at room temperature in the dark. D4-MG was synthesized, as previously described [35], using d6-acetone. The concentration and purity of the d4-MG stock solutions, as well as the identities of the contaminants, were determined from 1 H, 2 H and 13 C NMR spectra acquired at 298 K using a Bruker Avance II NMR spectrometer. The purities of the d4-MG stock solutions were 60–65% based on the integration of the signals in the 2 H NMR spectrum, adjusted according to the number of equivalent nuclei with the major contaminants being d3-acetate and d6-acetone. Representative spectra are shown in Additional File 1 Fig. S1.
Since α-dicarbonyls are highly reactive, we tested in a pilot study whether small differences in the time between blood collection and sample extraction or in the protocol of blood extraction could influence sample concentrations. Systematic variation of these parameters did not significantly affect results (Additional File 1 Fig. S2) suggesting that our protocol for α-dicarbonyl measurement is robust.
For the detection of glycated amino acids, 360 µl of acetonitrile with 0.1% formic acid (Biosolve) was added to 100 µl serum for protein precipitation. After the addition of 10 µl internal standard mix (d2-CML, d4-CEL, d3-MG-H1, 400 nM in H2O, PolyPeptide Group), the samples were mixed thoroughly and incubated for 30 min at -20 °C. After centrifugation (20,000 x g, 4° C, 10 min), the supernatant was transferred to glass vials for detection.
LC/MS analysis was performed on a TSQ Endura triple quadrupole mass spectrometer, equipped with a heated electrospray ionization source and coupled to a Dionex Ultimate 3000 UHPLC system (ThermoFisher Scientific, Bremen, Germany). For both methods water containing 0.1% formic acid as mobile phase A and 100% acetonitrile as phase B were used. All solvents were of LC-MS grade quality and were purchased from Merck (Darmstadt, Germany).
α-Dicarbonyls were measured as described previously with some modifications [20]. Briefly, quinoxaline derivatives were separated on an Ascentis Express C18 column (100 mm x 2.1 mm x 5 µM; Sigma-Aldrich) applying an isocratic gradient of 90% A and 10% B with a flow rate of 0.2 ml/min for 10 min. Afterwards, B increased to 100% between 10 and 11 min, which was followed by a washing step with 100% B between 11 and 15 min with a flow rate of 0.4 ml/min. Re-equilibration took place between 15 and 20 min back to 10% B. Following parameters were used in the positive ionization mode: ion spray voltage, 4600 V; vaporizer temperature, 100 °C; and ion transfer tube temperature, 300 °C.
For glycated amino acids, a BEH amide column was used (XBridge BEH Amide, 100 mm x 2.1 mm, 2.5 μm, Waters). The chromatographic separation was realized using following gradient at 40 °C and a flow rate of 0.4 ml/min: 95% B from 0 to 3 min, followed by a decrease to 60% B from 3 to 6 min and to 30% B from 6 to 12 min. After washing at 30% B from 12 to 16 min, re-equilibration took place by increasing to 95% B from 16 to 25 min and keeping 95% for another 5 min. Following parameters were used in the positive ionization mode: ion spray voltage, 4000 V; vaporizer temperature, 200 °C; and ion transfer tube temperature, 300 °C.
Multiple reaction monitoring (MRM) was used to identify quinoxaline derivates and glycated amino acids with collision-induced fragmentation at 2.5 mTorr using argon. Retention times, MRM transitions, scan parameters, limits of quantification, and inter batch variances are listed in Additional File 1 Table S3. Representative spectra are shown in Additional File 1 Fig. S3. Quantification was performed with an external calibration curve based on the ratio of the areas under the peaks to the internal standard. Each sample was measured as a singleton. Every 20–30 samples, one quality control sample was included in the measurements.
Glucose concentrations were measured in venous serum samples using the Glucose-Glo™ Assay (Promega, USA) according to the manufacturer’s instruction.
Statistics
To identify potential associations of glyoxal and methylglyoxal as well as glycated amino acids with stroke outcome and hyperglycaemia, we chose an explorative approach. For correlation analysis we used the Spearman test. Group differences were analysed using the Mann Whitney U-test. Stroke patients were stratified according to mRS90 values into good (0–2) and bad (3–6) outcome groups. Odds ratios (OR) for a bad outcome were estimated by defining groups according to the median concentration of glucose, α-dicarbonyls, or glycated amino acids (< or > = median). Categorical variables were compared with the Chi-square test.
To investigate whether glyoxal at day 2 was associated with outcome, we predicted outcome based on the logistic regression model by Weimar et al. that uses age and NIHSS at admission [36]. Overall accuracy was 82.9%, sensitivity 75%, and specificity 84.8%. We included Box-Cox transformed glyoxal concentrations as an additional covariable and compared both model fits using a likelihood-ratio test. Using fractional polynomials, we found no indication that the relationship between transformed glyoxal and outcome is non-linear. Finally, we identified no interactions between glyoxal and age as well as NIHSS in predicting outcome.
Results
For the investigation of α-dicarbonyls and their derivatives, we recruited 135 patients with acute ischemic stroke in cohort 1. Demographic data and clinical characteristics are presented in Additional File 1 Table S1. The median time between stroke onset and first blood sampling was 13.2 h (IQR = 6.3 − 20.0 h) (day 1). In 51 of 102 patients, for whom measurements in venous blood samples were available on admission, glucose concentrations were above 7.8 mM (140 mg/dl), including 20 diabetes mellitus patients and 31 patients without diagnosed diabetes (Additional File 1 Fig. S4). We analysed concentrations of α-dicarbonyls and glycated amino acids in relation to neurological scores or related metabolic parameters; the results are presented as a p-value heatmap (Fig. 2). As expected, α-dicarbonyls and glycated amino acids positively correlated with concentrations of glucose or HbA1c and were elevated in subjects with diabetes (Fig. 2A).
P-value heatmap of correlations and group differences of glucose, α-dicarbonyls, and glycated amino acids with diabetes-related parameters and neurological scores. Blood samples of four consecutive days after stroke have been analysed using LC-MS. (A) In patients of cohort 1, concentrations of the compounds were tested for an association with the history of diabetes or laboratory parameters of hyperglycaemia and diabetes (glucose, HbA1c, Spearman coefficient; diabetes mellitus, Mann Whitney U-test). (B) Correlations with neurological scores on admission (mRS, NIHSS) and later stages (mRS day 90, NIHSS discharge) in cohort 1 (Spearman coefficient). (C) Results were confirmed in cohort 2. mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale. White squares indicate no correlation, grey squares indicate parameters that were not measured (day 2–4, cohort 2) or for which a correlation with themselves (glucose) was meaningless
Interestingly, glyoxal, methylglyoxal and the glyoxal-derived G-H1 positively correlated with mRS or NIHSS on admission or the NIHSS at discharge from stroke unit (median, 4 days after onset of symptoms, Fig. 2B). In contrast, the methylglyoxal-derived glycated amino acids CEL, argpyrimidine and pyrraline showed a negative correlation, if any, with the mRS or NIHSS on admission or discharge from stroke unit (Fig. 2B). Food contains high amounts of AGEs which, after ingestion and digestion, reach the blood stream as glycated amino acids [28,29,30,31], but many patients do not eat because of dysphagia after stroke. In support of a dietary source, pyrraline and MG-H1 concentrations on day 1 inversely correlated with the time since the last meal of patients (Additional File 1 Fig. S5A). Furthermore, on day 3, pyrraline showed a positive correlation with 4-hydroxyproline, a marker for the intake of meat, which is a rich source of AGEs (Additional File 1 Fig. S5B) [37,38,39]. The inverse correlation between CEL, argpyrimidine and pyrraline and neurological scores at early timepoints could therefore be due to impaired oral food intake of patients with a severe stroke [40,41,42].
In line with the previously reported association between post-stroke hyperglycaemia and bad outcome, glucose concentrations on day 1 showed a trend toward correlation with mRS90 (p = 0.052, Fig. 2B) [2, 8, 43,44,45]. Interestingly, we also observed a positive correlation of glyoxal and methylglyoxal concentrations on day 2 with mRS90 (Fig. 2B). The delay may be accounted for by the time needed for the production of α-dicarbonyls from glucose. In addition, the glyoxal-derived glycated amino acid G-H1 on day 3 of admission showed a trend to a correlation with mRS90 values. In contrast, the methylglyoxal-derived glycated amino acids MG-H1, CEL and argpyrimidine were not correlated with mRS90 or were negatively correlated with mRS90 values (Fig. 2B). Pyrraline levels on day 3 and 4 were also inversely correlated with mRS90. In patients with a favourable outcome after stroke, high pyrraline concentrations may reflect early normalization of food intake.
We re-evaluated the association between α-dicarbonyls or glycated amino acids and stroke outcome in cohort 2 which included 61 acute ischemic stroke patients (demographic characteristics in Additional File 1 Table S2). Similar to the results of cohort 1, glyoxal, methylglyoxal, and G-H1 positively correlated with NIHSS or mRS on day 1, whereas MG-H1, CEL, and pyrraline showed a negative correlation (Fig. 2C). Again, glyoxal and G-H1 concentrations positively correlated with mRS90.
To assess the prognostic value of the various parameters, we dichotomized patients of cohort 1 based on serum concentrations either above or below the median (Additional File 1 Fig. S6). With a glucose concentration on day 1 above the median of 7.8 mmol/l, patients had an OR of 3.28 (95% CI 1.14–9.46, p = 0.023) for a poor outcome. Among the α-dicarbonyls, only high glyoxal concentrations on day 2 denoted a higher OR for a bad outcome, while there was no effect for methylglyoxal. Interestingly, increased levels of glyoxal-related glycated amino acids G-H1 and CML on day 1 and 2, respectively, also increased the risk for a bad outcome (Additional File 1 Fig. S6). Similarly, in cohort 2 high levels of G-H1 elevated the odds ratio for a poor outcome (Additional File 1 Fig. S7). In contrast, high concentrations of the methylglyoxal-related CEL on day 4 or pyrraline on day 3 were associated with a lower risk for a bad outcome, at least in cohort 1 (Additional File 1 Fig. S6). Overall, glyoxal and related glycated amino acids had the strongest association with a bad outcome after ischemic stroke. However, in a clinical prognostic model based on age and NIHSS [36], adding glyoxal concentrations on day 2 did not improve the prediction of outcome. This may be due to the fact that glyoxal plasma concentrations correlated with age.
When comparing patients from cohort 1 with a poor outcome (mRS90 ≥ 3) and a good outcome (mRS90 ≤ 2), we found higher serum concentrations of glucose on day 1, glyoxal and methylglyoxal on day 2 or 3 in the poor outcome group (Fig. 3, Additional File 1 Fig. S8). The sequence in which changes occurred may reflect the conversion of glucose to α-dicarbonyls. Because patients with a poor outcome were more likely to have a history of diabetes, the analysis was repeated after excluding diabetes patients. Despite the lower numbers of cases, most of the differences between the prognostic groups remained in patients without known diabetes, arguing that the metabolites reflect the acute stroke-induced hyperglycaemia (Additional File 1 Fig. S9). The analysis of cohort 2 confirmed elevated serum concentrations of glucose, glyoxal, and methylglyoxal in patients with a poor outcome (Additional File 1 Fig. S10).
Discussion
Hyperglycaemia after stroke is associated with a poor outcome and experimental evidence indicates that high blood levels of glucose aggravate ischemic brain damage [2,3,4, 8]. To explore the possibility that glucose worsens stroke outcome through reactive α-dicarbonyl metabolites and the resulting α-dicarbonyl stress, we measured the reactive glucose metabolites glyoxal and methylglyoxal as well as their glycated amino acid derivatives in acute stroke patients. For the analysis, we used a highly sensitive and specific LC-MS/MS approach. Prior to detection dicarbonyls were derivatised to increase the sensitivity, as previously described [46,47,48]. In line with earlier reports [2, 5,6,7], glucose concentrations on admission correlated with poor functional outcome after 3 months as measured by mRS90. Similarly, we found a correlation for glyoxal, methylglyoxal and the glyoxal-derived glycated amino acid G-H1 with mRS90 when investigating two independent cohorts of acute stroke patients. The association with a bad outcome as defined by mRS90 > 2 was strongest for glyoxal. However, the relationship between methylglyoxal and a bad outcome was not very robust. Differences between glyoxal and methylglyoxal are likely due to the way of how they are produced from glucose. While glyoxal is formed extracellularly by autoxidation from glucose, methylglyoxal is a by-product of intracellular glycolysis (Additional File 1 Fig. S11). After stroke, glucocorticoids and inflammatory mediators induce insulin resistance and hyperglycaemia [2]. We propose that insulin resistance and the blockage of glucose entry into insulin-sensitive tissues favour extracellular glyoxal production in plasma from glucose which consequently leads to the formation of glyoxal-associated glycated amino acids [2, 49].
α-Dicarbonyls are involved in the pathogenesis of atherosclerotic disease and are associated with a higher risk of stroke [50, 51]. In acute stroke, hyperglycaemia and other factors, such as hypoxia or lipid peroxidation, may increase the production of α-dicarbonyls and AGEs. In preclinical models, glyoxal and glyoxal-derived AGEs aggravate ischemic brain damage by RAGE-dependent or -independent mechanisms [19, 20, 52]. The AGEs receptor RAGE is expressed by monocyte-derived macrophages and determines their polarization towards an inflammatory cell type [19, 20]. In addition, activation of RAGE mediates the metabolic reprogramming of T cells and skews their differentiation in experimental stroke models [52]. These mechanisms may explain that RAGE mediates the deleterious effects of hyperglycaemia in stroke [20]. In addition, dicarbonyls have RAGE-independent effects on brain cells. In brain endothelial cells, they downregulate the expression of CSF-1 that induces a noninflammatory polarization of macrophages in the ischemic brain [20]. Glyoxal exposure has been reported to lead to the collapse of the mitochondrial membrane potential and compromise the survival of endothelial cells [53]. Toxic effects have also been found in neuronal cells with glyoxal and glyoxal-derived AGEs inducing apoptosis [54,55,56]. The correlation of glyoxal concentrations with a poor prognosis that we have observed in this study suggests that the proinflammatory and neurotoxic effects of glyoxal and glyoxal-derived AGEs are relevant in acute hyperglycaemic stroke.
Unexpectedly, although methylglyoxal showed a weak positive correlation with poor stroke outcome, methylglyoxal-derived glycated amino acids and pyrraline in serum inversely correlated with poor stroke outcome. This finding is likely due to the reduced food intake in patients with a severe stroke [40,41,42]. Food is a rich source of AGEs that are degraded to glycated amino acids and absorbed into the blood stream [28,29,30,31, 57]. In support of this explanation, pyrraline and MG-H1 inversely correlated with the time since the last meal. Whether α-dicarbonyls or glycated amino acids derived from AGEs in food have a detrimental effect on health, is still a contentious matter [57]. The fact that undernutrition in stroke is known to be associated with a poor outcome suggests that food-derived α-dicarbonyls or glycated amino acids do not have a strong effect on ischemic brain injury [40, 41].
The present study has some limitations. One problem relates to patient attrition. As not all patients remained on the stroke unit until day 4, the time course of concentrations has to be interpreted with caution. Furthermore, the overall number of patients was rather small, so we may have overlooked confounding factors. However, importantly, we found similar results in two independent cohorts of stroke patients. Obviously, the association between glyoxal and outcome does not prove causality. Interventions studies will be required to address this point.
Conclusions
As lowering elevated glucose concentrations with insulin had no significant effect on stroke prognosis [58], other strategies are needed to prevent the neurotoxic effects of glucose. Indeed, the formation of α-dicarbonyls or their action provides a rich treasure trove for potential pharmacological targets [59]. In preclinical stroke models, several of these interventions reduced ischemic brain damage [17, 19, 52]. Our results suggest that efforts to translate this strategy into the clinic and reduce α-dicarbonyls or counteract their effects may be successful in the treatment of acute stroke.
Data Availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CEL:
-
Nε-carboxyethyllysine
- CML:
-
Nε-carboxymethyllysine
- G-H1:
-
Nδ-(5-hydro-4-imidazolon-2-yl) ornithine
- LC-MS/MS:
-
Liquid chromatography coupled to tandem mass spectrometry
- MG-H1:
-
Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl) ornithine
- MRM:
-
Multiple reaction monitoring
- NIHSS:
-
National Institute of Health stroke scale
- mRS:
-
Modified Rankin scale
- RAGE:
-
Receptor for AGEs
References
Scott JF, Robinson GM, French JM, O’Connell JE, Alberti KG, Gray CS. Prevalence of admission hyperglycaemia across clinical subtypes of acute stroke. Lancet. 1999;353(9150):376–7.
Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6(3):145–55.
Rinkel LA, Nguyen TTM, Guglielmi V, Groot AE, Posthuma L, Roos YBWEM, Majoie CBLM, Lycklama À, Nijeholt GJ, Emmer BJ, Van Der Worp HB, et al. High admission glucose is Associated with Poor Outcome after Endovascular Treatment for ischemic stroke. Stroke. 2020;51(11):3215–23.
Perez-Vega C, Domingo RA, Tripathi S, Ramos-Fresnedo A, Kashyap S, Quinones-Hinojosa A, Lin MP, Fox WC, Tawk RG. Influence of glucose levels on clinical outcome after mechanical thrombectomy for large-vessel occlusion: a systematic review and meta-analysis. J neurointerventional Surg 2022, 14(1).
Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32(10):2426–32.
Stollberger C, Exner I, Finsterer J, Slany J, Steger C. Stroke in diabetic and non-diabetic patients: course and prognostic value of admission serum glucose. Ann Med. 2005;37(5):357–64.
Stead LG, Gilmore RM, Bellolio MF, Mishra S, Bhagra A, Vaidyanathan L, Decker WW, Brown RD Jr. Hyperglycemia as an independent predictor of worse outcome in non-diabetic patients presenting with acute ischemic stroke. Neurocrit Care. 2009;10(2):181–6.
Kruyt ND, Nys GM, van der Worp HB, van Zandvoort MJ, Kappelle LJ, Biessels GJ. Hyperglycemia and cognitive outcome after ischemic stroke. J Neurol Sci. 2008;270(1–2):141–7.
Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344 Pt 1:109–16.
Kalapos MP. Where does plasma methylglyoxal originate from? Diabetes Res Clin Pract. 2013;99(3):260–71.
Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–29.
Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnolzer M, Lasitschka F, et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med. 2012;18(6):926–33.
Thornalley PJ. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems–role in ageing and disease. Drug Metab Drug Interact. 2008;23(1–2):125–50.
Requena JR, Ahmed MU, Fountain CW, Degenhardt TP, Reddy S, Perez C, Lyons TJ, Jenkins AJ, Baynes JW, Thorpe SR. Carboxymethylethanolamine, a biomarker of phospholipid modification during the maillard reaction in vivo. J Biol Chem. 1997;272(28):17473–9.
Kang JH. Modification and inactivation of human Cu,Zn-superoxide dismutase by methylglyoxal. Mol Cells. 2003;15(2):194–9.
Wong A, Dukic-Stefanovic S, Gasic-Milenkovic J, Schinzel R, Wiesinger H, Riederer P, Munch G. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. Eur J Neurosci. 2001;14(12):1961–7.
Takizawa S, Izuhara Y, Kitao Y, Hori O, Ogawa S, Morita Y, Takagi S, van Ypersele de Strihou C, Miyata T. A novel inhibitor of advanced glycation and endoplasmic reticulum stress reduces infarct volume in rat focal cerebral ischemia. Brain Res. 2007;1183:124–37.
Wang B, Aw TY, Stokes KY. The protection conferred against ischemia-reperfusion injury in the diabetic brain by N-acetylcysteine is associated with decreased dicarbonyl stress. Free Radic Biol Med. 2016;96:89–98.
Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, et al. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28(46):12023–31.
Khan MA, Schultz S, Othman A, Fleming T, Lebron-Galan R, Rades D, Clemente D, Nawroth PP, Schwaninger M. Hyperglycemia in Stroke impairs polarization of Monocytes/Macrophages to a protective noninflammatory cell type. J Neurosci. 2016;36(36):9313–25.
Zimmerman GA, Meistrell M 3rd, Bloom O, Cockroft KM, Bianchi M, Risucci D, Broome J, Farmer P, Cerami A, Vlassara H, et al. Neurotoxicity of advanced glycation endproducts during focal stroke and neuroprotective effects of aminoguanidine. Proc Natl Acad Sci U S A. 1995;92(9):3744–8.
Chen W, Huang W, Yang Y, Li K. Methylglyoxal Scavengers Attenuate Angiogenesis Dysfunction Induced by Methylglyoxal and Oxygen-Glucose deprivation. Oxidative Med Cell Longev. 2022;2022:1–18.
Soboleva A, Modzel M, Didio A, Płóciennik H, Kijewska M, Grischina T, Karonova T, Bilova T, Stefanov V, Stefanowicz P, et al. Quantification of prospective type 2 diabetes mellitus biomarkers by stable isotope dilution with bi-labeled standard glycated peptides. Anal Methods. 2017;9(3):409–18.
Kielmas M, Kijewska M, Stefanowicz P, Szewczuk Z. Testing isotopic labeling with [(1)(3)C(6)]glucose as a method of advanced glycation sites identification. Anal Biochem. 2012;431(1):57–65.
Frolov A, Hoffmann R. Identification and relative quantification of specific glycation sites in human serum albumin. Anal Bioanal Chem. 2010;397(6):2349–56.
Soboleva A, Mavropulo-Stolyarenko G, Karonova T, Thieme D, Hoehenwarter W, Ihling C, Stefanov V, Grishina T, Frolov A. Multiple glycation Sites in blood plasma proteins as an Integrated Biomarker of type 2 diabetes Mellitus. Int J Mol Sci. 2019;20(9):2329.
Thornalley PJ, Rabbani N. Detection of oxidized and glycated proteins in clinical samples using mass spectrometry–a user’s perspective. Biochim Biophys Acta. 2014;1840(2):818–29.
Foerster A, Henle T. Glycation in food and metabolic transit of dietary AGEs (advanced glycation end-products): studies on the urinary excretion of pyrraline. Biochem Soc Trans. 2003;31(Pt 6):1383–5.
Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci. 2005;1043:461–6.
Semba RD, Ang A, Talegawkar S, Crasto C, Dalal M, Jardack P, Traber MG, Ferrucci L, Arab L. Dietary intake associated with serum versus urinary carboxymethyl-lysine, a major advanced glycation end product, in adults: the Energetics Study. Eur J Clin Nutr. 2012;66(1):3–9.
Maasen K, van Greevenbroek MMJ, Scheijen J, van der Kallen CJH, Stehouwer CDA, Schalkwijk CG. High dietary glycemic load is associated with higher concentrations of urinary advanced glycation endproducts: the cohort on diabetes and atherosclerosis maastricht (CODAM) study. Am J Clin Nutr. 2019;110(2):358–66.
Ikeda T, Maruyama K, Ito N, Utagawa A, Nagane M, Shiokawa Y. Serum pentosidine, an advanced glycation end product, indicates poor outcomes after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2012;21(5):386–90.
Ohnuki Y, Nagano R, Takizawa S, Takagi S, Miyata T. Advanced glycation end products in patients with cerebral infarction. Intern Med. 2009;48(8):587–91.
Leńska-Mieciek M, Korczak-Kowalska G, Bocian K, Fiszer U. Pentosidine, advanced glycation end product, in acute ischaemic stroke patients with and without atrial rhythm disturbances. Neurol Neurochir Pol. 2020;54(4):323–8.
Clelland JD, Thornalley P. Svnthesis of 14 C-labelled methylglyoxal and S-D-lactoylglutathione. J Label Compd Radiopharm. 1990;28:1455–64.
Weimar C, König IR, Kraywinkel K, Ziegler A, Diener HC, German Stroke Study C. Age and National Institutes of Health Stroke Scale score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke. 2004;35(1):158–62.
Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110(6):911–916e912.
Guan F, Du W, Zhang J, Su C, Zhang B, Deng K, Du S, Wang H. Amino acids and lipids Associated with Long-Term and Short-Term Red Meat Consumption in the Chinese Population: an untargeted Metabolomics Study. Nutrients 2021, 13(12).
Cuparencu C, Praticó G, Hemeryck LY, Sri Harsha PSC, Noerman S, Rombouts C, Xi M, Vanhaecke L, Hanhineva K, Brennan L, et al. Biomarkers of meat and seafood intake: an extensive literature review. Genes & nutrition. 2019;14:35.
Yoo SH, Kim JS, Kwon SU, Yun SC, Koh JY, Kang DW. Undernutrition as a predictor of poor clinical outcomes in acute ischemic stroke patients. Arch Neurol. 2008;65(1):39–43.
Collaboration FT. Poor nutritional status on admission predicts poor outcomes after stroke: observational data from the FOOD trial. Stroke. 2003;34(6):1450–6.
Gariballa SE, Parker SG, Taub N, Castleden M. Nutritional status of hospitalized acute stroke patients. Br J Nutr. 1998;79(6):481–7.
Melamed E. Reactive hyperglycemia in patients with Acute Stroke. J Neurol Sci. 1976;29(2–4):267–75.
Chamorro A, Brown S, Amaro S, Hill MD, Muir KW, Dippel DWJ, van Zwam W, Butcher K, Ford GA, den Hertog HM, et al. Glucose modifies the Effect of Endovascular Thrombectomy in patients with Acute Stroke. Stroke. 2019;50(3):690–6.
Muller C, Cheung NW, Dewey H, Churilov L, Middleton S, Thijs V, Ekinci EI, Levi C, Lindley R, Donnan G, et al. Treatment with exenatide in acute ischemic stroke trial protocol: a prospective, randomized, open label, blinded end-point study of exenatide vs. standard care in post stroke hyperglycemia. Int J Stroke. 2018;13(8):857–62.
Ohmori S, Mori M, Kawase M, Tsuboi S. Determination of methylglyoxal as 2-methylquinoxaline by high-performance liquid chromatography and its application to biological samples. J Chromatogr. 1987;414(1):149–55.
Hara S, Yamaguchi M, Takemori Y, Yoshitake T, Nakamura M. 1,2-diamino-4,5-methylenedioxybenzene as a highly sensitive fluorogenic reagent for α-dicarbonyl compounds. Anal Chim Acta. 1988;215:267–76.
Rabbani N, Thornalley PJ. Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat Protoc. 2014;9(8):1969–79.
Yokoyama K, Yamada T, Mitani H, Yamada S, Pu S, Yamanashi T, Matsumura H, Nakagome K, Kaneko K. Relationship between hypothalamic-pituitary-adrenal axis dysregulation and insulin resistance in elderly patients with depression. Psychiatry Res. 2015;226(2–3):494–8.
Lamprea-Montealegre JA, Arnold AM, Mc CRL, Mukamal KJ, Djousse L, Biggs ML, Siscovick DS, Tracy RP, Beisswenger PJ, Psaty BM, et al. Plasma levels of Advanced Glycation Endproducts and Risk of Cardiovascular events: findings from 2 prospective cohorts. J Am Heart Association. 2022;11(15):e024012.
Schalkwijk CG. Vascular AGE-ing by methylglyoxal: the past, the present and the future. Diabetologia. 2015;58(8):1715–9.
Zhang Y, Li F, Chen C, Li Y, Xie W, Huang D, Zhai X, Yu W, Wan J, Li P. RAGE-mediated T cell metabolic reprogramming shapes T cell inflammatory response after stroke. J Cereb Blood Flow Metab. 2022;42(6):952–65.
Xie MZ, Guo C, Dong JQ, Zhang J, Sun KT, Lu GJ, Wang L, Bo DY, Jiao LY, Zhao GA. Glyoxal damages human aortic endothelial cells by perturbing the glutathione, mitochondrial membrane potential, and mitogen-activated protein kinase pathways. BMC Cardiovasc Disord. 2021;21(1):603.
Takeuchi M, Bucala R, Suzuki T, Ohkubo T, Yamazaki M, Koike T, Kameda Y, Makita Z. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J Neuropathol Exp Neurol. 2000;59(12):1094–105.
Reber F, Kasper M, Siegner A, Kniep E, Seigel G, Funk RH. Alteration of the intracellular pH and apoptosis induction in a retinal cell line by the AGE-inducing agent glyoxal. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2002;240(12):1022–32.
Shen J, Wu Y, Xu JY, Zhang J, Sinclair SH, Yanoff M, Xu G, Li W, Xu GT. ERK- and akt-dependent neuroprotection by erythropoietin (EPO) against glyoxal-AGEs via modulation of Bcl-xL, bax, and BAD. Invest Ophthalmol Vis Sci. 2010;51(1):35–46.
Maasen K, Eussen SJPM, Scheijen JLJM, Carla JH, Dagnelie PC, Opperhuizen A, Stehouwer CDA, Marleen MJ, Schalkwijk CG. Higher habitual intake of dietary dicarbonyls is associated with higher corresponding plasma dicarbonyl concentrations and skin autofluorescence: the Maastricht Study. Am J Clin Nutr. 2022;115(1):34–44.
Johnston KC, Bruno A, Pauls Q, Hall CE, Barrett KM, Barsan W, Fansler A, Van de Bruinhorst K, Janis S, Durkalski-Mauldin VL, et al. Intensive vs Standard Treatment of Hyperglycemia and functional outcome in patients with Acute ischemic stroke: the SHINE Randomized Clinical Trial. JAMA. 2019;322(4):326–35.
Schalkwijk CG, Stehouwer CDA. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other Age-Related Diseases. Physiol Rev. 2020;100(1):407–61.
Rabbani N, Thornalley PJ. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem Biophys Res Commun. 2015;458(2):221–6.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the German Centre for Cardiovascular Research (DZHK) to M.S. (81Z0700109) and I.R.K. and from the German Research Foundation (DFG) to T.F. and P.P.N. (SFB1118, A04/S01).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
SR and JI performed mass spectrometry analyses. SR performed cell culture experiments. SR, JI, OH, RI, GR analysed and interpreted patient data. SR, AO, KB, TF and PPN established the LC-MS/MS technique. KDK performed the NMR acquisition and analysis. SR, JI, IRK and MS performed statistical analyses. SR, JI, OH and MS designed the study. SR and MS drafted the first manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
After approval of the study by the ethical committees of the medical faculty of the University of Heidelberg (cohort 1) and the University of Lübeck (cohort 2), the collection of clinical data and blood took place upon informed consent by the patient or legal guardian following the Declaration of Helsinki. The ethical committee of the University of Lübeck approved the analysis of the blood samples of cohort 1.
Consent for publication
Not applicable.
Competing interests
The authors declare the following conflict of interest: GR received speaker’s honoraria and reimbursement for congress traveling and accommodation from Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo and AstraZeneca. MS received speaker’s honoraria from AstraZeneca.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rhein, S., Inderhees, J., Herrmann, O. et al. Glyoxal in hyperglycaemic ischemic stroke – a cohort study. Cardiovasc Diabetol 22, 173 (2023). https://doi.org/10.1186/s12933-023-01892-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-01892-7