Abstract
Background
Exploratory analysis to determine the effect of semaglutide versus comparators on high-sensitivity C-reactive protein (hsCRP) in subjects with type 2 diabetes.
Methods
Trials of once-weekly subcutaneous (SUSTAIN 3) and once-daily oral (PIONEER 1, 2, 5) semaglutide with hsCRP data were analyzed. Subjects with type 2 diabetes (N = 2482) received semaglutide (n = 1328) or comparators (placebo, n = 339; exenatide extended-release, n = 405; empagliflozin, n = 410). hsCRP ratio to baseline at end-of-treatment was analyzed overall, by clinical cutoff (< 1.0, ≥ 1.0 to ≤ 3.0, or > 3.0 mg/L), by tertile, and by estimated glomerular filtration rate in PIONEER 5 (a trial which was conducted in a population with type 2 diabetes and chronic kidney disease [CKD]). Mediation analyses assessed the effect of change in glycated hemoglobin (HbA1c) and/or change in body weight (BW) on hsCRP reductions.
Results
Geometric mean baseline hsCRP was similar across trials (range 2.7–3.0 mg/L). Semaglutide reduced hsCRP levels by clinical cutoffs and tertiles from baseline to end-of-treatment in all trials versus comparators (estimated treatment ratios [ETRs] versus comparators: 0.70–0.76; p < 0.01) except versus placebo in PIONEER 5 (ETR [95% CI]: 0.83 [0.67–1.03]; p > 0.05). The effect of semaglutide on hsCRP was partially mediated (20.6–61.8%) by change in HbA1c and BW.
Conclusions
Semaglutide reduced hsCRP ratios-to-baseline versus comparators in subjects with type 2 diabetes (not significant with CKD). This effect was partially mediated via reductions in HbA1c and BW and potentially by a direct effect of semaglutide. Semaglutide appears to have an anti-inflammatory effect, which is being further investigated in ongoing trials.
Trial registrations: ClinicalTrials.gov identifiers: NCT01885208 (first registered June 2013), NCT02906930 (first registered September 2016), NCT02863328 (first registered August 2016), NCT02827708 (first registered July 2016).
Similar content being viewed by others
Background
Chronic, low-grade, systemic inflammation is a feature of type 2 diabetes, identifiable by the presence of circulating inflammatory markers [1, 2]. Elevated plasma glucose levels and dyslipidemia have been associated with proinflammatory cytokine production, suggesting a mechanistic link between type 2 diabetes, inflammation, and atherosclerotic cardiovascular (CV) disease [1, 2], and this inflammatory state may contribute to the development and progression of type 2 diabetes [2]. High-sensitivity C-reactive protein (hsCRP) is a sensitive biomarker of systemic inflammation reported to be elevated in patients with type 2 diabetes with other underlying comorbidities [3, 4]. The effects of statins on hsCRP have been studied in the JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial, which enrolled patients based on hsCRP levels, and showed that rosuvastatin reduced hsCRP levels and resulted in fewer CV events compared with placebo [5]. This has been confirmed in a recent meta-analysis that showed statins reduce hsCRP levels in patients with CV disease [6]. According to the Centers for Disease Control and Prevention/American Heart Association guidelines, hsCRP < 1 mg/L indicates low CV risk, hsCRP of 1–3 mg/L indicates intermediate CV risk, and hsCRP > 3 mg/L indicates high CV risk [7]. These hsCRP categories are also used to predict CV outcomes [8].
Semaglutide, a human glucagon-like peptide-1 receptor agonist (GLP-1RA) available in weekly subcutaneous (s.c.) and daily oral formulations, was investigated in the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) and Peptide Innovation for Early Diabetes Treatment (PIONEER) phase 3 clinical trial programs, against placebo and active comparators [9,10,11,12,13]. The CV outcomes trials, SUSTAIN 6 and PIONEER 6, which were part of these programs, demonstrated CV benefits with s.c. semaglutide and CV non-inferiority with oral semaglutide vs placebo, respectively [14, 15]. Although there have been several studies with GLP-1RAs demonstrating a significant reduction in hsCRP [16, 17], the impact of semaglutide on hsCRP has yet to be fully elucidated.
The aim of this exploratory analysis was to evaluate the effect of semaglutide on hsCRP, and the contribution of glucose control and weight loss to this effect, in subjects at different stages of type 2 diabetes.
Methods
Trial designs and patient populations
This analysis included phase 3 trials from the semaglutide (s.c. and oral) clinical development programs that collected hsCRP data, specifically SUSTAIN 3 and PIONEER 1, 2, and 5 [18,19,20,21]; hsCRP data collected in SUSTAIN 2, 4, and 5 trials [22,23,24] were not used due to technical issues during sample collection — the hsCRP analysis assay was found to have a negative bias (an under-recovery of up to –25% at hsCRP concentrations below 5 mg/L), which affected a proportion of the samples.
Study designs for these double-blind, randomized controlled trials have been published previously [18,19,20,21]. In brief, subjects with inadequately controlled type 2 diabetes were randomized to receive: once-weekly s.c. semaglutide 1.0 mg or once-weekly GLP-1RA exenatide extended-release (exenatide ER) 2.0 mg (SUSTAIN 3); once-daily oral semaglutide 3, 7, or 14 mg or placebo (PIONEER 1); once-daily oral semaglutide 14 mg or once-daily sodium–glucose cotransporter-2 (SGLT-2) inhibitor empagliflozin 25 mg (PIONEER 2); and once-daily oral semaglutide 14 mg or placebo (PIONEER 5) [18,19,20,21]. Treatment durations were 56 weeks (SUSTAIN 3), 52 weeks (PIONEER 2), and 26 weeks (PIONEER 1 and 5). All subjects in PIONEER 5 had chronic kidney disease (CKD, estimated glomerular filtration rate [eGFR] 30–59 mL/min/1.73 m2 only). Only approved maintenance doses of semaglutide were included in this analysis.
All trial protocols were approved by local independent ethics committees and institutional review boards at each site and trials were conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. All subjects provided written, informed consent prior to trial start.
hsCRP was measured at weeks 0 and 56 in SUSTAIN 3; weeks 0 and 26 in PIONEER 1; weeks 0, 26, and 52 in PIONEER 2; and weeks 0, 8, and 26 in PIONEER 5. Data were evaluated for subjects receiving trial drug without rescue medication (trial product estimand), to avoid confounding effects from other antihyperglycemic drugs. Data from SUSTAIN 3, PIONEER 1, and PIONEER 2 were analyzed individually for change in hsCRP but were pooled for baseline characteristics; PIONEER 5 data were analyzed separately because they included subjects with CKD (baseline mean eGFR 48 mL/min/1.73 m2) who had longer disease duration (mean 14 years) [21].
Baseline hsCRP categories
Baseline characteristics were analyzed by hsCRP clinical cutoff (< 1.0, ≥ 1.0 to ≤ 3.0, or > 3.0 mg/L) to identify differences or similarities between subgroups, using combined data for SUSTAIN 3, PIONEER 1, and PIONEER 2; for PIONEER 5, they were calculated separately. Comparisons between baseline hsCRP clinical cutoff subgroups were conducted using a chi-squared test for categorical parameters and a Kruskal–Wallis test for continuous parameters.
Handling of hsCRP values below lower limit of quantification
If hsCRP values were below the lower limit of quantification (LLoQ), a prespecified imputation rule was applied so that hsCRP values were imputed as LLoQ/2. In all three PIONEER trials, LLoQ was 0.1 mg/L and in SUSTAIN 3, it was 1.4 nmol/L (approx. 0.147 mg/L). The proportion of values that were below LLoQ ranged from 0–0.19% at baseline to 0–0.7% at end-of-treatment.
Analysis of change in hsCRP from baseline
Because type 2 diabetes is a pro-inflammatory disease and patients may therefore have background chronic inflammation, hsCRP data were analyzed as ratios to baseline at end-of-treatment, by baseline clinical cutoff subgroups (< 1.0, ≥ 1.0 to ≤ 3.0, or > 3.0 mg/L) and by hsCRP tertile. Furthermore, to account for potential sex-related differences on the inflammatory response [25], a sensitivity analysis for the effect of treatment (semaglutide or comparator) on ratios-to-baseline by sex (male/female) at baseline was also conducted. Finally, to account for the effect of statins, which are known to reduce hsCRP levels [5, 6], a sensitivity analysis was conducted for the effect of treatment (semaglutide or comparators) on ratios to baseline by statin use (yes/no) at baseline. Absolute mean changes were also assessed by treatment arm in each trial, but not for subgroups because absolute data are more affected by high variability and outliers. Ratios to baseline at end-of-treatment were analyzed by baseline eGFR for PIONEER 5.
Due to right-skewing of the data, hsCRP ratios to baseline at end-of-treatment (Yeot/baseline, where Yeot indicates the observed value at the end-of-treatment visit) were log-transformed and analyzed using a mixed model for repeated measurements (MMRM) with treatment arm as categorical fixed effect and baseline value (log-transformed) as covariate, all nested within visit, and an unstructured residual covariance matrix. Absolute change from baseline to end-of-treatment (Yeot–baseline) was analyzed by the same model using non-transformed data as a supplementary analysis. From these two models, the treatment ratio (semaglutide arms versus comparators) using log-transformed data and treatment difference (semaglutide arms versus comparators) were estimated. The estimated difference and 95% confidence interval (CI) on the log-scale for each treatment arm were calculated using log-transformed data and were transformed back using the exponential function to generate an average ratio-to-baseline and corresponding 95% CI for each treatment arm on the geometric mean scale. Ratios below one indicated reductions in geometric mean hsCRP. Percentage reductions were calculated using 100% multiplied by (1 – ratio-to-baseline).
In all models, the data for comparator arms were pooled. Observed values from the on-treatment without rescue medication period were included in the MMRM. In an MMRM, the missing values are automatically accounted for using a missing-at-random assumption. A p-value of < 0.05 was considered significant; no adjustment for multiplicity was performed.
Subgroup analyses of change in hsCRP from baseline to end-of-treatment
The subgroup analyses of hsCRP clinical cutoffs (< 1.0, ≥ 1.0 to ≤ 3.0, or > 3.0 mg/L), hsCRP tertiles, sex (male/female), statin use at baseline (yes/no), and baseline eGFR (< 45 or ≥ 45 mL/min/1.73 m2; PIONEER 5 data only) were conducted using MMRMs with treatment arm, subgroup, and treatment arm-by-subgroup interaction as fixed effects and baseline hsCRP value (log-transformed) as covariate, all nested within visit, and an unstructured residual/covariance matrix. The interaction p-value was evaluated at end-of-treatment. The proportion of subjects who moved between risk groups (defined as hsCRP ≤ 3.0 or > 3.0 mg/L) was also assessed.
Mediation analyses
Mediation analyses examine associations among known, measured variables and outcomes, but do not necessarily identify causality. In this analysis, potential mediators for change in hsCRP, adjusted for possible confounders, were investigated. Mediation analyses included changes in HbA1c and body weight (BW) as mediators, separately and combined, for pooled semaglutide arms (7 mg and 14 mg in PIONEER 1) versus comparators.
The direct effect is the effect of semaglutide versus comparator on change in hsCRP, independent of changes in HbA1c and/or BW. This was estimated at end-of-treatment using an MMRM similar to that described above, with log-transformed values of hsCRP adjusted for baseline hsCRP (log-transformed), change of the mediator and baseline of the mediator and treatment, all nested within visit. The indirect effect is the effect of semaglutide versus comparator on change in hsCRP associated with changes in HbA1c and/or BW. This was estimated at end-of-treatment using an MMRM with change in the mediator adjusted for baseline hsCRP (log-transformed) and the baseline of the mediator and treatment and other relevant confounders, all nested within visits. The total effect was calculated as the sum of the direct and indirect effects using the product method. From these two models, the proportion of the effect mediated by change in HbA1c and BW was estimated at end-of-treatment, respectively, as calculated by the indirect effect divided by the total effect × 100. Similarly, the combined indirect effect from change in HbA1c and BW was calculated using each mediator as an endpoint in separate MMRMs (as described above), including all mediators and log-transformed hsCRP as baseline values and other relevant baseline variables (confounders) as presented in VanderWeelle 2014 [26], and from these models the proportion mediated was estimated.
Results
Baseline characteristics
A total of 2471 of 2482 randomized subjects (99.6%) had an hsCRP measurement at baseline and were included in the analyses. Geometric mean (% coefficient of variation) baseline hsCRP values were 2.8 (153) mg/L in SUSTAIN 3, 2.9 (173) mg/L in PIONEER 1, 2.7 (171) mg/L in PIONEER 2, and 3.0 (141) mg/L in PIONEER 5. As expected, subjects in the combined SUSTAIN 3, PIONEER 1, and PIONEER 2 analysis were younger, had a shorter disease duration, higher eGFR and lower systolic blood pressure at baseline, and were more likely to be previous or current smokers than subjects with CKD in PIONEER 5. Mean BMI at baseline was similar across all four included trials (Table 1).
In SUSTAIN 3, PIONEER 1, PIONEER 2, and PIONEER 5, there were more subjects in the high clinical cutoff group (> 3.0 mg/L) than in each of the lower cutoff (< 1.0 and ≥ 1.0 to ≤ 3.0 mg/L) groups. Subjects in this group in the SUSTAIN 3, PIONEER 1, and PIONEER 2 populations were younger, with a higher HbA1c, BW, BMI, and eGFR, and a shorter diabetes duration versus those in lower clinical cutoff groups. In PIONEER 5, subjects in the high clinical cutoff were younger, had a higher BMI and a lower eGFR versus those in the lower clinical cutoffs.
Estimated treatment ratios and ratio-to-baseline at end-of-treatment by trial
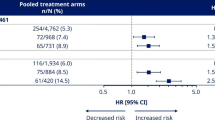
In SUSTAIN 3, PIONEER 1, and PIONEER 2, there was a significant reduction in hsCRP from baseline to end-of-treatment with semaglutide versus comparators. In SUSTAIN 3, the estimated treatment ratio (ETR) at week 56 for semaglutide 1.0 mg versus exenatide ER 2.0 mg was reduced by 25% (0.75 [95% CI, 0.65–0.88]; p < 0.001; Fig. 1). In PIONEER 1, the ETRs at week 26 for oral semaglutide 7 mg and 14 mg versus placebo were reduced by 28% and 24% (0.72 [95% CI, 0.59–0.89]; p < 0.01 and 0.76 [95% CI, 0.62–0.93]; p < 0.01, respectively; Fig. 1). In PIONEER 2, the ETR for semaglutide 14 mg versus empagliflozin 25 mg at week 52 was reduced by 30% (0.70 [95% CI, 0.61–0.80]; p < 0.0001; Fig. 1). In PIONEER 5, there was a nonsignificant trend of reduction, as the ETR at week 26 for oral semaglutide versus placebo was 0.83 [95% CI, 0.67–1.03]; p = 0.08; Fig. 1.
Ratios to baseline at end-of-treatment for hsCRP with semaglutide and comparators by trial. ‘On-treatment without rescue medication’ data from the full analysis set. Ratios to baseline were analyzed using a mixed model for repeated measurements with treatment as categorical fixed effect and baseline hsCRP value (log-transformed) as covariate, all nested within visit, and an unstructured residual covariance matrix on log-transformed values. CI confidence interval; exenatide ER exenatide extended-release; ETR estimated treatment ratio; hsCRP high-sensitivity C-reactive protein; N number of subjects with available hsCRP data; s.c. subcutaneous
Reductions of hsCRP from baseline were significant in all trials with semaglutide (ratio-to-baseline at end-of-treatment 0.55 to 0.82, p ≤ 0.01 for all), and with the two active comparators, exenatide ER and empagliflozin (ratio-to-baseline at end-of-treatment 0.72 and 0.91, p < 0.0001 and p = 0.0443, respectively; Fig. 1). hsCRP was not changed from baseline in the placebo arms of the trials with a ratio-to-baseline of 0.99 in both trials (Fig. 1).
Estimated treatment ratios and ratio-to-baseline by trial and subgroups
Clinical cutoffs of hsCRP at baseline
In all trials, the ETRs between semaglutide versus comparators were not affected by hsCRP cutoff group at baseline, as indicated by the nonsignificant interaction p-values for each trial (Fig. 2).
Ratio to baseline at end-of-treatment for hsCRP by trial according to clinical cutoffs. Panel (a) shows SUSTAIN 3 data, panel (b) PIONEER 1 data, panel (c) PIONEER 2 data, and panel (d) PIONEER 5 data. ‘On-treatment without rescue medication’ data from the full analysis set. Ratios to baseline were analyzed using a mixed model for repeated measurements with treatment by hsCRP groups as categorical fixed effects and baseline hsCRP value (log-transformed) as covariate, all nested within visit, and an unstructured residual covariance matrix on log-transformed values. Clinical cut-offs used in this analysis were < 1.0, ≥ 1.0 to ≤ 3.0, and > 3.0 mg/L. CI confidence interval; ETR estimated treatment ratio; exenatide ER exenatide extended-release; hsCRP high-sensitivity C-reactive protein; N number of subjects with available hsCRP data; s.c. subcutaneous
hsCRP tertiles at baseline
In all trials, the ETRs for semaglutide versus comparators were not affected by hsCRP tertile at baseline, as indicated by the interaction p-values for each trial, which were all nonsignificant, except for PIONEER 1 (pinteraction: 0.0074; Additional file 1: Fig. S1). The trend was for ratios-to-baseline at end-of-treatment to be lowest in the lowest tertiles for semaglutide and for the active comparators, i.e. the largest hsCRP reductions from baseline were observed in subjects with the lowest baseline hsCRP levels; the ratio-to-baseline increased progressively in the middle and high hsCRP tertile groups (Additional file 1: Fig. S1).
Stratification by sex
Sex (female or male) did not affect the ETRs for semaglutide versus comparators, as indicated by the interaction p-values in any of the trials (all pinteraction > 0.05) (Additional file 1: Fig. S2).
Statin use at baseline
Statin use at baseline (yes or no) did not affect the ETRs for semaglutide versus comparators, as indicated by the nonsignificant interaction p-values for each trial (Additional file 1: Fig. S3). No clear pattern in ratio-to-baseline was observed according to statin use at baseline for semaglutide and all comparators.
eGFR (< 45 versus ≥ 45 mL/min/1.73 m2) at baseline
eGFR group at baseline (< 45 or ≥ 45 mL/min/1.73 m2) did not affect the ETRs for semaglutide versus placebo in PIONEER 5. The ETR for semaglutide versus placebo for eGFR < 45 mL/min/1.73 m2 was 0.91 [95% CI, 0.64–1.30] and for eGFR ≥ 45 mL/min/1.73 m2 was 0.78 [95% CI, 0.59–1.02]. The pinteraction between treatment and eGFR subgroup was 0.48 (data not shown).
hsCRP cutoff at end-of-treatment versus baseline
In most study arms, a greater proportion of subjects moved to the ≤ 3.0 mg/L hsCRP category from the > 3.0 mg/L hsCRP category than moved in the opposite direction (this was not observed with placebo in PIONEER 5). The percentage of subjects moving from baseline hsCRP ≤ 3.0 mg/L to hsCRP > 3.0 mg/L at end-of-treatment was greater with comparators (exenatide ER, empagliflozin, and placebo) compared with semaglutide (except with oral semaglutide 7 mg versus placebo in PIONEER 1), and the percentage of subjects moving from baseline hsCRP > 3.0 mg/L to hsCRP ≤ 3.0 mg/L at end-of-treatment was greater with semaglutide compared with comparators (Additional file 1: Table S1).
Absolute hsCRP change from baseline
hsCRP was significantly reduced with oral semaglutide 7 mg versus placebo in PIONEER 1 (estimated treatment difference: −1.39 mg/L [95% CI, −2.56 to −0.22]; Additional file 1: Fig. S4). In the other trials, there was a trend for a negative absolute hsCRP change from baseline with oral semaglutide 14 mg versus placebo (PIONEER 5) and with semaglutide (s.c. 1.0 mg or oral 14 mg doses) versus active comparators (SUSTAIN 3 and PIONEER 2, respectively), although these differences did not reach statistical significance (p > 0.05; Additional file 1: Fig. S4).
Mediation analyses
Some of the effect of semaglutide (s.c. 1.0 mg, oral 7 mg, and oral 14 mg) on hsCRP was shown to be mediated by change in HbA1c (18.5–35.6%; Fig. 3a), with change in BW playing a lesser role in PIONEER 1 (10.9%) and PIONEER 2 (5.7%) but a similar or greater role in SUSTAIN 3 (35.8%) and PIONEER 5 (57.0%; Fig. 3b). When the two mediation parameters were considered together, some but not all of the effect of semaglutide on hsCRP was mediated by a change in HbA1c and BW (combined percentage mediated: 20.6–61.8%; Fig. 3c). The sum of the proportions mediated individually by change in BW and change in HbA1c was greater than the combined mediated proportion, which is likely to be caused by associations between HbA1c and BW.
Mediation of hsCRP by change in HbA1c and/or body weight. Panel (a) shows mediation of hsCRP by HbA1c, panel (b) by body weight, and panel (c) by HbA1c and body weight combined, all according to trial and with pooled semaglutide arms versus comparators. The indirect effect is the effect of semaglutide versus comparator on change in hsCRP that was caused by its effect through change on HbA1c and/or BW. The direct effect is the effect of semaglutide versus comparator on change in hsCRP independent of change in HbA1c and/or BW. The total effect was calculated by adding the indirect effect to the direct effect. The proportion mediated was calculated by dividing the indirect effect by the total effect × 100. Data from the ‘on-treatment without rescue medication’ period. Mediation was analyzed with mixed models for repeated measurements, adjusted for relevant mediators at baseline: HbA1c (panel a), BW (panel b), BW and HbA1c (panel c); and furthermore adjusted for additional covariates at baseline: log(hsCRP), age, sex, eGFR (Chronic Kidney Disease–Epidemiology Collaboration 2009), body mass index, statins (yes/no), insulin treatment (PIONEER 5 analyses only), metformin treatment (SUSTAIN 3 and PIONEER 5 analyses only), and HbA1c (panel b only). Mediator data were only taken from the visits where hsCRP was assessed for estimation of the direct effect. BW body weight; CI confidence interval; eGFR estimated glomerular filtration rate; HbA1c glycated hemoglobin; hsCRP high-sensitivity C-reactive protein
Discussion
This exploratory analysis suggests that s.c. and oral semaglutide significantly reduced hsCRP levels versus comparators in subjects with type 2 diabetes. The comparators were placebo in PIONEER 1 and PIONEER 5, and active comparators in SUSTAIN 3 (exenatide ER) and PIONEER 2 (empagliflozin). The effect of semaglutide on hsCRP was partially mediated via reductions in HbA1c and BW, which may therefore be involved in a potential anti-inflammatory effect of semaglutide. However, there may also be a direct effect of semaglutide on hsCRP, supporting potential direct anti-inflammatory action.
When stratified by hsCRP clinical cutoffs (< 1.0 mg/L, ≥ 1.0 to ≤ 3.0 mg/L, > 3.0 mg/L), subjects in the highest clinical cutoff were younger, with higher HbA1c, BW, and BMI (and higher eGFR in the pooled SUSTAIN 3, PIONEER 1 and 2 analysis only) at baseline. Treatment ratio reductions were generally similar across these categories, although some ETRs were not significant, possibly due to a lack of statistical power resulting from small sample size. Treatment ratios were also generally similar when subjects were stratified by tertile, sex, baseline statin use, or eGFR (data from PIONEER 5 only). Furthermore, more subjects treated with semaglutide moved from a higher to a lower hsCRP category compared with those treated with comparators, possibly indicating reduced inflammation. Finally, reductions in hsCRP appeared to be partially associated with change in HbA1c and also in BW (although to a lesser extent in PIONEER 1 and 2 than in the other trials); however, there are some supporting data for a direct effect of semaglutide.
There are several analyses of the effects of GLP-1RAs on hsCRP. Decreases in hsCRP have also been observed with other classes of antihyperglycemic drug (metformin, dipeptidyl peptidase-4 inhibitors, and SGLT-2 inhibitors), but with less consistency than with GLP-1RAs [27]. The beneficial effect of GLP-1RAs on hsCRP has been shown in a meta-analysis of small trials with liraglutide and exenatide [28]. In addition, analysis of a 52-week phase 2 trial investigated once-daily ≤ 0.4 mg doses of s.c. semaglutide (N = 957) and the STEP phase 3 trials investigated s.c. semaglutide 2.4 mg dose in subjects with overweight and obesity with or without type 2 diabetes (total N = 4684); these trials identified consistent reductions in hsCRP with once-daily semaglutide ≤ 0.4 mg and once-weekly s.c. semaglutide 2.4 mg [28,29,30,31,32]. An analysis of subjects with type 2 diabetes and obesity in the LYDIA trial found there was no significant difference in the effects of liraglutide on hsCRP compared with the dipeptidyl peptidase-4 inhibitor sitagliptin [33]. However, a recent meta-analysis, which included data from 40 randomized controlled trials in subjects with type 2 diabetes, found significant improvements with GLP-1RAs compared with standard diabetes therapies (including sulfonylureas, insulins, dipeptidyl peptidase-4 inhibitors, thiazolidinediones) and placebo in several clinical biomarkers, including CRP, tumor necrosis factor-alpha, and adiponectin (markers of inflammation) and malondialdehyde (a marker of oxidative stress) [34]. Our analyses not only confirm previous findings of hsCRP reductions with a GLP-1RA, but also expand knowledge on these reductions, which can occur regardless of baseline hsCRP, sex, and statin use. Reductions in hsCRP are highly clinically relevant: elevated hsCRP has been found to be predictive of major adverse CV events and death in individuals with or without type 2 diabetes and/or previous CV events [35]. The value of hsCRP as a CV risk factor appears similar to that of conventional CV risk factors – hsCRP is not a causal factor for CV disease and serves as a biomarker only [36].
As inflammation is an important process in CV disease [1, 2] and GLP-1RAs are hypothesized to lower CV risk via attenuation of atherosclerosis [37, 38], the hsCRP reduction observed with the GLP-1RA semaglutide suggests that it might exert anti-inflammatory effects. Some possible mechanisms for this action include GLP-1 receptor expression in the intestine, which would lead to an improved gut barrier function [39, 40], or reprogramming of macrophages [41]. However, the actual mechanism remains unknown.
Our analyses contained data from PIONEER 5, which included subjects with CKD. However, as hsCRP and other inflammatory markers are typically higher in patients with CKD [42], the data from PIONEER 5 were analyzed separately to those of the other trials. Mean baseline hsCRP in PIONEER 5 was similar to that in other trials, and hsCRP ratio to baseline was reduced at end-of-treatment. Furthermore, baseline eGFR categories were shown to have no effect on the hsCRP ratio to baseline at end-of-treatment. This is a clinically relevant result, because reductions in hsCRP have been linked to improved kidney outcomes [43]. In general, the pathological milieu is altered in patients with kidney impairment and may lead to differences in responses to drugs [44], although the renal safety of semaglutide presented in the PIONEER 5 primary publication was consistent with the suggested kidney-protective effects of GLP-1RAs overall in this population [21].
In the mediation analyses, which examined change in HbA1c and BW (both separately and combined) as potential mediators for the effect of semaglutide on hsCRP, the effect on hsCRP was partially mediated via the effect of semaglutide on HbA1c and BW, although HbA1c seemed to contribute slightly more than BW. Our findings are consistent with those from a longitudinal observational study in patients without diabetes, which found that there was a positive association between HbA1c and hsCRP levels [45]. Furthermore, as it is well-known that weight loss has an anti-inflammatory effect [30,31,32], partial mediation by BW is unsurprising.
Strengths of the current analysis include its large sample size of patient-level data from several randomized clinical trials of semaglutide. Another strength is that the same central laboratory analyzed hsCRP and other laboratory results.
Limitations of this analysis include its exploratory nature, lack of hard endpoints for evaluation, low hsCRP testing frequency, lack of data on other markers of inflammation, and the relatively short follow-up time (26–56 weeks). The analyses were performed using patient-level data for each individual trial as data could not be “pooled” due to differences in trial duration, although pooling was performed for baseline characteristics, with the exception of PIONEER 5 owing to its inclusion of a kidney-impaired population. In addition, hsCRP data are subject to a high degree of variability and outliers may dominate the absolute change in hsCRP data, based on the assumption of a normal distribution. This may explain why differences in the relative change in hsCRP were significant and some absolute changes were not; however, for lower levels of hsCRP at baseline, ratios to baseline may be overly sensitive to small changes. Another limitation is that the mediation analysis did not use all mediator values obtained during the trial that may have a direct relationship with hsCRP levels, as may be done in more complex longitudinal mediation analyses. Finally, these trials were not powered for mediation analyses specifically, and hence there was high variability in the statistics used.
Conclusions
Semaglutide appeared to reduce hsCRP ratios-to-baseline to a greater extent than comparators — another GLP-1RA (exenatide ER), an SGLT-2 inhibitor (empagliflozin), and placebo (not significant versus placebo in PIONEER 5) — in subjects with type 2 diabetes, and this was partially mediated via its effect on HbA1c and BW. The mediation analysis suggested that there may also be a direct semaglutide effect. Ongoing semaglutide CV and kidney outcome trials, such as SOUL (NCT03914326) investigating subjects with type 2 diabetes and CV disease, SELECT (NCT03574597) investigating subjects with obesity and CV disease, and FLOW (NCT03819153) investigating subjects with type 2 diabetes and renal impairment, may help further elucidate on the semaglutide impact on hsCRP and any possible association between hsCRP reduction and reductions in CV and kidney adverse outcomes. Furthermore, a dedicated CV mode of action trial with semaglutide in subjects with type 2 diabetes and CV disease is also ongoing, which includes inflammation in atherosclerosis as the primary endpoint (NCT04032197).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BW:
-
Body weight
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CV:
-
Cardiovascular
- eGFR:
-
Estimated glomerular filtration rate
- Exenatide ER:
-
Exenatide extended-release
- ETR:
-
Estimated treatment ratio
- GLP-1RA:
-
Glucagon-like peptide-1 receptor agonist
- HbA1c :
-
Glycated hemoglobin
- hsCRP:
-
High-sensitivity C-reactive protein
- JUPITER:
-
Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin
- LLoQ:
-
Lower limit of quantification
- MMRM:
-
Mixed model for repeated measurements
- PIONEER:
-
Peptide Innovation for Early Diabetes Treatment
- s.c.:
-
Subcutaneous
- SGLT-2:
-
Sodium–glucose cotransporter-2
- SUSTAIN:
-
Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes
- Yeot :
-
Observed value at the end-of-treatment visit
References
Masters SL, Latz E, O’Neill LA. The inflammasome in atherosclerosis and type 2 diabetes. Sci Transl Med. 2011;3:81ps17.
Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14:50–9.
Baldassarre MPA, Andersen A, Consoli A, Knop FK, Vilsbøll T. Cardiovascular biomarkers in clinical studies of type 2 diabetes. Diabetes Obes Metab. 2018;20:1350–60.
Bahceci M, Tuzcu A, Ogun C, Canoruc N, Iltimur K, Aslan C. Is serum C-reactive protein concentration correlated with HbA1c and insulin resistance in Type 2 diabetic men with or without coronary heart disease? J Endocrinol Invest. 2005;28:145–50.
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207.
Kandelouei T, Abbasifard M, Imani D, Aslani S, Razi B, Fasihi M, et al. Effect of statins on serum levels of hs-CRP and CRP in patients with cardiovascular diseases: a systematic review and meta-analysis of randomized controlled trials. Mediators Inflamm. 2022;2022:8732360.
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511.
Ridker PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol. 2016;67:712–23.
Aroda VR, Ahmann A, Cariou B, Chow F, Davies MJ, Jódar E, et al. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1–7 trials. Diabetes Metab. 2019;45:409–18.
Lingvay I, Catarig AM, Frias JP, Kumar H, Lausvig NL, le Roux CW, et al. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:834–44.
Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:356–67.
Capehorn MS, Catarig AM, Furberg JK, Janez A, Price HC, Tadayon S, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46:100–9.
Thethi TK, Pratley R, Meier JJ. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes Metab. 2020;22:1263–77.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44.
Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–51.
Wu Y, Lu Y, Yang S, Zhang Q. Effects of incretin-based therapy on high-sensitivity C-reactive protein in patients with type 2 diabetes: a systematic review and meta-analysis. Rev Invest Clin. 2021;73:100–10.
Seon MJ, Hwang SY, Son Y, Song J, Kim OY. Circulating GLP-1 levels as a potential indicator of metabolic syndrome risk in adult women. Nutrients. 2021;13:865.
Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41:258–66.
Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42:1724–32.
Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SØ, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42:2272–81.
Mosenzon O, Blicher TM, Rosenlund S, Eriksson JW, Heller S, Hels OH, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:515–27.
Ahrén B, Masmiquel L, Kumar H, Sargin M, Karsbøl JD, Jacobsen SH, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5:341–54.
Aroda VR, Bain SC, Cariou B, Piletic M, Rose L, Axelsen M, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:355–66.
Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103:2291–301.
Al-Salameh A, Chanson P, Bucher S, Ringa V, Becquemont L. Cardiovascular disease in type 2 diabetes: a review of sex-related differences in predisposition and prevention. Mayo Clin Proc. 2019;94:287–308.
VanderWeele TJ, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiol Methods. 2014;2:95–115.
Katsiki N, Ferrannini E. Anti-inflammatory properties of antidiabetic drugs: a “promised land” in the COVID-19 era? J Diabetes Complications. 2020;34:107723.
Mazidi M, Karimi E, Rezaie P, Ferns GA. Treatment with GLP1 receptor agonists reduce serum CRP concentrations in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Complications. 2017;31:1237–42.
Newsome P, Francque S, Harrison S, Ratziu V, Van Gaal L, Calanna S, et al. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment Pharmacol Ther. 2019;50:193–203.
Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989.
Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397:971–84.
Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325:1403–13.
Ahmad E, Waller HL, Sargeant JA, Webb MA, Htike ZZ, McCann GP, et al. Effects of liraglutide versus sitagliptin on circulating cardiovascular biomarkers, including circulating progenitor cells, in individuals with type 2 diabetes and obesity: analyses from the LYDIA trial. Diabetes Obes Metab. 2021;23:1409–14.
Bray JJH, Foster-Davies H, Salem A, Hoole AL, Obaid DR, Halcox JPJ, et al. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: a systematic review and meta-analysis of randomised controlled trials. Diabetes Obes Metab. 2021;23:1806–22.
Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25,979 participants from four UK prospective cohort studies. Diabetes Care. 2012;35:396–403.
Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–908.
Rakipovski G, Rolin B, Nøhr J, Klewe I, Frederiksen KS, Augustin R, et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE(-/-) and LDLr(-/-) mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. 2018;3:844–57.
Kaul S. Mitigating cardiovascular risk in type 2 diabetes with antidiabetes drugs: a review of principal cardiovascular outcome results of EMPA-REG OUTCOME, LEADER, and SUSTAIN-6 trials. Diabetes Care. 2017;40:821–31.
Yusta B, Baggio LL, Koehler J, Holland D, Cao X, Pinnell LJ, et al. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. 2015;64:2537–49.
Bang-Berthelsen CH, Holm TL, Pyke C, Simonsen L, Søkilde R, Pociot F, et al. GLP-1 induces barrier protective expression in Brunner’s glands and regulates colonic inflammation. Inflamm Bowel Dis. 2016;22:2078–97.
Bruen R, Curley S, Kajani S, Lynch G, O’Reilly ME, Dillon ET, et al. Liraglutide attenuates preestablished atherosclerosis in apolipoprotein E-deficient mice via regulation of immune cell phenotypes and proinflammatory mediators. J Pharmacol Exp Ther. 2019;370:447–58.
Stam F, van Guldener C, Schalkwijk CG, ter Wee PM, Donker AJ, Stehouwer CD. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol Dial Transplant. 2003;18:892–8.
Liu L, Gao B, Wang J, Yang C, Wu S, Wu Y, et al. Reduction in serum high-sensitivity C-reactive protein favors kidney outcomes in patients with impaired fasting glucose or diabetes. J Diabetes Res. 2020;2020:2720905.
Hahr AJ, Molitch ME. Management of diabetes mellitus in patients with chronic kidney disease. Clin Diabetes Endocrinol. 2015;1:2.
Ahmadi-Abhari S, Kaptoge S, Luben RN, Wareham NJ, Khaw KT. Longitudinal association of C-reactive protein and haemoglobin A1c over 13 years: the European Prospective Investigation into Cancer-Norfolk study. Cardiovasc Diabetol. 2015;14:61.
Acknowledgements
We thank all the subjects, investigators, and trial-site staff members who were involved in the conduct of the trial and Flavia Sciota, AXON Communications, for medical writing and editorial assistance (funded by Novo Nordisk).
Funding
This study was funded by Novo Nordisk A/S.
Author information
Authors and Affiliations
Contributions
S.R. performed statistical analyses. All authors acquired, analyzed, or interpreted data; drafted and critically revised the manuscript. ADR is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All trial protocols were approved by local independent ethics committees and institutional review boards at each site and trials were conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. All subjects provided written, informed consent prior to trial start.
Consent for publication
Not applicable.
Competing interests
OM reports personal fees for advisory board consultancy and speaker’s bureau from AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, and Sanofi; and research grant support from AstraZeneca and Novo Nordisk. MC reports being a partner at Clifton Medical Centre, a director at RIO Weight Management, Ltd, and a consultant for LighterLife and McDonald’s; he also reports research funding from Abbott, Boehringer Ingelheim/Lilly Alliance, Janssen, MSD, and Novo Nordisk; advisory board consultancy, consultancy and honoraria from Abbott, Boehringer Ingelheim/Lilly Alliance, and Novo Nordisk; and meeting support from Boehringer Ingelheim/Lilly Alliance and Novo Nordisk. ADR, SR, and PW are full-time employees of Novo Nordisk; SR also owns shares in Novo Nordisk. JR reports research funding from Applied Therapeutics Inc., Boehringer Ingelheim, Eli Lilly, Genentech, GlaxoSmithKline, Hanmi, Intarcia, Janssen, Lexicon, Merck, Metacrine, Novo Nordisk, Novartis, Oramed, Pfizer, and Sanofi; and advisory board consultancy, consultancy, and honoraria from Applied Therapeutics Inc., Boehringer Ingelheim, Eli Lilly, Hanmi, Intarcia, Janssen, Novo Nordisk, Oramed, Sanofi, and Zealand.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Additional information on SUSTAIN and PIONEER trials. Figure S1.
Ratios to baseline at end-of-treatment for hsCRP with semaglutide and comparators by trial according to baseline tertiles. Figure S2. Ratios to baseline at end-of-treatment for hsCRP by trial according to sex (female/male). Figure S3. Ratios to baseline at end-of-treatment for hsCRP by trial according to statin use (yes/no). Figure S4. Absolute change in hsCRP from baseline to end-of-treatment by trial (semaglutide vs comparator). Table S1. Percentage of subjects who moved between hsCRP risk groups according to hsCRP category by trial – change between baseline and end of treatment
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mosenzon, O., Capehorn, M.S., De Remigis, A. et al. Impact of semaglutide on high-sensitivity C-reactive protein: exploratory patient-level analyses of SUSTAIN and PIONEER randomized clinical trials. Cardiovasc Diabetol 21, 172 (2022). https://doi.org/10.1186/s12933-022-01585-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01585-7