Abstract
Background
Previous research has linked elevated low-density lipoprotein cholesterol (LDL-C) and remnant cholesterol (RC) with diabetes mellitus (DM). The present study aims to estimate the RC-related DM risk beyond LDL-C, and to investigate the extent to which the association of RC and DM is mediated via insulin resistance and inflammation.
Methods
We enrolled 7308 individuals without previous history of DM into the present study from the China Health and Nutrition Survey. Fasting RC was calculated as total cholesterol minus LDL-C and high-density lipoprotein cholesterol. Subjects were divided into four groups according to their LDL-C (100 mg/dL) and RC (24 mg/dL) levels to evaluate the role of LDL-C vs. RC on DM. A logistic regression analysis was then employed to evaluate the relationships between the discordant/concordant LDL-C and RC and DM. A mediation analysis was undertaken to identify potential mediators.
Results
Of all the participants, a total of 625 (8.55%) patients were newly diagnosed with DM. Compared to the high LDL-C/low RC group, the low LDL-C/high RC group was more common in DM patients. After a multivariate adjustment, elevated LDL-C and RC were associated with DM. Moreover, the low LDL-C/high RC group and the high LDL-C/low RC group manifested a 4.04-fold (95% CI 2.93–5.56) and 1.61-fold (95% CI 1.21–2.15) higher risk of DM, relative to those with low LDL-C/low RC. The subgroup analysis indicated that low LDL-C/high RC was more likely to be related to DM in females. Similar results were also shown when the sensitivity analyses were performed with different clinical cut-points of LDL-C. Insulin resistance and inflammation partially mediated the association between RC and DM.
Conclusions
Our findings provided evidence for RC beyond the LDL-C associations with DM that may be mediated via insulin resistance and the pro-inflammatory state. In addition, women are more susceptible to RC exposure-related DM.
Similar content being viewed by others
Background
Diabetes mellitus (DM) is a metabolic disorder that is characterized by persistent hyperglycemia due to defects in glucose metabolism. According to the International Diabetes Federation, 536.6 million people worldwide aged 21–79 were afflicted with DM in 2021 [1]. With rapid developments in the global economy and changes in diet, the high morbidity and disability exhibited in DM has become a significant public health issue that has reached alarming levels.
Previous studies have shown that dyslipidemia appears in the early stages of DM and continues to be involved in the disease progresses throughout its entire course [2,3,4]. Among the spectrum of lipoproteins, there is no doubt that low-density lipoprotein cholesterol (LDL-C) is the most important one. However, with the widespread use of statins in recent years, we have shifted our attention to triglyceride (TG)-rich lipoproteins (TRLs), one of the hallmarks of diabetic dyslipidemia that cannot be controlled by statins [5, 6]. There has been increasing evidence in recent years that TRLs are associated with the onset of diabetes [7]. A molecular lipid analysis showed that plasma TG levels were the only variable associated with DM in high-risk groups [8], and a high-fat diet increased the synthesis of TG in the liver, reduced relative high-density lipoprotein cholesterol (HDL-C), and accelerated the onset of DM [9]. In population cohort studies, a higher TG-related indicators were associated with DM independent of insulin resistance, highlighting the importance of TRLs in the development of DM [10,11,12].
Remnant cholesterol (RC) is the cholesterol content of TRLs that consists of very low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), and chylomicron remnants [13]. Although plasma TG can be used as a surrogate measure of TRLs or RC clinically, they represent different lipid disorders. In fact, RC is essentially a cholesterol nature. Cholesterol content in TRLs contributed more directly to cardiovascular disease (CVD) rather than TG, thus, RC was highlighted in the lipids management in recent years [14]. RC is more abundant, larger, and carries more cholesterol than LDL-C particles, so it can be inferred that RC is more harmful to pancreatic β-cells [8]; In addition, unlike LDL-C, TRLs exert their pathogenic effects through inflammatory pathways [15, 16]. Epidemiological evidence has suggested that higher RC levels were not only significantly associated with the development of DM, but also contributed to the comorbidities such as hypertension [17, 18]. In recent years, high levels of RC have been proposed as part of the explanation for the residual risk after optimal LDL-C was proposed as a viewpoint in primary prevention [19, 20]. Interestingly, as research continues, it appears that the association between RC and CVD extends beyond LDL-C [21, 22], introducing a new point of the discordant/concordant LDL-C and RC. It will become increasingly important to investigate the discordance/concordance of LDL-C and RC, especially in the context of the increasing prevalence of DM, as both are on the rise in DM patients. Specific TRLs and LDL-C particles have been linked to DM based on the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study [8], but the association between the discordant/concordant LDL-C and RC and DM requires further elucidation.
The prevalence of DM in China ranks first among the world’s countries and continues to exhibit an annual upward trend [1, 23] that is closely related to the rapid development of the economy and the changes in the dietary structure. The prevalence of obesity and dyslipidemia increased significantly during this same period [24]. The disparate prognostic outcomes of the LDL-C and RC discordance/concordance prompt further exploration of the high-risk population and the potential mechanism for this phenomenon. In this study, we aimed to identify the discordant/concordant LDL-C and RC associations with DM and explore whether insulin resistance and inflammation mediate this relationship, based on the China Health and Nutrition Survey.
Methods
Study setting and population
The China Health and Nutrition Survey (CHNS) is an ongoing longitudinal community-based cohort study carried out by the national and local governments of China, and the study details were described in our previous article [25]with the relevant protocol published elsewhere [26]. The CHNS is a collaborative project between the Carolina Population Center, University of North Carolina at Chapel Hill and the National Institute for Nutrition and Health (NINH, former National Institute of Nutrition and Food Safety), Chinese Center for Disease Control and Prevention. The study protocols and ethics approval were derived from the Institutional Review Committees of the University of North Carolina at Chapel Hill and the National Institute for Nutrition and Health at Beijing and so, were attributed to the two centers. Each participant was enrolled in China and provided written informed consent.
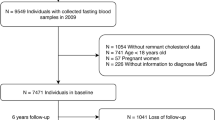
In 2009, the CHNS collected 9549 fasting blood samples. We excluded 841 participants who were under 18 years, 62 pregnant women, 281 participants previously diagnosed with DM, and 89 who were previously diagnosed with myocardial infarction—thus avoiding the influence of possible lipid-lowering therapies. We also declined to enroll 917 patients who were missing RC information or whose RC was less than or equal to 0, and 51 patients who lacked information regarding their glycosylated hemoglobin A1c (HbA1c) or fasting blood glucose (FBG) with respect to the DM diagnosis. Our current study population eventually comprised 7308 individuals (Additional file 1: Fig. S1), and we listed the patient information that includes the baseline characteristics, healthy behaviors, and laboratory examinations.
Measures
RC (mmol/L) was calculated from a standard lipid profile of the patient in a fasting state as total cholesterol (TC) (mmol/L) minus LDL-C (mmol/L) minus HDL-C (mmol/L) [27]. According to the American Diabetes Association criteria, DM was defined as exhibiting a previous DM history or a FBG ≥ 7.0 mmol/L and/or an HbA1c ≥ 6.5% [28].
Definitions
Height and weight were measured while the subjects were wearing light clothing and standing without shoes. We calculated the body mass index (BMI) as weight (kg)/height (m)2. Health behaviors (smoking and alcohol consumption) were self-reported. Smoking was defined as any previous smoking (yes/no), and alcohol consumption was defined as imbibition greater than three times per week (yes/no).
Participants were asked to maintain a regular pattern of life as well as normal emotional stability for at least three days, and to fast for 8–12 h prior to blood sample collection. From the blood samples, the white blood cells (WBCs) was measured with a Beckman Coulter LH751 (Beckman Coulter, USA). The LDL-C, HDL-C, TC, TG, apolipoprotein B (apoB), apolipoprotein A (apoA), FBG, uric acid, and serum creatinine were measured using a Hitachi 7600 machine (Randox, UK and Kyowa, Japan). The HbA1c was determined by the HLC-723 G7/D10/PDQ A1c (Tosoh, Japan/Bio-Rad, USA/Primus, USA), and the high-sensitivity C-reactive protein (Hs-CRP) was detected with a Hitachi 7600 (Denka Seiken, Japan). Elevated Hs-CRP was indicated as ≥ 5 mg/L. We calculated the estimated glomerular filtration rate (eGFR) (mL/min 1.73 m− 2) employing the Chronic Kidney Disease—Epidemiology Collaboration (CKD-EPI) equation, and the CKD was diagnosed based on an eGFR of < 60 mL/min 1.73 m− 2. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated by the formula HOMA-IR = serum insulin (mU/mL) * FBG (mmol/L)/22.5 [29], while the homeostasis model assessment of insulin sensitivity (HOMA-IS) was calculated by the formula HOMA-IS = 1/HOMA-IR.
Statistical analyses
To assess the impact of RC beyond the LDL-C in DM patients, we selected 2.6 mmol/L (100 mg/dL) and 0.62 mmol/L (24 mg/dL) as the cut-off points for the LDL-C and RC, respectively, according to the recommended LDL-C target from the 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias [30] and a recent primary prevention study of RC [21]. Hence, we divided all the participants into four groups (Group 1: low LDL-C/low RC, Group 2: low LDL-C/high RC, Group 3: high LDL-C/low RC, and Group 4: high LDL-C/high RC).
Participant characteristics were described according to whether DM was present and commensurate with the discordance/concordance of LDL-C and RC. Continuous variables are expressed as means ± standard deviation for normal distributions or medians and interquartile range (25–75%) for skewed distributions, and categorical variables are presented as relative frequencies (percentages). We employed t-tests for normally distributed data and the Mann-Whitney U/Kruskal-Wallis rank test for non-normally distributed data; The Chi-squared test/Fisher’s exact-probability test for categorical variables was used to determine significant differences between the groups. The independent association of the discordance/concordance of the LDL-C and RC with the presented DM was evaluated using logistic models with odds ratios (ORs) and 95% confidence intervals (CIs). Potential covariates that were significant in the baseline comparison or that we considered to be of clinical importance were included in the multivariate models. We constructed four main models for the covariate adjustment, i.e., Model 1, unadjusted; Model 2, adjusted for age, sex, and BMI; and Model 3, adjusted for the variables in Model 1, educational level, smoking, alcohol consumption, and CKD. Since previous studies have reported that RC is closely related to TG, we adjusted for the TG in Model 4 for further validation. In addition, as previous anti-hypertensive drug use and the LDL-C size that can be reflected by the LDL-C/Apo-B ratio and are associated with the development of DM, we also adjusted for the use of anti-hypertensive drugs and the LDL-C/Apo-B ratio in Model 5.
The subgroup analysis was executed to examine the effect of the discordant/concordant LDL-C and RC on DM in the various subgroups, including age (</≥ 65 years) and sex (male/female). We tested the modifications and interactions of the subgroups using the likelihood-ratio test. For sensitivity analysis, using the same model, we assessed the relationship between the discordant/concordant RC and LDL-C groups and DM using a more stringent clinical cut-point for LDL-C (1.80 mmol/L).
Given that the pro-inflammatory state and insulin resistance have been identified as pathways through which DM may affect the metabolism of serum lipids [31, 32], we investigated whether the association between the discordant/concordant LDL-C and RC and DM was mediated by WBCs, hs-CRP, and HOMA-IR. The Sobel test was used to assess the effects of these mediators [33].
The proportion of missing data in the analytic sample was not greater than 2%, and the missing data were then interpolated using the method of the last observation carried forward, or using the means and medians for the continuous variables and skewed variables. Comparisons where P was < 0.05 (two-sided) were considered to be statistically significant. We performed all analyses with Stata 15.0, R (version 3.4.3) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA).
Results
Baseline characteristics
The distribution of LDL-C and RC by the discordant/concordant groups is shown in Fig. 1. A high level of RC accounted for a relatively small proportion of the participants with low or high levels of LDL-C, while higher RC was more distributed in the low LDL-C group. Baseline characteristics of participants according to the discordance/concordance of the LDL-C and RC levels are shown in Table 1. Compared with patients in Group 3 (high LDL-C/low RC), those in Group 2 (low LDL-C/high RC) tended to be younger, were males, smokers and drinkers, and had higher BMI and blood pressure. The prevalence of DM was higher in Group 2 (15.93%) than in Group 3 (7.51%). As for the cardiometabolic biomarkers, Group 2 had lower levels of HDL-C TC and lipoprotein (a) than Group 3, while TG in Group 2 is lower than that in Group 3. Group 2 was also accompanied by an increase in the HOMA-IR, WBCs, and hs-CRP. Baseline information of our enrolled participants by DM and non-DM groups was also summarized in Table 2, exhibiting significantly higher LDL-C and RC levels in DM compared with the control group. DM patients tended to be older, and also tended to possess a higher BMI, blood pressure, WBC count, and hs-CRP. In addition, they manifested lower HDL-C and lipoprotein (a). For the discordance/concordance of LDL-C and RC, Group 2 (low LDL-C/high RC) and Group 4 (high LDL-C/high RC) were more common in DM patients.
The distribution of LDL-C and RC by the discordant/concordant groups. a The box plot of the different LDL-C and RC levels. b the relative proportions of the discordant/concordant groups. Groups 1–4 represent the low LDL-C/low RC group, low LDL-C/high RC group, high LDL-C/low RC group and the high LDL-C/high RC group, respectively. LDL-C low-density lipoprotein cholesterol, RC remnant cholesterol
Inter-relation of the discordant/concordant LDL-C and RC and DM
Univariate analysis showed that age, BMI, educational level, smoking, alcohol consumption, CKD, the discordant/concordant LDL-C and RC, elevated WBC count and hs-CRP were all significantly associated with DM (Additional file 1: Table S1). Using the multivariate adjusted model for age, sex, BMI, educational level, smoking, alcohol consumption and CKD, we observed that participants in Group 2, Group 3 and Group 4 were associated with 304%, 61%, and 198%, respectively, increased risk for DM (OR: 4.04, 95% CI 2.93–5.56 for Group 2; OR: 1.61, 95% CI 1.21–2.15 for Group 3; and OR: 2.98, 95% CI 2.18–4.07 for Group 4) relative to Group 1 (Fig. 2). Other models in which we considered TG, anti-hypertensive drugs and LDL-C/Apo-B ratio in the analysis also revealed that Group 2 and Group 4 still related with the increased risk for DM (Additional file 1: Table S2).
Association of the discordance/concordance of LDL-C (2.60 mmol/L cutoffs) and RC (0.62 mmol/L cutoffs) with DM. a The proportion of the discordant/concordant groups in DM and non-DM population. b The odds ratios (95% CIs) of DM according to the discordance/concordance of LDL-C and RC. Model adjusted for age, sex, BMI, educational level, smoking, alcohol consumption, and chronic kidney disease. DM diabetes mellitus, LDL-C low-density lipoprotein cholesterol, RC remnant cholesterol, OR odds ratio, CI confidence interval
Subgroup and sensitivity analysis
In the subgroup analysis, the test for interactions was significant for sex (p for interactions < 0.05), while we noted no significant interactions for age (Additional file 1: Fig. S2). Sex modified the effect of the discordant/concordant LDL-C and RC on DM, with a low LDL-C/high RC ratio more likely to be related to DM in females. We evaluated the association between the discordant/concordant LDL-C and RC groups and DM using stricter clinical LDL-C cut-points (1.80 mmol/L). In this sensitivity analysis, after adjusting the variables in Model 3, we found that a low LDL-C/high RC was still the one most closely related to DM (Additional file 1: Fig. S3).
Mediator analysis
When we assessed the potential mediation effects in the correlation between RC and DM, we noted that HOMA-IR, WBCs, and hs-CRP were significant mediators of the relationship between RC and DM using the Sobel test. We observed significant indirect effects of HOMA-IR, WBCs, and hs-CRP between RC and the risk of DM with the three indices mediated at 14.13%, 2.10%, and 1.30% of the association, respectively (Fig. 3).
Discussion
We herein found that the discordant/concordant LDL-C and RC were associated with DM to varying degrees. Among them, the low LDL-C/high RC, but not high LDL-C/low RC, was highly associated with DM, suggesting the role of RC beyond LDL-C in DM among the general population. More importantly, this relationship was stronger in females. In addition, the mediation of insulin resistance and the pro-inflammatory state may be the reason why RC is more closely related to DM. Early recognition of the discordant/concordant LDL-C and RC can help distinguish high-risk patients who are predisposed to DM.
As TRLs are cholesterol-rich, RC has attracted attention for its pathogenic capability due to a recently proposed concept—that of cholesterol toxicity—and this has engendered novel perspectives on the development of DM [34]. Previous cohort studies of the general population and coronary artery disease patients suggested that RC was a predictor of new-onset hyperglycemia, superior to other traditional lipid parameters [17, 18, 35]. Another study conducted in a kidney transplant cohort also demonstrated a significant association of baseline RC with new onset diabetes after transplantation [36].
Our results confirmed the previous studies in which elevated LDL-C and RC were implicated in the development of DM. Importantly, we further clarified the role of RC beyond that of LDL-C in the contribution of cholesterol to DM, expanding our knowledge of the pathogenesis of RC. As two large primary prevention clinical studies indicated that RC, but not LDL-C, was associated with the morbidity of atherosclerotic cardiovascular disease [21, 37], the adverse effects of RC may thus be far greater than those with LDL-C. However, there are currently fewer studies that have focused on the relationship between the LDL-C and RC discordance/concordance and DM in the general population. Our result indicated that discordance in LDL-C and RC could be a surrogate for DM, which is a beneficial supplement to the development of DM. Moreover, we have also demonstrated that females are more sensitive to DM from RC exposure, which was consistent with a previous study [18]. Currently, sex differences in discordance/concordance in LDL-C and RC have not been well studied, and we consider that this difference may be related to different dietary structures and cholesterol metabolism. It’s worth noting that women require stricter RC management.
Although the mechanisms underlying the RC-induced disturbance of blood glucose metabolism are not fully understood, the damage of cholesterol overload to β-cells is well documented. Animal experiments have shown that a high-cholesterol environment inhibits the survival of pancreatic β-cells, resulting in the inhibition of insulin secretion. However, cholesterol-lowering strategies benefit pancreatic β-cell function [38,39,40,41]. Excess cholesterol is also able to induce endoplasmic reticulum stress and mitochondrial dysfunction and promote reactive oxygen species (ROS) production in pancreatic β-cells, ultimately leading to structural changes in insulin-containing granules [34]. RC is more abundant, larger, and carries more cholesterol than LDL-C particles; hence, it can be inferred that RC is more harmful to pancreatic β-cells [8]. Furthermore, unlike LDL-C, the unique pro-inflammatory characteristics of RC may significantly contribute to abnormal glucose metabolism by exacerbating insulin resistance and a systemic pro-inflammatory state [20, 42]. Apart from these effects, the particle size of TRLs has drawn great interest from researchers, and an increasing body of evidence suggests that the particle size of TRLs is the key factor that contributes to its pathogenicity [8, 43]. Specific particle properties of TRLs and LDL-C are associated with β-cell function and incident DM [8].
There is currently no clear evidence that supports a critical role for insulin resistance and pro-inflammation in the relationship between RC and DM. Ohnishi et al. and Funada et al. previously reported a correlation between RC and insulin resistance [44, 45]. Modern lipoprotein subclass studies have also depicted particle sizes and concentrations of VLDL and LDL-C (as the principal components of RC) as being associated with insulin resistance in a prediabetic population [3, 4, 46]. Insulin resistance, then, also affects RC metabolism. Insulin resistance in the liver can impair the translocation of LRP1 from intracellular vesicles to the hepatocyte plasma membrane, and this then leads to a reduced clearance of TRLs [47]. In addition, an attenuated clearance of TRLs by hepatocytes results in an accumulation of RC [48]. These results may explain the role of insulin resistance in mediating the relationship between RC and DM. In addition, numerous studies have revealed that increased inflammatory-cell infiltration of pancreatic islets in type 2 DM is generally associated with the degree of β-cell dysfunction during the development of diabetes [49]. Islet-resident macrophages and islet-cell inflammation induced by the overproduction of cytokines and ROS are components of the etiology and pathogenesis of human type 2 DM [31, 50]. In our study, we observed that elevated RC was associated with increased WBCs and hs-CRP, suggesting that high levels of RC were often accompanied by a pro-inflammatory state. The mediation analyses showed that the WBC count and CRP concentrations were mediators of the relationship between RC and DM, but that each aspect’s mediating effect was relatively weak. Therefore, we posit that the systemic pro-inflammatory response caused by elevated RC may be involved in the onset and development of DM.
The relationship between RC and DM may also be regulated by mechanisms of RC clearance. For example, peripheral lipoprotein lipase (LPL) and adiponectin are associated with the metabolism of TRLs and involved in the clearance of RC [51, 52]. Shirakawa et al. found that the residual-lipoprotein particle size (which is regulated by circulating LPL and adiponectin) was significantly larger in type 2 DM patients than that in healthy individuals [53]. This finding implicated a potential role for RC accumulation in DM due to inactive LPL and adiponectin, and this might explain the closer relationship between RC and DM from another perspective.
Limitations
We acknowledge several limitations of the present study. First, we could not draw causal conclusions regarding the pathogenesis of RC and DM due to the cross-sectional nature of the study design. Second, limited by the data provided by CHNS, information on previous statin use could not be obtained. Therefore, the effect of statin use on the relationship between the discordant/concordant LDL-C and RC and DM requires further investigation. In addition, the particle size of the TRLs were not available for more precise classification and assessment of RC. Third, although we executed our analyses via different methods to support our findings, caution must be exercised in interpreting these results as some observed associations may have been incidental. Fourth, RC includes the cholesterol component of VLDL and IDL in the fasting state. However, in the non-fasting blood sample, RC would contain the additional cholesterol component of chylomicron remnants than fasting state. The contribution of RC to DM beyond LDL-C in the non-fasting state needs to be clarified in future research. Finally, the mediation analysis assumed a certain sequence of effects, and our cross-sectional study design limited our directional determination of these effects, thus serving as a potential limitation that is encountered in cross-sectional studies in general.
Conclusions
The present study provided evidence for the association between the discordance/concordance in LDL-C and RC levels and DM in the general population, and this relationship was partially mediated by insulin resistance and the pro-inflammatory state. In addition, females are more sensitive to DM associated with elevated RC. These findings have important clinical implications for the pathogenesis underlying the RC-induced impairment of glucose metabolism.
Availability of data and materials
The data analyzed in this study can be available at: https://www.cpc.unc.edu/projects/china.
Abbreviations
- apoA:
-
Apolipoprotein A
- apoB:
-
Apolipoprotein B
- BMI:
-
Body mass index
- CHNS:
-
China health and nutrition survey
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- FBG:
-
Fasting blood glucose
- HbA1c:
-
Glycosylated hemoglobin A1c
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- HOMA-IS:
-
Homeostasis model assessment of insulin sensitivity
- Hs-CRP:
-
High-sensitivity C-reactive protein
- LDL-C:
-
Low-density lipoprotein cholesterol
- LPL:
-
Lipoprotein lipase
- OR:
-
Odds ratio
- RC:
-
Remnant cholesterol
- ROS:
-
Reactive oxygen species
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- TRLs:
-
Triglyceride-rich lipoproteins
- VLDL:
-
Very low-density lipoproteins
- IDL:
-
Intermediate-density lipoproteins
- WBC:
-
White blood cell
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Wang J, Stancakova A, Soininen P, Kangas AJ, Paananen J, Kuusisto J, Ala-Korpela M, Laakso M. Lipoprotein subclass profiles in individuals with varying degrees of glucose tolerance: a population-based study of 9399 Finnish men. J Intern Med. 2012;272(6):562–72.
Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52(2):453–62.
Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, Haffner SM. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111(25):3465–72.
Sascău R, Clement A, Radu R, Prisacariu C, Stătescu C. Triglyceride-rich lipoproteins and their remnants as silent promoters of atherosclerotic cardiovascular disease and other metabolic disorders: a review. Nutrients. 2021. https://doi.org/10.3390/nu13061774.
Boden WE, Bhatt DL, Toth PP, Ray KK, Chapman MJ, Luscher TF. Profound reductions in first and total cardiovascular events with icosapent ethyl in the REDUCE-IT trial: why these results usher in a new era in dyslipidaemia therapeutics. Eur Heart J. 2020;41(24):2304–12.
Jialal I, Singh G. Management of diabetic dyslipidemia: an update. World J Diabetes. 2019;10(5):280–90.
Sokooti S, Flores-Guerrero JL, Heerspink HJL, Connelly MA, Bakker SJL, Dullaart RPF. Triglyceride-rich lipoprotein and LDL particle subfractions and their association with incident type 2 diabetes: the PREVEND study. Cardiovasc Diabetol. 2021;20(1):156.
Rabinovich-Nikitin I, Dhingra R, Kirshenbaum LA. Activation of mitophagy in high-fat diet-induced diabetic cardiomyopathy. Circ Res. 2019;124(9):1288–90.
Gong R, Liu Y, Luo G, Liu W, Jin Z, Xu Z, Li Z, Yang L, Wei X. Associations of TG/HDL ratio with the risk of prediabetes and diabetes in Chinese adults: a Chinese population cohort study based on open data. Int J Endocrinol. 2021;2021:9949579.
Li X, Li G, Cheng T, Liu J, Song G, Ma H. Association between triglyceride-glucose index and risk of incident diabetes: a secondary analysis based on a Chinese cohort study: TyG index and incident diabetes. Lipids Health Dis. 2020;19(1):236.
Kim J, Shin SJ, Kim YS, Kang HT. Positive association between the ratio of triglycerides to high-density lipoprotein cholesterol and diabetes incidence in Korean adults. Cardiovasc Diabetol. 2021;20(1):183.
Jorgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjaerg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34(24):1826–33.
Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. 2019;40(2):537–57.
Bornfeldt KE, Linton MF, Fisher EA, Guyton JR. JCL roundtable: lipids and inflammation in atherosclerosis. J Clin Lipidol. 2021;15(1):3–17.
Varbo A, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128(12):1298–309.
Hadi Alijanvand M, Aminorroaya A, Kazemi I, Amini M, Aminorroaya Yamini S, Mansourian M. Prevalence and predictors of prediabetes and its coexistence with high blood pressure in first-degree relatives of patients with type 2 diabetes: a 9-year cohort study. J Res Med Sci. 2020;25:31.
Xie G, Zhong Y, Yang S, Zou Y. Remnant cholesterol is an independent predictor of new-onset diabetes: a single-center cohort study. Diabetes Metab Syndr Obes. 2021;14:4735–45.
Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–35.
Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118(4):547–63.
Quispe R, Martin SS, Michos ED, Lamba I, Blumenthal RS, Saeed A, Lima J, Puri R, Nomura S, Tsai M, et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur Heart J. 2021;42(42):4324–32.
Castañer O, Pintó X, Subirana I, Amor AJ, Ros E, Hernáez Á, Martínez-González MÁ, Corella D, Salas-Salvadó J, Estruch R, et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol. 2020;76(23):2712–24.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98.
Wang L, Wang H, Zhang B, Popkin BM, Du S. Elevated fat intake increases body weight and the risk of overweight and obesity among Chinese adults: 1991–2015 trends. Nutrients. 2020. https://doi.org/10.3390/nu12113272.
Hu X, Appleton AA, Ou Y, Zhang Y, Cai A, Zhou Y, Dong H. Abdominal volume index trajectories and risk of diabetes mellitus: results from the China health and nutrition survey. J Diabetes Investig. 2021. https://doi.org/10.1111/jdi.13733.
Zhang B, Zhai FY, Du SF, Popkin BM. The China health and nutrition survey, 1989–2011. Obes Rev. 2014;15(Suppl 1):2–7.
Qian S, You S, Sun Y, Wu Q, Wang X, Tang W, Dong X, Liu CF, Xu T, Cao Y, et al. Remnant cholesterol and common carotid artery intima-media thickness in patients with ischemic stroke. Circ Cardiovasc Imaging. 2021;14(4):e010953.
Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62-69.
Dematteis M, Julien C, Guillermet C, Sturm N, Lantuejoul S, Mallaret M, Levy P, Gozal E. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med. 2008;177(2):227–35.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Boni-Schnetzler M, Meier DT. Islet inflammation in type 2 diabetes. Semin Immunopathol. 2019;41(4):501–13.
Ohnishi HSS, Takagi S, Ohata J, Isobe T, Kikuchi Y, Takeuchi H, Shimamoto K. Relationship between insulin-resistance and remnant-like particle cholesterol. Atherosclerosis. 2002;164(1):167–70.
Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociolo Methodol. 1982;13:290–312.
Song Y, Liu J, Zhao K, Gao L, Zhao J. Cholesterol-induced toxicity: an integrated view of the role of cholesterol in multiple diseases. Cell Metab. 2021;33(10):1911–25.
Saely CH, Rein P, Leiherer A, Vonbank A, Zanolin D, Drexel H. Remnant cholesterol predicts the development of type 2 diabetes mellitus in patients with established coronary artery disease. J Am Coll Cardiol. 2017;69(11):1673.
Szili-Torok T, Sokooti S, Osté MCJ, Gomes-Neto AW, Dullaart RPF, Bakker SJL, Tietge UJF. Remnant lipoprotein cholesterol is associated with incident new onset diabetes after transplantation (NODAT) in renal transplant recipients: results of the TransplantLines Biobank and cohort Studies. Cardiovasc Diabetol. 2022;21(1):41.
Castañer OPX, Subirana I, Amor AJ, Ros E, Hernáez Á, Martínez-González MÁ, Corella D, Salas-Salvadó J, Estruch R, et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol. 2020;76(23):2712–24.
Chen Z, Liu S, Li C, Sun S, Liu Q, Lei L, Gao L, Shen Z. Atorvastatin helps preserve pancreatic β cell function in obese C57BL/6 J mice and the effect is related to increased pancreas proliferation and amelioration of endoplasmic-reticulum stress. Lipids Health Dis. 2014;13:98.
Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic beta-cell dysfunction. Diabetes. 2007;56(9):2328–38.
Agouridis AP, Kostapanos MS, Elisaf MS. Statins and their increased risk of inducing diabetes. Expert Opin Drug Saf. 2015;14(12):1835–44.
Lu X, Liu J, Hou F, Liu Z, Cao X, Seo H, Gao B. Cholesterol induces pancreatic beta cell apoptosis through oxidative stress pathway. Cell Stress Chaperones. 2011;16(5):539–48.
Wagner R, Jaghutriz BA, Gerst F, Barroso Oquendo M, Machann J, Schick F, Loffler MW, Nadalin S, Fend F, Konigsrainer A, et al. Pancreatic steatosis associates with impaired insulin secretion in genetically predisposed individuals. J Clin Endocrinol Metab. 2020. https://doi.org/10.1210/clinem/dgaa435.
Carvalho LSF, Benseñor IM, Nogueira ACC, Duncan BB, Schmidt MI, Blaha MJ, Toth PP, Jones SR, Santos RD, Lotufo PA, et al. Increased particle size of triacylglycerol-enriched remnant lipoproteins, but not their plasma concentration or lipid content, augments risk prediction of incident type 2 diabetes. Diabetologia. 2021;64(2):385–96.
Ohnishi H, Saitoh S, Takagi S, Ohata J-i, Isobe T, Kikuchi Y, Takeuchi H, Shimamoto K. Relationship between insulin-resistance and remnant-like particle cholesterol. Atherosclerosis. 2002;164(1):167–70.
Funada J, Sekiya M, Otani T, Watanabe K, Sato M, Akutsu H. The close relationship between postprandial remnant metabolism and insulin resistance. Atherosclerosis. 2004;172(1):151–4.
Shalaurova I, Connelly MA, Garvey WT, Otvos JD. Lipoprotein insulin resistance index: a lipoprotein particle-derived measure of insulin resistance. Metab Syndr Relat Disord. 2014;12(8):422–9.
Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46(6):733–49.
Haas ME, Attie AD, Biddinger SB. The regulation of ApoB metabolism by insulin. Trends Endocrinol Metab. 2013;24(8):391–7.
Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and beta-cell abnormalities. Nat Rev Endocrinol. 2020;16(2):81–90.
Di Marco E, Jha JC, Sharma A, Wilkinson-Berka JL, Jandeleit-Dahm KA, de Haan JB. Are reactive oxygen species still the basis for diabetic complications? Clin Sci. 2015;129(2):199–216.
Chan DC, Watts GF, Ng TW, Uchida Y, Sakai N, Yamashita S, Barrett PH. Adiponectin and other adipocytokines as predictors of markers of triglyceride-rich lipoprotein metabolism. Clin Chem. 2005;51(3):578–85.
Dallinga-Thie GM, Franssen R, Mooij HL, Visser ME, Hassing HC, Peelman F, Kastelein JJ, Peterfy M, Nieuwdorp M. The metabolism of triglyceride-rich lipoproteins revisited: new players, new insight. Atherosclerosis. 2010;211(1):1–8.
Shirakawa T, Nakajima K, Yatsuzuka S, Shimomura Y, Kobayashi J, Machida T, Sumino H, Murakami M. The role of circulating lipoprotein lipase and adiponectin on the particle size of remnant lipoproteins in patients with diabetes mellitus and metabolic syndrome. Clin Chim Acta. 2015;440:123–32.
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
Our research was supported by the National Key Research and Development Program of China (No. 2016YFC1301202), the Key Project of Natural Science Foundation of Guangdong Province (2017B030311010) and Guangzhou Municipal Science and Technology Project (202002030088).
Author information
Authors and Affiliations
Contributions
XMH and QZL drafted the manuscript and XMH prepared tables and figures. XMH, WMW, BYY and BJL collected and analyzed the data. YLZ, HJD and JJL contributed to funding acquisition. HJD and JJL supervised the study. XYG, HJD and JJL revised the manuscript. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Study protocols and ethics approval were derived from the Institutional Review Committees of the University of North Carolina at Chapel Hill and the National Institute for Nutrition and Health at Beijing and so, were attributed to the two centers. Each participant in our study was enrolled in China and assigned the informed consent.
Consent for publication
All the authors gave their consent to publication.
Competing interests
The authors declare no confict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Univariate and multivariate analysis for DM.TableS2. The correlation between discordant/concordant LDL-C and RC and DM. Figure S1. Study flowchart. Figure S2.Subgroup analyses stratified by patient characteristics. Figure S3. Associationof discordance/concordance of LDL-C (1.80 mmol/L cutoffs) and RC (0.62 mmol/Lcutoffs) with DM.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, X., Liu, Q., Guo, X. et al. The role of remnant cholesterol beyond low-density lipoprotein cholesterol in diabetes mellitus. Cardiovasc Diabetol 21, 117 (2022). https://doi.org/10.1186/s12933-022-01554-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01554-0