Abstract
Background
Empagliflozin has been reported to protect endothelial cell function, regardless of diabetes status. However, the role of empagliflozin in microvascular protection during myocardial ischemia reperfusion injury (I/R) has not been fully understood.
Methods
Electron microscopy, western blots, immunofluorescence, qPCR, mutant plasmid transfection, co-immunoprecipitation were employed to explore whether empagliflozin could alleviate microvascular damage and endothelial injury during cardiac I/R injury.
Results
In mice, empagliflozin attenuated I/R injury-induced microvascular occlusion and microthrombus formation. In human coronary artery endothelial cells, I/R injury led to adhesive factor upregulation, endothelial nitric oxide synthase inactivation, focal adhesion kinase downregulation, barrier dysfunction, cytoskeletal degradation and cellular apoptosis; however, empagliflozin treatment diminished these effects. Empagliflozin improved mitochondrial oxidative stress, mitochondrial respiration and adenosine triphosphate metabolism in I/R-treated human coronary artery endothelial cells by preventing the phosphorylation of dynamin-related protein 1 (Drp1) and mitochondrial fission 1 protein (Fis1), thus repressing mitochondrial fission. The protective effects of empagliflozin on mitochondrial homeostasis and endothelial function were abrogated by the re-introduction of phosphorylated Fis1, but not phosphorylated Drp1, suggesting that Fis1 dephosphorylation is the predominant mechanism whereby empagliflozin inhibits mitochondrial fission during I/R injury. Besides, I/R injury induced Fis1 phosphorylation primarily by activating the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) pathway, while empagliflozin inactivated this pathway by exerting anti-oxidative effects.
Conclusions
These results demonstrated that empagliflozin can protect the microvasculature by inhibiting the DNA-PKcs/Fis1/mitochondrial fission pathway during myocardial I/R injury.
Similar content being viewed by others
Background
Revascularization of occluded coronary arteries is a standard therapeutic approach for patients with myocardial infarction. However, revascularization also induces ischemia/reperfusion (I/R) injury in heart tissues, including cardiomyocytes and endothelial cells [1, 2]. I/R injury-induced endothelial dysfunction is associated with microvascular injury, which is characterized by perfusion defects, microvascular spasms, microthrombus formation and increased vascular permeability [3,4,5]. Cardiac microvascular I/R injury expands the infarction size and thus increases the peri-operative mortality of patients receiving revascularization therapy [6, 7]. Thus, despite the great success of therapies to reduce post-ischemic cardiomyocyte injury, additional molecular studies and therapeutic drugs are needed to address cardiac microvascular dysfunction during I/R injury.
Empagliflozin is an inhibitor of sodium/glucose cotransporter 2. Based on the findings of the EMPA-REG OUTCOME study, empagliflozin was originally used to treat type-2 diabetes in clinical practice [8]. However, based on the promising results of the EMPEROR-Reduced trial [9] and the EMPEROR-Preserved trial [10], the US Food and Drug Administration has also approved empagliflozin for the broader treatment of heart failure, regardless of diabetes status. Intensive research has been conducted to explain the cardioprotective potential of empagliflozin, and the anti-inflammatory [11], anti-oxidative [12], anti-apoptotic [13] and metabolic [14] effects of empagliflozin have been widely reported. These molecular mechanisms, in addition to hypoglycemic effects, may be the means by which empagliflozin enhances cardiomyocyte structure/function and thus suppresses the major adverse cardiovascular event rate in patients with heart failure [15, 16]. More importantly, several studies [17,18,19] have revealed that empagliflozin can protect endothelial cell function, regardless of diabetes status. The beneficial impact of empagliflozin on cardiac microvascular function has also been confirmed using non-invasive Doppler ultrasound imaging to assess the coronary flow velocity reserve [20]. Despite these exciting findings, the effects of empagliflozin on cardiac microvascular I/R injury are not fully understood.
Many studies have identified mitochondrial damage as a core pathogenic contributor to I/R injury-induced cardiac microvascular dysfunction [21,22,23]. Mitochondria-derived superoxides induce the oxidation of tetrahydrobiopterin to dihydrobiopterin, which prevents tetrahydrobiopterin from binding to endothelial nitric oxide synthase (eNOS), thus uncoupling eNOS and reducing nitric oxide production [24]. Moreover, when mitochondrial pro-apoptotic proteins such as cytochrome c and Smac infiltrate the cytosol, they activate caspase family members to promote apoptosis [25]. Mitochondria are also involved in endothelial mobilization, senescence, growth and proliferation [26,27,28].
It is now recognized that mitochondrial structural disorder precedes mitochondrial dysfunction [29, 30]. Increased mitochondrial fission reduces the mitochondrial membrane potential (MMP), augments mitochondrial reactive oxygen species (mtROS) production and activates mitochondria-dependent apoptotic pathways during cardiac microvascular I/R injury [30, 31]. Interestingly, our previous research and other studies have demonstrated that empagliflozin inhibits mitochondrial fission by suppressing the phosphorylation of dynamin-related protein 1 (Drp1) [32,33,34]. However, endothelial mitochondrial fission is induced by the phosphorylation of not only Drp1, but also mitochondrial fission 1 protein (Fis1) [35] and/or mitochondrial fission factor (Mff) [36]. It is poorly understood whether empagliflozin alters Fis1 or Mff phosphorylation in cardiac microvascular I/R injury.
Recently, we reported that Fis1 phosphorylation is an essential step in mitochondrial fission[37]. Oxidative stress promotes DNA damage [38, 39] and thus activates the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), which binds to and phosphorylates Fis1. Phosphorylated Fis1 has an increased affinity for cytosolic Drp1, regardless of whether Drp1 is phosphorylated [35]. In light of the anti-oxidative activity of empagliflozin, we hypothesized that empagliflozin may prevent DNA-PKcs-induced Fis1 phosphorylation in endothelial cells, thus inhibiting mitochondrial fission, improving endothelial function and alleviating microvascular I/R injury.
Materials and methods
Murine myocardial I/R injury
All experimental procedures that involved animals were approved by the University of Wyoming College of Health Sciences and Chinese PLA General Hospital, performed in accordance with the Animal Use Guidelines of Chinese PLA General Hospital, and consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No 85-23). 12-week-old C57BL/6J male mice were anesthetized with inhaled isoflurane (1.5–2%), intubated and ventilated with a small animal respirator (Harvard Apparatus). Then, a left thoracotomy was performed to expose the heart, and the left anterior descending coronary artery was ligated with an 8.0 surgical silk suture for 45 min to induce ischemia, followed by 2 h of reperfusion, as we previously reported [40]. Empagliflozin (10 mg/kg/d) was administered for seven days before myocardial I/R injury, in accordance with a previous study [32].
Electron microscopy
Heart tissues (infarcted zone) were immerged in a fixative (2.5% glutaraldehyde) and stored overnight at 4 °C. The samples were then processed and analyzed as we previously described [41].
Immunohistochemistry
Heart tissues (infarcted zone) were embedded in paraffin and dewaxed, as for hematoxylin and eosin staining. The sections were placed in a 3% H2O2 methanol solution at room temperature for 20 min to inactivate endogenous hydrogen peroxide. For the unmasking of antigens, the sections were microwaved for 15 min in 10 mmol/L citrate buffer, pH 6.0. Then, immunostaining was performed using the avidin: biotinylated enzyme complex method with an antibody against ICAM1 (1:500, Abcam, #179707). After being washed, the slices were incubated with an appropriate biotin-conjugated secondary antibody for one hour at room temperature, and then the color was developed using 3,3’-diaminobenzidine tetrahydrochloride as a chromogen. The sections were counterstained with Gill-2 hematoxylin (Thermo-Shandon, Pittsburgh, PA, USA). After being stained, the sections were dehydrated in increasing concentrations of ethanol and xylene. Immunohistochemical staining images were analyzed using Aperio ImageScope v12.32.8013 (Leica Biosystems).
HCAEC culture and sI/R injury in vitro
HCAECs (American Type Culture Collection, PCS-100-020™) were cultured with vascular basal cell medium containing 5 ng/mL vascular endothelial growth factor, 5 ng/mL epidermal growth factor, 5 ng/mL fibroblastic growth factor, 15 ng/mL insulin-like growth factor 1, 10 mM L-glutamine, 0.75 U/mL heparin sulfate, 1 µg/mL hydrocortisone, 50 µg/mL ascorbic acid, 1% amphotericin B, 1% penicillin-streptomycin and 10% fetal bovine serum. Hypoxia/reoxygenation was used to induce sI/R injury in vitro. In brief, the medium was changed to an ischemia-mimicking solution containing 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10 mM 2-deoxy-D-glucose, 139 mM NaCl, 12 mM KCl, 0.5 mM MgCl2, 1.3 mM CaCl2 and 20 mM lactic acid, pH 6.2, and then the cells were incubated under 100% nitrogen (O2 < 1%) at 37 °C for 45 min. The cultures were then returned to normal culture conditions (10% fetal bovine serum, 37 °C ambient air, 5% CO2) for 2 h. Empagliflozin (10 µM) was added to the HCAECs 12 h before the sI/R injury. For the induction of oxidative stress, HCAECs were treated with 0.3 mM hydrogen peroxide for six hours. NAC (10 mM) was added to the medium of HCAECs to reduce oxidative stress-induced DNA-PKcs activation in the presence of sI/R, based on a previous study [42].
RNA extraction and quantitative polymerase chain reaction (PCR)
Total mRNA was isolated using a Total RNA Kit (Omega) and reverse transcribed using a Reverse Transcription Kit (Vazyme) according to the manufacturers’ instructions. Then, mRNA levels were determined using quantitative PCR with SYBR Green (Vazyme). The amount of each cDNA relative to the endogenous control (β-actin) was calculated using the 2−ΔΔCt method. The following primer pairs were used: TNFα (Forward, 5’-AGATGGAGCAACCTAAGGTC-3’; Reverse, 5’-GCAGACCTCGCTGTTCTAGC-3’), IL-6 (Forward, 5’-CAGACTCGCGCCTCTAAGGAGT-3’; Reverse, 5’-GATAGCCGATCCGTCGAA-3’), MCP1 (Forward, 5’-GGATGGATTGCACAGCCATT-3’; Reverse, 5’-GCGCCGACTCAGAGGTGT-3’).
Western blot analysis
Proteins from lysed cells were fractionated via sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Nonspecific binding sites were blocked with 5% bovine serum albumin in Tris-buffered saline with Tween 20 (TBST: 120 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.05% Tween 20) for two hours at room temperature. The blots were incubated with specific primary antibodies (each diluted 1:1000) overnight at 4 °C. β-actin was used as a loading control. The membranes were then washed three times with TBST and incubated with horseradish peroxidase-conjugated secondary antibodies. Proteins were visualized using the Immobilon™ Western Chemiluminescent HRP substrate (Millipore, USA). The following antibodies were obtained from Abcam: ET-1 (#ab178454), eNOS (#ab199956), p-eNOS (#ab215717), Fak (#ab40794), Src (#ab133283), Drp1 (#ab184247), Fis1 (#ab156865), GAPDH (#ab8245), Tom20 (#ab186735), DNA-PKcs (#ab32566), F-actin (#ab130935) and Met (#ab51067). The following antibodies were obtained from Cell Signaling Technology: p-Drp1 (#4494), Mff (#86,668) and p-Mff (#49,281).
Confocal analysis of the mitochondrial membrane potential (MMP), mitochondrial morphology and mPTP opening
The MMP was measured using an MMP assay kit (Solarbio, Beijing, China). Cells were stained with the unique fluorescence probe JC-1 at 37 °C for 20 min, and then were washed twice with phosphate-buffered saline. The fluorescence intensity of the cells was observed using a flow cytometer (BD FACSCalibur, NJ, USA) and a confocal laser scanning microscope (Olympus, Japan). Then, the average fluorescence intensity of green monomers and red aggregates was determined, and the ratio was calculated.
MitoTracker™ was used to detect changes in mitochondrial dynamics. For mitochondrial labeling, the cell samples were incubated with a 100 nM solution of MitoTracker™ Green FM (Thermo Fisher Scientific, MA, USA) for 30 min at 37 °C, 5% CO2. Images were obtained using a Nikon confocal microscope system and camera (Nikon Instruments, NY, USA). The fluorescent dye and length of mitochondria were measured using Image J software. We selected 10 random fluorescence fields from each group.
To track mPTP opening, we treated HCAECs with tetramethylrhodamine ethyl ester, based on our previous study. The fluorescent signal of tetramethylrhodamine ethyl ester was determined using a Nikon confocal microscope system and camera.
Plasmids, lentiviruses, siRNA and reagents
Eukaryotic expression vectors encoding Drp1, Fis1, DNA-PKcs and Met were generated through the insertion of PCR-amplified fragments into a pcDNA3 vector (Invitrogen, CA, USA). Serine 616 was replaced with aspartic acid to establish the Drp1 phosphorylation-mimetic mutant (Drp1S616D). Threonine 34 was replaced with aspartic acid to construct the Fis1 phosphorylation-mimetic mutant (Fis1T34D). For lentiviral production, HEK293T cells were co-transfected with recombinant lentiviral vectors and a pPACK Packaging Plasmid Mix (System Biosciences) using MegaTran reagent (OriGene, Beijing, China). The target cells were infected with the lentiviruses according to the manufacturer’s instructions. siRNA against DNA-PKcs was established and transfected into cells according to our recent reports [37, 43].
Immunoprecipitation assays
HCAECs were transfected with the indicated plasmids. After 24 h, the cells were lysed with ice-cold immunoprecipitation buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1% Triton X-100 and 0.5% sodium deoxycholate) containing protease inhibitor cocktail tablets (04693132001, Roche), and the samples were centrifuged at 13,000xg for 15 min. Then, the cell lysates were incubated with Protein G-agarose beads (AA104307, Bestchrom) and the indicated antibodies overnight at 4 °C. After being washed with immunoprecipitation buffer, the immunocomplexes were collected and immunoblotted using the indicated primary antibodies and corresponding secondary antibodies. Clean-Blot IP Detection Reagent (21,230, Thermo Fisher Scientific) was used to prevent interference from denatured immunoprecipitation antibody fragments.
Immunofluorescence staining, TUNEL assay and mitochondrial ROS detection
Samples were fixed with 4% paraformaldehyde and permeabilized with 0.1% saponin. The cells were then incubated with primary antibodies and the corresponding secondary antibodies. Images were acquired using a confocal microscope (LCS-SP8-STED, Leica). The TUNEL assay (Abcam, #ab66108) was conducted based on the manufacturer’s instructions. MtROS levels in HCAECs were measured using a Mitochondrial ROS Detection Assay Kit (Cayman, Cat#701,600) according to the manufacturer’s recommendations.
ELISA
The activity levels of DNA-PKcs (DNA-PK Kinase Enzyme System, Cat. No. #V4106, Promega Corporation, USA), Met (MET Kinase Enzyme System, Cat. No. #V3361, Promega Corporation), caspase-3 (Human Cleaved Caspase-3 ELISA Kit, Abcam, #220,655), mitochondrial respiratory complex I (Complex I Enzyme Activity Microplate Assay Kit, Abcam, #ab109721) and mitochondrial respiratory complex II (Complex II Enzyme Activity Microplate Assay Kit, Abcam, #ab109908) were determined according to the manufacturers’ protocols for the respective kits. Intracellular ATP production was measured with an ATP Assay Kit (Abcam, #83,355) based on the manufacturer’s protocol. Serum Biochemical Analysis. Serum TnI (Elabscience, E-EL-M0086c), LDH activity (Lactate dehydrogenase (LDH) assay kit, Nanjing, jiancheng, China), and serum creatine kinase-MB (CK-MB) (creatine kinase MB isoenzyme Assay Kit, Nanjing jiancheng, China) were measured with an ATP Assay Kit (Abcam, #83,355) based on the manufacturer’s protocol.
Statistical analyses
All data in this study were used to determine the means and standard deviations. Student’s t test was used to assess the differences between two groups. One-way analysis of variance was conducted to evaluate differences among three or more groups. Bonferroni analysis and Tamhane’s T2 analysis were performed as appropriate. P values < 0.05 were considered statistically significant.
Results
Empagliflozin reduces I/R injury-induced cardiac microvascular damage
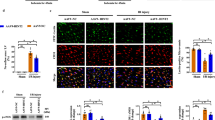
To assess the effects of empagliflozin on cardiac microvascular damage during I/R injury, we treated mice with or without empagliflozin (10 mg/kg/d) for seven days before subjecting them to myocardial I/R injury or a sham operation. Then, we used electron microscopy to observe the microvascular structure. In mice exposed to I/R injury, the microvessel walls became thickened and narrowed; however, empagliflozin treatment reversed these alterations (Fig. 1A).
Empagliflozin reduces I/R injury-induced cardiac microvascular damage. Mice were subjected to 45 min of ischemia followed by two hours of reperfusion to induce cardiac I/R injury. Empagliflozin (EMPA, 10 mg/kg/d) was administered for seven days before myocardial I/R injury. A Electron microscopy was used to detect structural alterations in cardiac microvessels. Yellow arrows indicate the thickened wall and narrowed lumen. B, C. Immunofluorescence was used to detect fibrin accumulation in microvessels. D, E Proteins were isolated from reperfused heart and the expression of fibrin was determined by western blots. F, G Heart samples were collected after I/R injury for immunohistochemical analysis of ICAM1. H –J Total mRNA was isolated from reperfused hearts, and the mRNA levels of IL-6, MCP-1 and TNFα were determined using quantitative PCR. Data are shown as mean ± SEM, n = 6 mice per group. *p < 0.05
To determine whether the occluded microcirculation had induced microthrombus formation, we performed immunofluorescence analyses of fibrin in the cardiac microvessels. As expected, fibrin accumulation was greater in reperfused hearts than in sham-operated hearts (Fig. 1B, C). However, empagliflozin prevented the deposition of fibrin in the microcirculation, suggesting that this drug has anti-thrombotic effects (Fig. 1B, C). To validate these findings, we performed Western blotting, which confirmed that I/R injury promoted fibrin accumulation in heart tissues, whereas empagliflozin reversed this alteration (Fig. 1D, E).
In addition to luminal stenosis, the expression of adhesive factors such as intercellular adhesion molecule 1 (ICAM1) can increase the likelihood of microthrombus formation. As shown in Fig. 1F, G, I/R injury increased ICAM1 expression on the surface of microvessels, whereas empagliflozin supplementation reduced the abundance of ICAM1 in the microcirculation. Upregulated adhesive factors and increased microvessel permeability can stimulate the inflammatory response during I/R injury. Transcriptional analyses demonstrated that I/R injury augmented interleukin 6 (IL-6), tumor necrosis factor alpha (TNFα) and monocyte chemoattractant protein 1 (MCP1) mRNA levels, while empagliflozin treatment reduced the transcription of these inflammatory factors in heart tissues (Fig. 1H–J). Besides, the levels of cardiac injury markers (such as TnI, CK-MB and LDH) were also normalized by EMPA in the presence of I/R injury (Table 1). These results demonstrated that empagliflozin attenuates I/R injury-induced cardiac microvascular dysfunction.
Empagliflozin sustains cardiac microvascular endothelial function after simulated I/R injury
Next, we evaluated the influence of empagliflozin on human coronary artery endothelial cells (HCAECs) following simulated I/R (sI/R) injury. Considering that the balance between endothelin 1 (ET-1) and eNOS fine-tunes endothelium-dependent vascular relaxation, we used Western blotting to examine the expression of these proteins in HCAECs. The results demonstrated that sI/R upregulated ET-1 expression and inhibited eNOS activity, whereas empagliflozin normalized the balance between these two proteins in sI/R-treated HCAECs (Fig. 2A–C). Src and focal adhesion kinase (Fak), the regulators of endothelial integrity and barrier function, were also downregulated in HCAECs upon sI/R injury, but were expressed at near-normal levels following empagliflozin treatment (Fig. 2A–E).
Empagliflozin sustains cardiac microvascular endothelial function after I/R injury. HCAECs were subjected to 45 min of hypoxia followed by two hours of reoxygenation to induce sI/R injury in vitro. The cells were incubated with empagliflozin (EMPA, 10 µM) for 12 h before sI/R injury. A–E Western blotting was used to assess the protein levels of ET-1, p-eNOS, Fak and Src in HCAECs following sI/R injury or empagliflozin treatment. F, G FITC-dextran clearance and TER assays were performed to determine the alterations of endothelial barrier function and integrity. H, I An immunofluorescence assay was used to observe cytoskeletal changes in HCAECs following sI/R injury. J–L Western blotting was used to determine F-actin protein levels in HCAECs following sI/R injury or empagliflozin treatment. M An ELISA was used to determine the activity of caspase-3, a marker of cell apoptosis. N TUNEL assay was used to observe the number of apoptotic endothelial cells in the presence of sI/R. Data are shown as mean ± SEM, n = ten independent cell isolations per group. *p < 0.05
To further assess the effects of empagliflozin on barrier function and integrity, we performed fluorescein isothiocyanate (FITC)-dextran clearance and transendothelial electrical resistance (TER) assays. The remaining FITC-dextran content in HCAECs was significantly elevated following sI/R injury, indicating that endothelial hyperpermeability accelerated FITC-dextran accumulation (Fig. 2F–G). In addition, the TER value was reduced in sI/R-treated HCAECs, demonstrating that endothelial gap junctions were weakened. Empagliflozin administration not only reduced FITC-dextran deposition, but also enhanced the TER value (Fig. 2F–G), suggesting that empagliflozin can protect endothelial barrier function and integrity.
Previous research has indicated that cytoskeletal degradation, resulting from F-actin depolymerization into G-actin, is an early sign of endothelial dysfunction because it impairs cellular mobilization and stimulates apoptosis [44]. In HCAECs exposed to sI/R injury, the F-actin cytoskeleton became disorganized (Fig. 2H, I) and F-actin expression was reduced (Fig. 2J–L). On the other hand, a well-arranged F-actin cytoskeleton (Fig. 2H, I) and increased F-actin levels (Fig. 2J–L) were detected in empagliflozin-treated sI/R-injured HCAECs. The activity of the apoptosis marker caspase-3 increased upon sI/R injury, but decreased following empagliflozin treatment (Fig. 2M). Consistent with this observation, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analyses revealed that the number of apoptotic HCAECs increased upon sI/R stress and decreased in the presence of empagliflozin (Fig. 2N, O). These results demonstrated that empagliflozin sustains HCAEC function during sI/R injury.
Empagliflozin restores mitochondrial homeostasis in endothelial cells
Mitochondrial damage has been associated with endothelial dysfunction during sI/R injury [Figs. 3, 4]. Thus, we explored the effects of empagliflozin on endothelial mitochondrial function. We found that sI/R injury triggered mtROS production, whereas empagliflozin treatment neutralized excessive mtROS in HCAECs (Fig. 3A, B). Oxidative stress may damage the mitochondrial genome. As shown in Fig. 3 C, D, sI/R injury reduced the mitochondrial DNA copy number and transcription in HCAECs, while empagliflozin treatment attenuated these effects. Since the mitochondrial respiratory complexes are partly encoded by mitochondrial DNA, we measured mitochondrial respiratory complex I and II activity levels. The activities of these two complexes in HCAECs were reduced upon sI/R injury, but were sustained following empagliflozin supplementation (Fig. 3E, F).
Empagliflozin restores mitochondrial homeostasis in endothelial cells. HCAECs were subjected to 45 min of hypoxia followed by two hours of reoxygenation to induce sI/R injury in vitro. The cells were incubated with empagliflozin (EMPA, 10 µM) for 12 h before sI/R injury. A, B. The mtROS levels in HCAECs were measured using a Mitochondrial ROS Detection Assay Kit. C, D qPCR was used to analyze the alterations of mitochondrial copy and transcription. E, F ELISAs were used to detect alterations in mitochondrial respiratory complexes I and II. G, H The MMP was measured using an MMP assay kit with the JC-10 fluorescence probe. I ATP production was measured with an ELISA. J The mPTP opening rate was determined using a tetramethylrhodamine ethyl ester fluorescence assay. Data are shown as mean ± SEM, n = ten independent cell isolations per group. *p < 0.05
The mitochondrial membrane potential (MMP) is primarily determined by electron transmission through the mitochondrial respiratory complexes. We found that sI/R injury depolarized the MMP, whereas empagliflozin stabilized the MMP in sI/R-treated HCAECs (Fig. 3G, H). Since the MMP drives adenosine triphosphate (ATP) synthesis, we also assessed ATP production. ATP levels were reduced in sI/R-treated HCAECs; however, empagliflozin supplementation maintained the normal energy output following sI/R injury (Fig. 3I). Moreover, ATP reduction is also a result of mitochondrial membrane hyperpermeability. We found that sI/R promoted, whereas empagliflozin inhibited, the opening of mitochondrial permeability transition pore (mPTP) (Fig. 3J) in HCAECs.These results revealed that empagliflozin preserves mitochondrial function in HCAECs under sI/R stress.
Empagliflozin suppresses mitochondrial fission by inhibiting Fis1 phosphorylation
Our previous studies identified mitochondrial fission as an initial signal of mitochondrial dysfunction [45,46,47]. Thus, we wondered whether empagliflozin could maintain mitochondrial homeostasis by inhibiting mitochondrial fission in HCAECs. Immunofluorescence analyses of mitochondrial structure revealed that HCAECs exposed to sI/R injury contained fragmented and round mitochondria (Fig. 4A–C). Empagliflozin treatment elongated the mitochondria and maintained the highly-connected mitochondrial networks in HCAECs (Fig. 4A–C). At the molecular level, mitochondrial fission is primarily induced by Drp1 and its receptors, including Mff and Fis1 [48]. Western blotting demonstrated that sI/R injury induced Drp1 phosphorylation and Fis1 phosphorylation, but had no influence on Mff phosphorylation (Fig. 4D–G). Empagliflozin treatment inhibited the phosphorylation of not only Drp1, but also Fis1 (Fig. 4D–G).
Empagliflozin suppresses mitochondrial fission by inhibiting Fis1 phosphorylation. HCAECs were subjected to 45 min of hypoxia followed by two hours of reoxygenation to induce sI/R injury in vitro. The cells were incubated with empagliflozin (EMPA, 10 µM) for 12 h before sI/R injury. A–C MitoTracker™ was used to detect changes in mitochondrial dynamics. The number cardiomyocyte with fragmented mitochondria as well as the average length of mitochondria were recorded. At least 100 mitochondria from 10 HCAECs were used to evaluate the number of HCAECs with fragmented mitochondria. D–G Western blotting was used to determine p-Drp1, p-Fis1 and p-Mff protein levels in HCAECs following sI/R injury or empagliflozin treatment. H–J Western blotting was used to assess cytoplasmic and mitochondrial Drp1 protein levels. HCAECs were transfected with a Drp1 phosphorylation-mimetic mutant (Drp1S616D) or a Fis1 phosphorylation-mimetic mutant (Fis1T34D) in the presence of empagliflozin. K–L The binding between Drp1 and Fis1 was determined using a co-immunoprecipitation assay in the presence of empagliflozin. HCAECs were transfected with Drp1S616D or Fis1T34D. M–O Immunofluorescence staining was used to observe the mitochondrial morphology in HCAECs transfected with Drp1S616D or Fis1T34D. Data are shown as mean ± SEM, n = ten independent cell isolations per group. *p < 0.05
The phosphorylation of Drp1 accelerates its translocation from the cytoplasm to the surface of the mitochondria [49]. On the other hand, the phosphorylation of Fis1 increases its affinity for cytoplasmic Drp1 [35]. To determine whether empagliflozin repressed mitochondrial fission through one or both of these mechanisms, we used a phosphorylation-mimetic Drp1 mutant (Drp1S616D, in which serine 616 was replaced with aspartic acid to mimic p-Drp1Ser616) and a phosphorylation-mimetic Fis1 mutant (Fis1T34D, in which threonine 34 was replaced with aspartic acid to mimic p-Fis1Thr34). We transfected HCAECs with these mutant constructs in the presence of empagliflozin, and then evaluated Drp1 translocation (from the cytoplasm to mitochondria) and mitochondrial fission following sI/R injury.
Of note, Fis1T34D transfection enhanced mitochondrial Drp1 (mito-Drp1) expression in HCAECs, despite the treatment of these cells with empagliflozin; however, Drp1S616D transfection did not have this effect (Fig. 4H–J). Furthermore, a co-immunoprecipitation assay revealed that empagliflozin impaired the binding between Drp1 and Fis1 in sI/R-injured HCAECs, and this effect was abolished by Fis1T34D rather than by Drp1S616D transfection (Fig. 4K–L). A morphological immunofluorescence assay of mitochondria indicated that empagliflozin mostly repressed mitochondrial fission in sI/R-treated HCAECs. Transfection of HCAECs with Fis1T34D prevented empagliflozin from inhibiting mitochondrial fission; however, transfection with Drp1S616D did not have this effect (Fig. 4M–O). These findings suggested that empagliflozin suppresses mitochondrial fission under sI/R injury primarily by promoting Fis1 dephosphorylation, although empagliflozin can also induce Drp1 dephosphorylation.
Fis1T34D mutant transfection prevents empagliflozin from protecting HCAEC mitochondria
To confirm that Fis1 dephosphorylation was the main mechanism whereby empagliflozin protected HCAECs and their mitochondria, we either transfected empagliflozin-treated HCAECs with the Fis1T34D mutant, or transfected sI/R-injured HCAECs with a Fis1 phosphorylation-defective mutant (Fis1T34A, in which threonine 34 was replaced with alanine, as the negative control group). Then, we evaluated mitochondrial homeostasis and endothelial function. As shown in Fig. 5A, B, empagliflozin administration or Fis1T34A transfection had similar effects in sustaining the MMP of sI/R-injured HCAECs. However, empagliflozin failed to maintain the MMP of sI/R-injured HCAECs transfected with Fis1T34D (Fig. 5A, B). Empagliflozin supplementation also inhibited mtROS generation (Fig. 5C, D) and enhanced ATP production (Fig. 5E) in sI/R-injured HCAECs, while Fis1T34D transfection abrogated these effects. Moreover, empagliflozin suppressed the mitochondrial permeability transition pore (mPTP) opening rate after sI/R injury in normal HCAECs, but not in HCAECs transfected with Fis1T34D (Fig. 5F).
Fis1 mutant transfection prevents empagliflozin from protecting HCAEC mitochondria. HCAECs were subjected to 45 min of hypoxia followed by two hours of reoxygenation to induce sI/R injury in vitro. The cells were incubated with empagliflozin (EMPA, 10 µM) for 12 h before sI/R injury. HCAECs were transfected with a Fis1 phosphorylation-mimetic mutant (Fis1T34D) to activate mitochondrial fission in empagliflozin-treated sI/R-injured HCAECs, or were transfected with a Fis1 phosphorylation-defective mutant (Fis1T34A) to inhibit mitochondrial fission in sI/R-treated HCAECs. A,B The MMP was measured using an MMP assay kit with the JC-10 fluorescence probe. C,D The mtROS levels in HCAECs were measured using a Mitochondrial ROS Detection Assay Kit. E ATP production was measured with an ELISA. F. The mPTP opening rate was determined with a tetramethylrhodamine ethyl ester fluorescence assay. G–I Western blotting was used to assess ET-1 and p-eNOS protein levels in HCAECs following sI/R injury or empagliflozin treatment. J,K FITC-dextran clearance and TER assays were performed to determine the alterations of endothelial barrier function and integrity. Data are shown as mean ± SEM, n = ten independent cell isolations per group. *p < 0.05
Western blotting demonstrated that empagliflozin treatment downregulated ET-1 but upregulated eNOS activity in sI/R-injured HCAECs; however, these alterations were undetectable in cells transfected with Fis1T34D (Fig. 5G–I). Additionally, FITC-dextran clearance and TER assays confirmed that empagliflozin protected endothelial barrier function and integrity in HCAECs subjected to sI/R injury, whereas Fis1T34D transfection nullified these beneficial actions (Fig. 5J and K). These data suggested that empagliflozin maintains mitochondrial homeostasis and endothelial function by suppressing Fis1 phosphorylation.
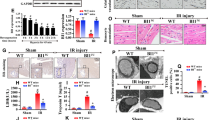
Empagliflozin inhibits oxidative stress-induced DNA-PKcs activation
Previous studies have identified Met tyrosine kinase [35] and DNA-PKcs[37] as potential upstream triggers of Fis1 phosphorylation; thus, we focused on these two pathways to understand how empagliflozin inhibits Fis1 phosphorylation. Enzyme-linked immunosorbent assays (ELISAs) demonstrated that DNA-PKcs activity was significantly elevated in HCAECs subjected to sI/R injury, while Met activity was not altered (Fig. 6A, B). Additionally, Western blotting illustrated that sI/R injury induced the phosphorylation of DNA-PKcs, but did not alter the expression of Met in HCAECs (Fig. 6C–E). As expected, empagliflozin treatment had no effect on Met activity (Fig. 6 A-B) or expression (Fig. 6C–E), but obviously inhibited DNA-PKcs activity (Fig. 6A, B) and phosphorylation (Fig. 6C–E) in sI/R-treated HCAECs.
Empagliflozin inhibits oxidative stress-induced DNA-PKcs activation. HCAECs were subjected to 45 min of hypoxia followed by two hours of reoxygenation to induce sI/R injury in vitro. The cells were incubated with empagliflozin (EMPA, 10 µM) for 12 h before sI/R injury. A, B ELISAs were used to analyze the kinase activities of DNA-PKcs and Met in HCAECs following sI/R injury or empagliflozin treatment. C–G Western blotting was used to determine DNA-PKcs, Met and p-Fis1 protein levels in HCAECs following sI/R injury or empagliflozin treatment. siRNAs were transfected into HCAECs to knock down DNA-PKcs oe Met. H–J MitoTracker™ was used to detect changes in mitochondrial dynamics. The number cardiomyocyte with fragmented mitochondria as well as the average length of mitochondria were recorded. K–M To induce oxidative stress, HCAECs were treated with 0.3 mM hydrogen peroxide for six hours to activate DNA-PKcs. NAC (10 mM) was added to the medium in the presence of hydrogen peroxide to reduce oxidative stress-induced DNA-PKcs activation. Then, DNA-PKcs activity was determined with an ELISA. Data are shown as mean ± SEM, n = 10 mice per group or ten independent cell isolations per group. *p < 0.05
We then performed loss-of-function assays by using lentiviruses to knock down DNA-PKcs or Met in HCAECs. Knocking down DNA-PKcs suppressed Fis1 phosphorylation in HCAECs subjected to sI/R injury, while knocking down Met did not have this effect (Fig. 6F, G). Similarly, sI/R injury-induced mitochondrial fission could be attenuated by empagliflozin treatment or DNA-PKcs knockdown, but not by Met knockdown in HCAECs (Fig. 6H–J). These data confirmed that DNA-PKcs phosphorylates Fis1 during I/R injury.
Several factors are thought to activate DNA-PKcs, including oxidative stress and DNA damage [50]. Considering that empagliflozin has anti-oxidative properties, we assessed whether empagliflozin might inhibit DNA-PKcs by neutralizing oxidative stress. We added supraphysiological hydrogen peroxide concentrations to the media of empagliflozin-treated sI/R-injured HCAECs. As a negative control group, we treated sI/R-injured HCAECs with the anti-oxidant N-acetylcysteine (NAC). As shown in Fig. 6K, either empagliflozin or NAC treatment could attenuate sI/R injury-induced DNA-PKcs activation; however, exogenous hydrogen peroxide abrogated the ability of empagliflozin to inactivate DNA-PKcs. Likewise, either empagliflozin or NAC supplementation could attenuate sI/R injury-induced mitochondrial fission (Fig. 6J) and Fis1 phosphorylation in HCAECs, but exogenous hydrogen peroxide nullified this effect of empagliflozin (Fig. 6L and M). These results indicated that empagliflozin prevents DNA-PKcs activation by reducing oxidative stress in HCAECs during sI/R injury.
Discusssion
In the present study, we found that empagliflozin protected against cardiac microvascular I/R injury by reducing mitochondrial fission and inactivating the DNA-PKcs/Fis1 pathway. In vivo, empagliflozin administration improved the microvascular structure, reduced luminal stenosis, prevented microthrombus formation and attenuated endothelial hyperpermeability-related inflammatory responses following I/R injury. In vitro, empagliflozin enhanced eNOS activity, improved barrier function, maintained the endothelial cytoskeletal integrity and reduced apoptosis in HCAECs subjected to sI/R injury. Mitochondrial dysfunction was found to induce cardiac microvascular endothelial I/R injury. Specifically, an overload of mtROS damaged mitochondrial DNA, thus suppressing the transcription of mitochondria-encoded respiratory complexes and reducing the MMP in HCAECs. However, empagliflozin treatment preserved mitochondrial homeostasis by repressing Fis1-induced mitochondrial fission in these cells. Although empagliflozin also inhibited Drp1 phosphorylation, we found that Fis1 dephosphorylation was the predominant mechanism whereby empagliflozin prevented sI/R injury-induced mitochondrial fission in HCAECs. Indeed, the re-introduction of phosphorylated Fis1 abrogated the protective effects of empagliflozin on mitochondrial homeostasis and endothelial function. Fis1 was primarily phosphorylated by DNA-PKcs in sI/R-injured HCAECs, while empagliflozin could inactivate DNA-PKcs through its anti-oxidative effects. This is the first study to illustrate the mechanism whereby empagliflozin ameliorates cardiac microvascular I/R injury, and suggests that DNA-PKcs/Fis1/mitochondrial fission could be potential targets for the treatment of cardiac microvascular I/R injury.
Empagliflozin has exhibited multiple protective functions in cardiovascular disease studies [51,52,53]. In myocardia from patients with heart failure with preserved ejection fraction (HFpEF), empagliflozin treatment suppressed the production of inflammatory factors (e.g., ICAM1, vascular cell adhesion molecule 1, TNFα and IL-6) and reversed changes in redox parameters (e.g., H2O2, 3-nitrotyrosine, glutathione and lipid peroxides) [11]. Through its anti-oxidative effects, empagliflozin attenuated oxidized protein kinase GIα levels, improved myofilament function, reduced cardiomyocyte stiffness and increased the diastolic capacity of the heart [11]. In a murine model of myocardial I/R injury [54], empagliflozin reduced the myocardial infarct size and improved cardiac contractility by stimulating the 5’ adenosine monophosphate-activated protein kinase pathway, independent of its hypoglycemic effects. Empagliflozin was also found to attenuate adverse post-infarcted left ventricular remodeling by elevating the mitophagic flux and enhancing mitochondrial biogenesis [55]. In a mouse model of ventricular fibrillation-induced cardiac arrest [56], empagliflozin treatment improved left ventricular function and increased the survival time, partly by increasing myocardial ketone levels and β-hydroxy butyrate dehydrogenase 1 protein expression. Chronic hyperlipidemia was found to augment the cardiomyocyte area and left ventricular thickness, while empagliflozin alleviated this pathological phenotype by inhibiting the myocardial renin angiotensin system, endoplasmic reticular stress and abnormal oxidative reactions [55].
Though numerous studies have examined the effects of empagliflozin on cardiomyocytes, relatively few studies have explored the effects of this drug on endothelial homeostasis and microvascular function, especially under non-diabetic conditions. In heart tissues from HFpEF patients, oxidated eNOS expression was induced, soluble guanylyl cyclase activity was diminished and cyclic guanosine monophosphate levels were reduced relative to control subjects; however, these alterations were undetectable in HFpEF patients receiving empagliflozin [11]. In TNFα-induced endothelial inflammatory injury, empagliflozin inhibited the Na+/H+ exchanger and reduced cytoplasmic Na+ levels, thus suppressing ROS generation [17]. In addition to exerting anti-oxidative effects, empagliflozin was found to activate protein kinase G and voltage-dependent K+ channels to dilate the rabbit aorta [57]. Empagliflozin has also been shown to ameliorate other forms of endothelial dysfunction, such as endothelial senescence [58], cellular apoptosis [59], endothelium-leukocyte interactions [60] and endothelial barrier dysfunction [52]. Despite these promising findings, the effects of empagliflozin on cardiac microvascular I/R injury have not been fully understood. Our results suggested that empagliflozin may be an effective tool to attenuate endothelial dysfunction and microvascular injury. Similar to the previous findings, our study demonstrated that empagliflozin can inhibit oxidative stress, inflammation, apoptosis and thrombosis, suggesting that this drug sustains endothelial homeostasis and microvascular integrity through multiple actions.
Mitochondrial damage has been reported as a core pathogenic contributor to endothelial dysfunction under I/R injury [21,22,23]. Mitochondrial fission is regarded as an early change in mitochondrial morphology that precedes mitochondrial dysfunction. Reperfusion injury was shown to promote Drp1 phosphorylation at Ser616 and rapidly increase the number of fragmented mitochondria, correlating with elevated mtROS production, a reduced MMP and activated mitochondrial apoptosis [41, 61]. On the other hand, inhibiting mitochondrial fission by depleting Drp1 in endothelial cells markedly reduced mtROS generation, restored mitochondrial energy metabolism and promoted cell survival following I/R injury [31]. Moreover, suppressing mitochondrial fission by activating mitophagy or mitochondrial fusion in the endothelium restored eNOS activity [62], improved vascular permeability[63] and normalized endothelium-dependent vascular relaxation [64]. Due to its effects on mitochondrial homeostasis, mitochondrial fission seems to be a good target for the prevention of microvascular I/R injury. Unfortunately, only a few drugs have been reported to inhibit mitochondrial fission and thus protect against microvascular I/R injury – for instance, melatonin [46], angiotensin-converting enzyme inhibitors [65], glucagon-like peptide-1 receptor agonists [66] and green tea (Camellia sinensis) [67]. In the present study, we identified empagliflozin as a novel suppressor of mitochondrial fission during I/R-induced endothelial dysfunction. Thus, empagliflozin may be able to attenuate perioperative microvascular injury in patients receiving revascularization treatment.
Empagliflozin has already been widely reported to inhibit mitochondrial fission in various cell types, including adipocytes [68], renal tubular cells [69] and endothelial cells[32]. It is generally accepted that empagliflozin suppresses mitochondrial fission by reducing Drp1 phosphorylation [33, 34]. In the present study, we also found that empagliflozin prevented Drp1 phosphorylation in HCAECs subjected to sI/R injury. After translocating from the cytoplasm to the surface of mitochondria, Drp1 consumes ATP and divides mitochondria into several fragments [49]. Although phosphorylation increases the mobility of Drp1 [70], the binding of cytoplasmic Drp1 to mitochondria requires several receptors, including Mff and Fis1 [71, 72]. The phosphorylation of Fis1 [35] and Mff [36] increases their affinity for cytoplasmic Drp1, and thus is important for inducing mitochondrial fission, regardless of whether Drp1 itself is phosphorylated. In the present study, we found that the phosphorylation of Fis1, but not Mff, was induced by sI/R injury and inhibited by empagliflozin in HCAECs. Transfection of a Fis1 phosphorylation-mimetic mutant, rather than a Drp1 phosphorylation-mimetic mutant, prevented empagliflozin from inhibiting mitochondrial fission in sI/R-injured HCAECs. These data suggested that Fis1 dephosphorylation, rather than Drp1 dephosphorylation, is the primary mechanism whereby empagliflozin inhibits mitochondrial fission in the endothelium following I/R injury. Lastly, we demonstrated that empagliflozin blocked Fis1 phosphorylation by preventing DNA-PKcs activation in sI/R-injured HCAECs; however, exogenous hydrogen peroxide treatment offset this effect. Therefore, through its anti-oxidative properties, empagliflozin prevented DNA-PKcs activation and hindered its kinase activity, ultimately reducing Fis1 phosphorylation.
This study had several limitations. First, it is difficult to evaluate microvascular function in vivo, so we only observed structural changes in microvessels following empagliflozin treatment. Second, we were only able to perform molecular investigations in vitro due to the fatality of Fis1 or DNA-PKcs transgenic mice.
Conclusions
Our results demonstrated that empagliflozin can protect against cardiac microvascular I/R injury. Empagliflozin administration prevented oxidative stress-induced DNA-PKcs activation, thus suppressing Fis1 phosphorylation and mitochondrial fission in sI/R-injured HCAECs. By inhibiting mitochondrial fission, empagliflozin sustained mitochondrial homeostasis, thereby improving endothelial function and protecting the microcirculation during cardiac I/R injury. Based on our results, empagliflozin should be considered as a potential microvascular protective drug that inhibits the DNA-PKcs/Fis1/mitochondrial fission pathway.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Davidson SM, Ferdinandy P, Andreadou I, Bøtker HE, Heusch G, Ibáñez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, et al. Multitarget strategies to reduce myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2019;73(1):89–99.
Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106(3):360–8.
Chang X, Lochner A, Wang HH, Wang S, Zhu H, Ren J, Zhou H. Coronary microvascular injury in myocardial infarction: perception and knowledge for mitochondrial quality control. Theranostics. 2021;11(14):6766–85.
Wang J, Toan S, Zhou H. New insights into the role of mitochondria in cardiac microvascular ischemia/reperfusion injury. Angiogenesis. 2020;23(3):299–314.
Wang J, Toan S, Zhou H. Mitochondrial quality control in cardiac microvascular ischemia-reperfusion injury: new insights into the mechanisms and therapeutic potentials. Pharmacol Res. 2020;156: 104771.
Forman MB, Puett DW, Virmani R. Endothelial and myocardial injury during ischemia and reperfusion: pathogenesis and therapeutic implications. J Am Coll Cardiol. 1989;13(2):450–9.
Kang S, Yang Y. Coronary microvascular reperfusion injury and no-reflow in acute myocardial infarction. Clin Invest Med. 2007;30(3):E133-145.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Anker SD, Butler J, Filippatos G, Khan MS, Marx N, Lam CSP, Schnaidt S, Ofstad AP, Brueckmann M, Jamal W, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-Reduced trial. Circulation. 2021;143(4):337–49.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61.
Kolijn D, Pabel S, Tian Y, Lódi M, Herwig M, Carrizzo A, Zhazykbayeva S, Kovács Á, Fülöp G, Falcão-Pires I, et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc Res. 2021;117(2):495–507.
Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T, et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18(1):15.
Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, Kyrou I, Mantzoros CS, Kyriakopoulos G, Chatzigeorgiou A, Kalotychou V, Randeva MS, Chatha K, et al. Empagliflozin attenuates non-alcoholic fatty liver disease (NAFLD) in High Fat Diet Fed ApoE((-/-)) mice by activating autophagy and reducing ER stress and apoptosis. Int J Mol Sci. 2021;22(2):818.
Tsapas A, Avgerinos I, Karagiannis T, Malandris K, Manolopoulos A, Andreadis P, Liakos A, Matthews DR, Bekiari E. Comparative effectiveness of glucose-lowering drugs for Type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020;173(4):278–86.
Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Sattar N, Brueckmann M, Jamal W, Cotton D, et al. Empagliflozin in patients with heart failure, reduced ejection fraction, and volume overload: EMPEROR-reduced trial. J Am Coll Cardiol. 2021;77(11):1381–92.
Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, et al. Effect of empagliflozin on left ventricular volumes in patients with Type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. 2021;143(6):516–25.
Uthman L, Li X, Baartscheer A, Schumacher CA, Baumgart P, Hermanides J, Preckel B, Hollmann MW, Coronel R, Zuurbier CJ, et al. Empagliflozin reduces oxidative stress through inhibition of the novel inflammation/NHE/[Na(+)](c)/ROS-pathway in human endothelial cells. Biomed Pharmacother. 2022;146: 112515.
Hasan A, Hasan R. Empagliflozin relaxes resistance mesenteric arteries by stimulating multiple smooth muscle cell voltage-gated K(+) (K(V)) channels. Int J Mol Sci. 2021;22(19):10842.
Park SH, Belcastro E, Hasan H, Matsushita K, Marchandot B, Abbas M, Toti F, Auger C, Jesel L, Ohlmann P, et al. Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: protective effect of gliflozins. Cardiovasc Diabetol. 2021;20(1):65.
Adingupu DD, Göpel SO, Grönros J, Behrendt M, Sotak M, Miliotis T, Dahlqvist U, Gan LM, Jönsson-Rylander AC. SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob(-/-) mice. Cardiovasc Diabetol. 2019;18(1):16.
Daiber A, Andreadou I, Oelze M, Davidson SM, Hausenloy DJ. Discovery of new therapeutic redox targets for cardioprotection against ischemia/reperfusion injury and heart failure. Free Radical Biol Med. 2021;163:325–43.
Jiang X, Wu D, Jiang Z, Ling W, Qian G. Protective effect of nicorandil on cardiac microvascular injury: role of mitochondrial integrity. Oxid Med Cell Longev. 2021;2021:4665632.
Zhong J, Ouyang H, Sun M, Lu J, Zhong Y, Tan Y, Hu Y. Tanshinone IIA attenuates cardiac microvascular ischemia-reperfusion injury via regulating the SIRT1-PGC1α-mitochondrial apoptosis pathway. Cell Stress Chaperones. 2019;24(5):991–1003.
Kar S, Kavdia M. Modeling of biopterin-dependent pathways of eNOS for nitric oxide and superoxide production. Free Radical Biol Med. 2011;51(7):1411–27.
Zhang F, Zhang L, Sun LL, Meng XL, Zhao Y, Jin X. Effects of fluid shear stress on expression of Smac/DIABLO in human umbilical vein endothelial cells. Curr Ther Res Clin Exp. 2013;74:36–40.
Kluge MA, Fetterman JL, Vita JA. Mitochondria and endothelial function. Circ Res. 2013;112(8):1171–88.
Kirkman DL, Robinson AT, Rossman MJ, Seals DR, Edwards DG. Mitochondrial contributions to vascular endothelial dysfunction, arterial stiffness, and cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2021;320(5):H2080–100.
Shao Y, Li X, Wood JW, Ma JX. Mitochondrial dysfunctions, endothelial progenitor cells and diabetic retinopathy. J Diabetes Complications. 2018;32(10):966–73.
Groschner LN, Waldeck-Weiermair M, Malli R, Graier WF. Endothelial mitochondria–less respiration, more integration. Pflugers Arch. 2012;464(1):63–76.
Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu S, Ren J, Chen Y. NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res Cardiol. 2018;113(4):23.
Chen Y, Liu C, Zhou P, Li J, Zhao X, Wang Y, Chen R, Song L, Zhao H, Yan H. Coronary endothelium no-reflow injury is associated with ROS-modified mitochondrial fission through the JNK-Drp1 signaling pathway. Oxid Med Cell Longev. 2021;2021:6699516.
Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335–46.
Lee WC, Chau YY, Ng HY, Chen CH, Wang PW, Liou CW, Lin TK, Chen JB. Empagliflozin protects HK-2 cells from high glucose-mediated injuries via a mitochondrial mechanism. Cells. 2019;8(9):1085.
Mizuno M, Kuno A, Yano T, Miki T, Oshima H, Sato T, Nakata K, Kimura Y, Tanno M, Miura T. Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol Rep. 2018;6(12): e13741.
Yu Y, Peng XD, Qian XJ, Zhang KM, Huang X, Chen YH, Li YT, Feng GK, Zhang HL, Xu XL, et al. Fis1 phosphorylation by Met promotes mitochondrial fission and hepatocellular carcinoma metastasis. Signal Transduct Target Ther. 2021;6(1):401.
Pangou E, Bielska O, Guerber L, Schmucker S, Agote-Arán A, Ye T, Liao Y, Puig-Gamez M, Grandgirard E, Kleiss C, et al. A PKD-MFF signaling axis couples mitochondrial fission to mitotic progression. Cell Rep. 2021;35(7): 109129.
Wang S, Zhu H, Li R, Mui D, Toan S, Chang X, Zhou H. DNA-PKcs interacts with and phosphorylates Fis1 to induce mitochondrial fragmentation in tubular cells during acute kidney injury. Sci Signal. 2022;15(725):eabh1121.
Chen BP, Li M, Asaithamby A. New insights into the roles of ATM and DNA-PKcs in the cellular response to oxidative stress. Cancer Lett. 2012;327(1–2):103–10.
Das U, Manna K, Khan A, Sinha M, Biswas S, Sengupta A, Chakraborty A, Dey S. Ferulic acid (FA) abrogates γ-radiation induced oxidative stress and DNA damage by up-regulating nuclear translocation of Nrf2 and activation of NHEJ pathway. Free Radic Res. 2017;51(1):47–63.
Tan Y, Mui D, Toan S, Zhu P, Li R, Zhou H. SERCA overexpression improves mitochondrial quality control and attenuates cardiac microvascular ischemia-reperfusion injury. Mol Ther Nucleic Acids. 2020;22:696–707.
Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, Ma S, Zhu H, Ren J, Zhou H. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol. 2018;14:576–87.
Zhou H, Toan S, Zhu P, Wang J, Ren J, Zhang Y. DNA-PKcs promotes cardiac ischemia reperfusion injury through mitigating BI-1-governed mitochondrial homeostasis. Basic Res Cardiol. 2020;115(2):11.
Zhou H, Zhu P, Wang J, Toan S, Ren J. DNA-PKcs promotes alcohol-related liver disease by activating Drp1-related mitochondrial fission and repressing FUNDC1-required mitophagy. Signal Transduct Target Ther. 2019;4(1):56.
Gourlay CW, Ayscough KR. The actin cytoskeleton: a key regulator of apoptosis and ageing? Nat Rev Mol Cell Biol. 2005;6(7):583–9.
Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu P, Ma Q, Tian F, Chen Y. Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc. 2017;6(3):005328.
Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q, Jin Q, Cao F, Tian F, Chen Y. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res. 2017;63(1):e12413.
Zhou H, Shi C, Hu S, Zhu H, Ren J, Chen Y. BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis. 2018;21(3):599–615.
Kalia R, Wang RY, Yusuf A, Thomas PV, Agard DA, Shaw JM, Frost A. Structural basis of mitochondrial receptor binding and constriction by DRP1. Nature. 2018;558(7710):401–5.
Kraus F, Roy K, Pucadyil TJ, Ryan MT. Function and regulation of the divisome for mitochondrial fission. Nature. 2021;590(7844):57–66.
Mohiuddin IS, Kang MH. DNA-PK as an emerging therapeutic target in cancer. Front Oncol. 2019;9:635.
Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose Cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136(17):1643–58.
Cooper S, Teoh H, Campeau MA, Verma S, Leask RL. Empagliflozin restores the integrity of the endothelial glycocalyx in vitro. Mol Cell Biochem. 2019;459(1–2):121–30.
Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18(1):165.
Lu Q, Liu J, Li X, Sun X, Zhang J, Ren D, Tong N, Li J. Empagliflozin attenuates ischemia and reperfusion injury through LKB1/AMPK signaling pathway. Mol Cell Endocrinol. 2020;501: 110642.
Cotrin JC, de Souza GSM, Petito-da-Silva TI, Cardoso LEM, Souza-Mello V, Barbosa-da-Silva S. Empagliflozin alleviates left ventricle hypertrophy in high-fat-fed mice by modulating renin angiotensin pathway. J Renin Angiotensin Aldosterone Syst. 2022;2022:8861911.
Tan Y, Yu K, Liang L, Liu Y, Song F, Ge Q, Fang X, Yu T, Huang Z, Jiang L, et al. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function after cardiac arrest in rats by enhancing mitochondrial energy metabolism. Front Pharmacol. 2021;12: 758080.
Seo MS, Jung HS, An JR, Kang M, Heo R, Li H, Han ET, Yang SR, Cho EH, Bae YM, et al. Empagliflozin dilates the rabbit aorta by activating PKG and voltage-dependent K(+) channels. Toxicol Appl Pharmacol. 2020;403: 115153.
Khemais-Benkhiat S, Belcastro E, Idris-Khodja N, Park SH, Amoura L, Abbas M, Auger C, Kessler L, Mayoux E, Toti F, et al. Angiotensin II-induced redox-sensitive SGLT1 and 2 expression promotes high glucose-induced endothelial cell senescence. J Cell Mol Med. 2020;24(3):2109–22.
Nakao M, Shimizu I, Katsuumi G, Yoshida Y, Suda M, Hayashi Y, Ikegami R, Hsiao YT, Okuda S, Soga T, et al. Empagliflozin maintains capillarization and improves cardiac function in a murine model of left ventricular pressure overload. Sci Rep. 2021;11(1):18384.
Canet F, Iannantuoni F, Marañon AM, Díaz-Pozo P, López-Domènech S, Vezza T, Navarro B, Solá E, Falcón R, Bañuls C, et al. Does empagliflozin modulate leukocyte-endothelium interactions, oxidative stress, and inflammation in Type 2 diabetes? Antioxidants (Basel, Switzerland). 2021;10(8):1228.
Huang M, Wei R, Wang Y, Su T, Li P, Chen X. The uremic toxin hippurate promotes endothelial dysfunction via the activation of Drp1-mediated mitochondrial fission. Redox Biol. 2018;16:303–13.
Totzeck M, Hendgen-Cotta UB, Rassaf T. Nitrite-Nitric oxide signaling and cardioprotection. Adv Exp Med Biol. 2017;982:335–46.
Herr DJ, Baarine M, Aune SE, Li X, Ball LE, Lemasters JJ, Beeson CC, Chou JC, Menick DR. HDAC1 localizes to the mitochondria of cardiac myocytes and contributes to early cardiac reperfusion injury. J Mol Cell Cardiol. 2018;114:309–19.
Li S, Chen J, Liu M, Chen Y, Wu Y, Li Q, Ma T, Gao J, Xia Y, Fan M, et al. Protective effect of HINT2 on mitochondrial function via repressing MCU complex activation attenuates cardiac microvascular ischemia-reperfusion injury. Basic Res Cardiol. 2021;116(1):65.
Wang G, Zhang Q, Yuan W, Wu J, Li C. Sildenafil protects against myocardial ischemia-reperfusion injury following cardiac arrest in a porcine model: possible role of the renin-angiotensin system. Int J Mol Sci. 2015;16(11):27015–31.
Zhai R, Xu H, Hu F, Wu J, Kong X, Sun X. Exendin-4, a GLP-1 receptor agonist regulates retinal capillary tone and restores microvascular patency after ischaemia-reperfusion injury. Br J Pharmacol. 2020;177(15):3389–402.
Din USU, Sian TS, Deane CS, Smith K, Gates A, Lund JN, Williams JP, Rueda R, Pereira SL, Atherton PJ, et al. Green tea extract concurrent with an oral nutritional supplement acutely enhances muscle microvascular blood flow without altering leg glucose uptake in healthy older adults. Nutrients. 2021;13(11):3895.
Xu L, Xu C, Liu X, Li X, Li T, Yu X, Xue M, Yang J, Kosmas CE, Moris D, et al. Empagliflozin induces white adipocyte browning and modulates mitochondrial dynamics in KK Cg-Ay/J mice and mouse adipocytes. Front Physiol. 2021;12: 745058.
Liu X, Xu C, Xu L, Li X, Sun H, Xue M, Li T, Yu X, Sun B, Chen L. Empagliflozin improves diabetic renal tubular injury by alleviating mitochondrial fission via AMPK/SP1/PGAM5 pathway. Metabolism. 2020;111: 154334.
Hu C, Huang Y, Li L. Drp1-dependent mitochondrial fission plays critical roles in physiological and pathological progresses in mammals. Int J Mol Sci. 2017;18(1):144.
Yu R, Lendahl U, Nistér M, Zhao J. Regulation of mammalian mitochondrial dynamics: opportunities and challenges. Front Endocrinol (Lausanne). 2020;11:374.
El-Hattab AW, Suleiman J, Almannai M, Scaglia F. Mitochondrial dynamics: Biological roles, molecular machinery, and related diseases. Mol Genet Metab. 2018;125(4):315–21.
Acknowledgements
None.
Funding
This study is supported by the National Natural Science Foundation of China (NO. 82102262 and NO. 82170241) and Guangdong Basic and Applied Basic Research Foundation (NO. 2021A1515010977 and NO. 2022A1515012528).
Author information
Authors and Affiliations
Contributions
WTS, RJZ and JXQ contributed to the study designation. NZ and ND performed experiments and data analysis. HZ and XXC contributed to manuscript writing and editing. LM conducted a critical revision of the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Animal experiments were performed according to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH Publication 8th edition, 2011). The experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee of Chinese PLA General Hospital (Beijing, China, approval number: 2022033SPLA-31).
Consent for publication
None.
Competing interests
The authors state that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zou, R., Shi, W., Qiu, J. et al. Empagliflozin attenuates cardiac microvascular ischemia/reperfusion injury through improving mitochondrial homeostasis. Cardiovasc Diabetol 21, 106 (2022). https://doi.org/10.1186/s12933-022-01532-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01532-6