Abstract
Background
The identification of circulating biomarkers associated with the risk of type 2 diabetes (T2D) is useful for improving the current prevention strategies in the most at-risk patients. Here, we aimed to investigate the association of plasma apolipoprotein concentrations in prediabetes subjects with the incidence of new-onset T2D during follow-up.
Methods
In the IT-DIAB prospective study, 307 participants with impaired fasting glucose levels (fasting plasma glucose [FPG]: 110–125 mg/dL) were followed yearly for 5 years. The onset of T2D was defined as a first FPG value ≥ 126 mg/dL during follow-up. Apolipoprotein (apo)A-I, A-II, A-IV, B100, C-I, C-II, C-III, C-IV, D, E, F, H, J, L1, M, and (a) plasma concentrations were determined by mass spectrometry. Correlations between apolipoproteins and metabolic parameters at baseline were assessed by Spearman’s coefficients. Kaplan–Meier curves were drawn using a ternary approach based on terciles and incident T2D. The association between plasma apolipoproteins concentrations and the incidence of T2D was determined using Cox proportional-hazards models.
Results
During a median follow-up of 5-year, 115 participants (37.5%) developed T2D. After adjustment for age, sex, body mass index, FPG, HbA1c, and statin use, the plasma levels of apoC-I, apoC-II, apoC-III, apoE, apoF, apoH, apoJ, and apoL1 were positively associated with a high risk for T2D. After further adjustment for plasma triglycerides, only apoE (1 SD natural-log-transformed hazard ratio: 1.28 [95% confidence interval: 1.06; 1.54]; p = 0.010), apoF (1.22 [1.01; 1.48]; p = 0.037), apoJ (1.24 [1.03; 1.49]; p = 0.024), and apoL1 (1.26 [1.05; 1.52]; p = 0.014) remained significantly associated with the onset of T2D. Kaplan–Meier survival curves also showed that the lower third of plasma apoE levels (< 5.97 mg/dL) was significantly associated with a lower risk of conversion to T2D (log-rank test, p = 0.002) compared to the middle and upper thirds.
Conclusions
The plasma apoE levels are positively associated with the risk of T2D in prediabetes subjects, independently of traditional risk factors. The possible associations of apoF, apoJ, and apoL1 with T2D risk also pave the way for further investigations.
Trial registration This trial was registered at clinicaltrials.gov as NCT01218061 and NCT01432509
Similar content being viewed by others
Background
Currently, 463 million people are living with diabetes worldwide [1], and recent predictions estimate that the number of affected people may increase to 578 million by the year 2030 [2]. Type 2 diabetes (T2D) is the predominant form of the disease and results from complex interactions between modifiable and unmodifiable risk factors. T2D is characterized by chronic hyperglycemia leading to serious tissue damage that reduces both the quality of life and life expectancy of affected individuals, especially by promoting cardiovascular and renal complications [3, 4]. Thus, T2D is considered as a major health burden worldwide and one of the most important modifiable cardiovascular risk factors [5]. Hence, the identification of early biomarkers associated with T2D risk is required for improving the current prevention strategies and for gaining a better understanding of the disease [6].
Abnormalities in lipid and lipoprotein metabolism have been identified as risk factors for T2D. Low concentrations of high-density lipoprotein cholesterol (HDL-C) and high concentrations of plasma triglycerides (TG) are associated with an increased risk of T2D, and they are usually preceded by the gradual onset of insulin resistance [7, 8]. Clinical trials also have reported that the statin-induced reduction of low-density lipoprotein cholesterol (LDL-C) slightly increases the risk of new-onset T2D [9,10,11]. Moreover, Mendelian randomization studies have shown that loss-of-function variants in the 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) gene, the molecular target of statins, and in the proprotein convertase subtilisin/kexin type 9 (PCSK9) gene, a natural inhibitor of the LDL receptor, are associated with an increased risk of T2D [12]. In contrast, T2D prevalence is halved in patients with familial hypercholesterolemia, especially in carriers of the most severe LDL receptor mutations [13], suggesting a direct association between the prevalence of T2D and the upregulation of the LDL receptor pathway.
The metabolism of lipids and lipoproteins is dependent on apolipoproteins, a class of multifunctional proteins that govern the assembly of lipoprotein particles, maintain their structure, and direct their metabolism through binding to cell-surface receptors and regulating enzyme activity [14]. Several studies have demonstrated that the plasma levels of apolipoproteins better predict T2D than traditional plasma lipids, independently of classical risk factors [15,16,17]. Large cohort studies drawn from the general population have shown that the apolipoprotein (apo)B100-to-LDL-C, apoA-I-to-HDL-C, and apoA-II-to-HDL-C ratios are better predictors of worsening glycemia and incident T2D than LDL-C and HDL-C levels, respectively [15, 16]. More recently, another prospective population-based cohort study has revealed that the serum levels of apoC-III and the apoC-III-to-apoA-I ratio are strongly associated with incident T2D [7].
However, such studies only focused on major apolipoprotein species. Meanwhile, several reports have pointed out that other apolipoproteins (e.g., apoC-I, apoC-II, apoD, apoF, apoH, apoJ, apoL1, and apoM), whose metabolic functions are less understood, deserve further investigation [14, 18]. In this ancillary analysis from the prospective IT-DIAB study, we investigated the association between plasma concentrations of 16 apolipoproteins determined by a validated mass-spectrometry multiplex assay [19] with the risk of new-onset T2D in individuals with prediabetes.
Methods
Study population
The IT-DIAB study (Innovation Thérapeutique-Diabète NCT01218061 & NCT01432509) is a 5-year prospective, observational study designed to identify new biomarkers of T2D risk in a population with prediabetes. Details on the study design, recruitment, and procedures have been reported elsewhere [20, 21]. The institutional ethics committee approved the protocol, and all of the reported investigations were carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008. All subjects underwent a baseline visit between June 2010 and February 2013, including a medical interview, signing of the informed consent, self-administered questionnaire of diabetes risk score, physical examination (including body weight, height, waist, and hip circumference measurements), and blood sampling. Patients without a history of diabetes and with an impaired fasting glucose (IFG) level, defined as a fasting plasma glucose (FPG) level of 110–125 mg/dL (according to the World Health Organization classification), were eligible for the IT-DIAB study. The main exclusion criteria were a history of treatment with antidiabetic agents or insulin (with the exception of gestational diabetes), severe coagulation disorder or thrombocytopenia (platelet level < 100,000/mm3), severe renal insufficiency (defined using the Modification of Diet in Renal Disease equation as an estimated glomerular filtration rate < 30 mL/min/1.73 m2), severe liver impairment (prothrombin ratio < 50%), severe psychiatric disorder, alcohol abuse (estimated to be > 30 g/day), subject’s opposition, and an inability to participate for at least 5 years in the study. For the present analysis, 307 subjects with at least one follow-up visit were considered (Fig. 1).
Follow-up and conversion to new-onset diabetes
The end of the follow-up period occurred at the fifth yearly visit, or prematurely if the subject met one of the following criteria: subject withdrawal or lost to follow-up, inappropriate prescription of an antidiabetic agent, bariatric surgery, or death. New-onset diabetes was defined as a FPG value ≥ 126 mg/dL and/or a plasma glucose concentration ≥ 200 mg/dL after a 2-h 75-g oral glucose tolerance test.
Biochemical analysis
During the baseline visit, peripheral venous blood samples were obtained in the morning after an overnight fast for biological analyses. Standard biological analyses included FPG, glycated hemoglobin (HbA1c), and the lipid profile (total cholesterol (TC), HDL-C, and TG). LDL-C was calculated using the Friedewald equation, and non-HDL-C was calculated as TC minus HDL-C. Frozen heparinized plasma was used for the insulin measurement by an electro-chemiluminescent enzyme immunoassay (ECLIA) using the Cobas e-automated clinical analyzer system (Roche Diagnostics, Meylan, France). Plasma high-molecular-weight adiponectin levels were measured by ECLIA on the Lumipulse G600 automated clinical analyzer system (Fujirebio, Les Ulis, France). Homeostasis model assessments of insulin resistance (HOMA-IR) and beta-cell function (HOMA-β) were defined according to the equations proposed by Matthews et al. [22].
Apolipoprotein measurements
Plasma apolipoproteins A-I, A-II, A-IV, B100, C-I, C-II, C-III, C-IV, D, E, F, H, J, L1, M, and (a) as well as apoE phenotyping were determined by liquid chromatography-tandem mass spectrometry, as described previously [19]. Briefly, the apolipoproteins were quantified in 40-µL aliquots (EDTA plasma) using trypsin proteolysis and the subsequent analysis of proteotypic peptides. The apoE phenotypes (E2/E3/E4) were determined using a combination of five proteolytic peptides, as described previously [19, 23]. The intra- and inter-assay variabilities did not exceed 9.4%.
Statistical analysis
All categorical parameters were expressed as the number (%). All quantitative parameters were expressed as the mean ± standard deviation (SD) or, when the distribution was considered as skewed, median [25th percentile; 75th percentile]. The correlation between the baseline characteristics was studied as a cross-sectional study. Spearman’s rank correlation coefficients were calculated between plasma apolipoprotein concentrations and the clinical characteristics, glucose homeostasis, and lipid profile. The associations between the plasma lipids, plasma apolipoproteins, and final diabetic status (no diabetes vs. new-onset diabetes) were first analyzed using the univariate logistic regression model. Kaplan–Meier curves were drawn using a ternary approach based on terciles and the incidence of new-onset diabetes as the event of interest. Then, we used univariate and multiple Cox regression models with the following adjustments: no adjustment (model 1); adjusted for baseline values of age, sex, body mass index (BMI), FPG and HbA1c (model 2); model 2 and the use of statins or fibrates (model 3); and model 3 and TG (model 4). In the Cox analyses, we favored a systematic approach using log-natural transformation and standardization for all quantitative variables. The only exception was apoC-IV, which was used as a binary datum (detectable vs. not detectable). The proportional hazards assumption was tested based on Schoenfeld residuals. The first-order risk was set to 5%. The analyses were conducted on complete cases, without imputation. No correction was considered for multiple testing. All analyses were performed using R software, version 4.0.0 [24].
Results
Baseline characteristics of the study participants
The baseline characteristics of the study population are reported in Table 1. On average, the study population was middle aged (age: 57.3 ± 9.9 years old) and overweight (BMI: 29.8 ± 6.2 kg/m2). The median FPG was 115 [112; 119] mg/dL. Regarding lipid-lowering treatment, 83 participants (27%) declared to be on routine lipid lowering drug therapy (mostly under statins: 84%). The plasma HDL-C (53 ± 15 mg/dL), LDL-C (137 ± 36 mg/dL), and TG (119 [86; 167] mg/dL) levels were in the normal range (Table 2). During the 5-year follow-up (IQR 25–62 months, 262/307 patients (85.3%) until the 5th visit), 115 participants (37.5%) developed new-onset T2D.
Baseline concentrations of plasma lipids and apolipoproteins
The baseline concentrations of plasma lipids and apolipoproteins, according to the diabetes status (diabetes vs. no diabetes) at the end of the individual follow-ups, are reported in Table 2. The plasma TG concentrations were higher in the participants who developed T2D (146 ± 73 vs. 129 ± 76 mg/dL; odds ratios (OR) [95% confidence interval (CI)] 1.33 [1.05–1.69], p = 0.020), whereas the HDL-C levels were significantly lower (50 ± 13 vs. 55 ± 16 mg/dL; OR = 0.79 [0.62–1.00], p = 0.046) compared to those who did not develop T2D. No significant difference was found for baseline TC, LDL-C, or non-HDL-C concentrations according to the final diabetes status. In addition, univariate analyses showed no statistically significant difference in the baseline levels of apoA-I, apoA-II, apoA-IV, apoB100, apoC-I, apoC-IV, apoD, apoF, apoH, apoJ, apoM, or apo(a) between the participants who developed new-onset T2D during follow-up and the others. In contrast, the baseline concentrations of some apolipoproteins were significantly higher in subjects with new-onset T2D vs. the others: apoC-II (2.84 [2.08; 3.62] vs. 2.46 [1.88; 3.48] mg/dL, OR = 1.35 [1.06–1.70], p = 0.013); apoC-III (6.73 [5.23; 9.22] vs. 6.04 [4.88; 7.51] mg/dL, OR = 1.27 [1.01–1.61], p = 0.043), apoE (7.61 [6.11; 9.14] vs. 6.45 [5.44; 8.35] mg/dL, OR = 1.31 [1.04–1.66], p = 0.023), and apoL1 (1.20 [0.95; 1.52] vs. 1.09 [0.91; 1.28] mg/dL, OR = 1.43 [1.13–1.81], p = 0.0032). Interestingly, additional univariate analyses carried out after the exclusion of subjects on statin therapy at baseline (n = 237 subjects remaining) showed similar results (Additional file 1: Table S1).
Associations between plasma apolipoprotein levels and metabolic parameters
Spearman’s rank correlation coefficients were calculated between the plasma apolipoproteins and the metabolic parameters at baseline for participants without lipid-lowering therapy (Fig. 2, Additional file 1: Table S2). As anticipated, the plasma apolipoprotein concentrations were correlated with the plasma lipid levels. Plasma apoA-I, apoA-II, apoA-IV, apoD, and apoM were positively correlated with HDL-C (Spearman’s coefficient [R] = 0.16–0.60, p < 0.05), whereas plasma apoC-I, apoC-II, apoC-III, apoE, and apoL1 were positively correlated with plasma TG (R = 0.22–0.53, p < 0.05), and plasma apoB100 was correlated with LDL-C (R = 0.38, p < 0.0001). In addition, the HDL-related apolipoproteins (apoA-I, apoA-II, apoA-IV, apoD, and apoM) were negatively correlated with the BMI (R = − 0.17 to − 0.42, p < 0.05). None of the plasma apolipoproteins were associated with HbA1c, and only plasma apoB100 was negatively correlated with FPG (R = − 0.15, p = 0.026). The HDL-related apolipoproteins (apoA-I, apoA-IV, and apoD) were negatively correlated (R = − 0.14 to − 0.34, p < 0.05), while the TG-rich lipoprotein-related apolipoproteins (apoC-I and apoC-III) were positively correlated with the fasting plasma insulin level (R = 0.19–0.21, p < 0.01). Moreover, several plasma apolipoproteins were significantly correlated with insulin resistance (HOMA-IR) (apoC-I and apoC-III; R = 0.21 and 0.23, respectively, p < 0.001) and insulin secretion (HOMA-β) indexes (apoA-IV and apoJ; R = − 0.16 and 0.13, respectively, p < 0.05), or both (apoA-I, apoD, apoH, and apoL1, R = − 0.37–0.15; p < 0.05).
Spearman correlations between plasma apolipoprotein concentrations and biochemical parameters in IT-DIAB participants without statin treatment at baseline. BMI: body mass index; FPG: Fasting Plasma Glucose; HOMA-β: Homeostatic Model Assessment of Insulin beta-cell function; HOMA-IR: Homeostatic Model Assessment of Insulin Resistance; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; WHR: Waist/hip circumference ratio
Plasma apolipoprotein levels and risk of T2D during follow-up
Results of the univariate and multivariable Cox model analyses are depicted in Fig. 3 and detailed in Additional file 1: Table S3. The baseline plasma concentrations of apoA-I, apoA-II, apoA-IV, apoB100, apoC-IV, apoD, apoM, apo(a), TC, LDL-C, and non-HDL-C were not associated with the onset of T2D during follow-up, both before (model 1) and after multiple adjustments (models 2–4). In contrast, before any adjustment, the baseline plasma concentrations of other apolipoproteins were associated with the conversion to T2D: apoC-II (hazard ratio (HR) per 1 SD [95% CI] 1.26 [1.06; 1.51], p = 0.010), apoC-III (HR = 1.23 [1.04; 1.46], p = 0.019), apoE (HR = 1.24 [1.06; 1.47], p = 0.009), apoF (HR = 1.24 [1.02; 1.49], p = 0.029), apoH (HR = 1.22 [1.02; 1.45], p = 0.029), apoL1 (HR = 1.31 [1.10; 1.56], p = 0.003). Furthermore, low HDL-C concentrations (HR per 1 SD = 0.80 [0.66; 0.97], p = 0.025) and high TG concentrations (HR per 1 SD = 1.21 [1.03; 1.43], p = 0.024) were associated with T2D risk. For HDL-C, plasma TG, and plasma apoH, such an association with new-onset diabetes did not remain after adjustment for sex, age, BMI, FPG, and HbA1c (model 2). After additional adjustment for lipid-lowering therapy (model 3), the baseline plasma concentrations of apoC-I, apoC-II, apoC-III, apoE, apoF, apoH, apoJ, and apoL1 were significantly associated with the conversion to T2D; but only baseline apoE, apoF, apoJ, and L1 remained significant in the last model with additional adjustment for TG (HR: 1.28 [1.06; 1.54], p = 0.010; 1.22 [1.01; 1.48], p = 0.037; 1.24 [1.03; 1.49], p = 0.024; 1.26 [1.05; 1.52], p = 0.014; respectively). The Kaplan–Meier survival curves (Fig. 4) showed that the plasma concentrations of apoF, apoJ, and apoL1 (classified as terciles) were not significantly associated with the risk of conversion to T2D. In contrast, lower plasma concentrations of apoE (tercile 1) at baseline were significantly associated with a reduced risk of T2D (log-rank test, p = 0.002). Of note, no significant differences were found for the apoE phenotype distribution (apoE2/E3/E4) according to the final diabetes status of the participants at the final visit (p = 0.67, Additional file 1: Table S4). Finally, no interaction was observed between sex and plasma apoE concentrations regarding the risk of new-onset T2D during follow-up (p-value for interaction ≥ 0.90 in all three adjustment models, data not shown).
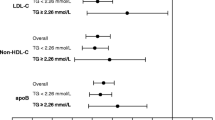
Association between plasma apolipoproteins and plasma lipids at baseline and the incidence of new-onset diabetes during follow-up (Cox models based on the proportional hazards assumption). Hazard ratios (HRs) are calculated per 1 SD after natural-log transformation. Red dots indicate significance with p < 0.05. Model 1: not adjusted (univariate). Model 2: adjusted for baseline values of age, sex, body mass index, fasting plasma glucose, and HbA1c. Model 3: model 2 with additional adjustment for the use of statins or fibrates. Model 4: model 3 with additional adjustment for triglycerides (TG). TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol
Survival curves for new-onset diabetes in the IT-DIAB cohort according to the baseline apoE, apoF, apoJ, and apoL1 plasma concentrations. The groups were created according to terciles. ApoE, tercile 1: 5.97 mg/dL; tercile 2: 8.06 mg/dL. ApoF, tercile 1: 0.63 mg/dL; tercile 2: 1.08 mg/dL. ApoJ, tercile 1: 7.07 mg/dL; tercile 2: 8.91 mg/dL. ApoL1, tercile 1: 0.96 mg/dL; tercile 2: 1.25 mg/dL
Discussion
The present study aimed to deeply investigate the association of the plasma concentrations of a broad panel of apolipoproteins with the risk of new-onset T2D in individuals with prediabetes. After multivariable adjustment for traditional risk factors for T2D, including plasma TG levels, only the plasma apoE, apoF, apoJ, and apoL1 concentrations remained positively and significantly associated with new-onset T2D. The Kaplan–Meier survival curves showed that lower plasma levels of apoE at baseline were associated with a lower occurrence of T2D during follow-up.
Several studies have previously assessed the link between plasma lipid parameters and the risk of T2D, highlighting that both high TG and low HDL-C levels are independent risk factors for new-onset T2D in different populations [25, 26]. Recently, a large study performed in 451,933 participants of the UK Biobank confirmed that both low HDL-C and apoA-I levels were significantly associated with the risk of new-onset diabetes after multivariable-adjusted regressions and two-sample Mendelian randomization analyses [27]. Although we also found such an inverse association between low HDL-C levels and the risk of new-onset T2D by univariate analysis, it did not remain after further adjustments for BMI and glucose homeostasis (FPG, HbA1C). Beyond classical plasma lipid parameters, only a few studies have investigated the associations between plasma apolipoprotein levels and the risk of T2D, and there are even fewer reports focusing on the target population of subjects with prediabetes [7, 15,16,17]. Moreover, most of these studies focused only on major structural species like apoA-I and apoA-II for HDL, and apoB100 for very-low-density lipoprotein (VLDL) and LDL. Interestingly, some of them showed that apolipoproteins can better predict the risk of T2D than classical plasma lipid parameters in the general population [15, 16].
Thanks to a high-throughput mass spectrometry-based assay, we were able to extend the analysis to 16 apolipoprotein species [19]. After adjustment for classical diabetes risk factors, glucose parameters (FPG, HbA1C), and plasma TG, we ultimately identified 4 apolipoproteins amongst 16 that remain significantly associated with new-onset T2D in individuals with prediabetes: apoE, apoF, apoJ, and apoL1. With a similar approach, another prospective population-based cohort study also extended the analysis of apolipoproteins to apoA-I, apoC-III, apoD, and apoE [7]. After a long-term median follow-up of 13.5 years, 11.3% of individuals (110/971) developed T2D. While both apoC-III and apoE were significantly associated with the risk of T2D after adjustments for traditional risk factors, only apoC-III remained to be significantly associated with the risk of T2D after further adjustment for TG [7]. Our findings reinforce the hypothesis that apoC-III impacts glucose homeostasis through the regulation of TG and TG-rich lipoprotein metabolism. In accordance with such a role of apoC-III in the pathophysiology of T2D, a pilot study demonstrated that volanesorsen, an antisense oligonucleotide targeting APOCIII mRNA, improves insulin sensitivity and reduces HbA1C in patients with T2D [28]. These promising findings need to be confirmed in larger randomized trials in patients with T2D and hypertriglyceridemia.
While apoE is a major human apolipoprotein that circulates primarily in association with HDL and VLDL [29], apoF, apoJ, and apoL1 are minor species in human plasma. ApoF circulates primarily as a component of HDL and LDL, and has been proposed to serve as a natural inhibitor of cholesterol ester transfer protein (CETP), with preferential inhibition of CETP activity with LDL [30]. There are no previous reports on the role of apoF in glucose metabolism. In our study, we did not find significant correlations between the plasma levels of apoF and metabolic parameters. However, it has been reported that pharmacological CETP inhibition with dalcetrapib or anacetrapib is associated with a reduced incidence of new-onset diabetes [31, 32]. ApoJ (also called clusterin) exists as multiple protein isoforms with contrasting properties. ApoJ circulates mainly as a component of HDL, and it has been implicated in a wide range of pathophysiological disorders [33]. In accordance with the role of apoJ in glucose homeostasis, apoJ can affect insulin signaling and inflammation [34]. Moreover, it has been reported that apoJ-knockout mice are insulin sensitive [35] and that the plasma levels of apoJ are elevated in obese subjects and positively associated with the BMI [36]. Finally, a Japanese study has found that a single nucleotide polymorphism of apoJ (rs22795590) is associated with a higher risk of T2D through both an increase in insulin resistance and an impairment of insulin secretion [37]. Surprisingly, we found that the apoJ levels were positively and significantly associated with HOMA-β but not with HOMA-IR, despite a trend (p = 0.062). It should be noted, however, that we were not able to discriminate the multiple isoforms of apoJ in the present study. ApoL1 also circulates primarily as a component of HDL [38]. Its plasma levels are correlated with plasma TG [39], a finding we also observed in our study population. The plasma levels of apoL1 have been reported to be increased in patients with metabolic syndrome, and an in vitro study has demonstrated that the insulin signaling pathway regulates both the synthesis and the secretion of apoL1 in hepatocytes [38]. In agreement with previous findings [38], we found a significant and positive correlation between apoL1 and HOMA-IR as well as an inverse correlation with plasma adiponectin, reinforcing its potential role in insulin resistance.
Finally, apoE was the apolipoprotein with the most robust association with new-onset T2D in our study. Indeed, we showed that a lower concentration (1st tercile) of apoE was associated with a reduced risk of new-onset T2D during the follow-up. ApoE predominantly acts as a ligand for the LDL receptor, the LDL receptor-related protein 1, and heparan sulfate proteoglycans, which mediate the clearance of triglyceride-rich lipoproteins and their remnants [23, 29]. A stable isotope kinetic study performed in obese patients has revealed that the VLDL and HDL apoE concentrations are increased in those with T2D as a result of increased production rates. In addition, HOMA-IR and HbA1c were positively correlated with the VLDL apoE production rate in those patients [40]. While the plasma apoE concentrations were positively associated with both TG and LDL-C in our study, we failed to find significant correlations with markers of glucose homeostasis. A meta-analysis has shown that the APOε2 allele is a moderate risk factor for T2D [41], in contrast to another study indicating that the APOε4 allele is associated with T2D risk [42]. A recent study also has reported that the ε2/ε2, ε3/ε4, and ε4/ε4 APOE genotypes are associated with an increased risk of T2D [43]. However, due to the sample size of our study and the low frequency of the apoE2 and apoE4 isoforms, we were not able to clearly rule on these contradictory results.
Importantly, our results are in accordance with previous studies highlighting a relationship between plasma apoE levels and metabolic complications. As discussed above, Brahimaj et al. have reported a positive association between apoE and T2D risk, even if the statistical significance was lost after adjustment for plasma TG [7]. In the German population-based KORA F4/FF4 study (including people with prediabetes), Huth et al. also found that apoE is positively associated with new-onset diabetes beyond classical risk factors such as HbA1c, age, and sex [44]. Moreover, a plasma proteome analysis combined with Mendelian randomization analysis further suggested causal effects of apoE2 protein on the development of metabolic syndrome in two cohorts (KORA and HUNT3) [45].
Our study presents some limitations that must be acknowledged. Due to its exploratory approach, the alpha risk was set to 5% for each test without taking into account multiple testing. In addition, the relatively small size of our cohort limited the statistical power. Consequently, replication cohorts should be analyzed to assess the reproducibility of our results, especially for apoF, apoJ, and apoL1. Besides, our first objective was to investigate the possible associations between plasma concentrations of apolipoproteins and the risk of new-onset T2D in individuals with prediabetes beyond traditional risk markers. Hence, we did not consider this issue from the perspective of the added benefit of these biomarkers in prediction models (presenting area under the receiver operating characteristic (AUROC) or integrated discrimination index (IDI) calculation) as we did not aim to create a new predictive model of the risk of T2D. However, a major strength of our study is the simultaneous analysis of 16 apolipoproteins thanks to a multiplexed assay that has been carefully validated in terms of linearity, specificity, intra- and inter-assay precisions, accuracy, and stability after long-term storage and after multiple freeze/thaw cycles, making it suitable for cohort studies [19]. To the best of our knowledge, this study is the first to investigate so many apolipoproteins in a well-characterized at-risk population.
Conclusions
Here, we showed that the plasma levels of apoE are associated with the occurrence of T2D in individuals with prediabetes, independently of traditional risk factors. Aside from the reported association between HDL-C or plasma TG with the risk of T2D in the general population, these findings strengthen the predictive value of plasma apolipoproteins in the development of metabolic diseases. However, additional studies are required to further determine the importance of apoE concentrations in triglyceride-rich lipoproteins and HDL for T2D risk assessment and for deciphering the underlying pathophysiological mechanisms. Furthermore, this study unraveled possible associations of minor plasma apolipoproteins (apoF, apoJ, and apoL1) with T2D, which paves the way for further investigations.
Availability of data and materials
The datasets associated with the current study are not publicly available but may be provided by the corresponding author on reasonable request.
Abbreviations
- Apo:
-
Apolipoprotein
- BMI:
-
Body mass index
- CETP:
-
Cholesterol ester transfer protein
- CI:
-
Confidence interval
- ECLIA:
-
Electro-chemiluminescent enzyme immunoassay
- FPG:
-
Fasting plasma glucose
- HbA1c :
-
Glycated hemoglobin
- HDL:
-
High-density lipoprotein
- HDL-C:
-
HDL cholesterol
- HMGCR:
-
3-Hydroxy-3-methylglutaryl-CoA reductase
- HOMA-IR:
-
Homeostasis model assessments of insulin resistance
- HOMA-β:
-
Homeostasis model assessments of beta-cell function
- HR:
-
Hazard ratio
- IT-DIAB:
-
Innovation Thérapeutique-Diabète
- LDL:
-
Low-density lipoprotein
- LDL-C:
-
LDL cholesterol
- OR:
-
Odds ratio
- PCSK9:
-
Proprotein convertase subtilisin/kexin type 9
- SD:
-
Standard deviation
- T2D:
-
Type 2 diabetes
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- VLDL:
-
Very-low-density lipoprotein
References
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843.
Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol. 2011;8:228–36.
Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–88.
Ferreira JP, Lamiral Z, Xhaard C, Duarte K, Bresso E, Devignes M-D, et al. Circulating plasma proteins and new-onset diabetes in a population-based study: proteomic and genomic insights from the STANISLAS cohort. Eur J Endocrinol. 2020;183:285–95.
Athyros VG, Doumas M, Imprialos KP, Stavropoulos K, Georgianou E, Katsimardou A, et al. Diabetes and lipid metabolism. Horm Athens Greece. 2018;17:61–7.
Backholer K, Peeters A, Herman WH, Shaw JE, Liew D, Ademi Z, et al. Diabetes prevention and treatment strategies: are we doing enough? Diabetes Care. 2013;36:2714–9.
Brahimaj A, Ligthart S, Ikram MA, Hofman A, Franco OH, Sijbrands EJG, et al. Serum levels of apolipoproteins and incident type 2 diabetes: a prospective cohort study. Diabetes Care. 2017;40:346–51.
Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol. 2017;13:572–87.
Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207.
Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet Lond Engl. 2010;375:735–42.
Kohli P, Waters DD, Nemr R, Arsenault BJ, Messig M, DeMicco DA, et al. Risk of new-onset diabetes and cardiovascular risk reduction from high-dose statin therapy in pre-diabetics and non-pre-diabetics: an analysis from TNT and IDEAL. J Am Coll Cardiol. 2015;65:402–4.
Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375:2144–53.
Besseling J, Kastelein JJP, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA. 2015;313:1029–36.
Dominiczak MH, Caslake MJ. Apolipoproteins: metabolic role and clinical biochemistry applications. Ann Clin Biochem. 2011;48:498–515.
Fizelova M, Miilunpohja M, Kangas AJ, Soininen P, Kuusisto J, Ala-Korpela M, et al. Associations of multiple lipoprotein and apolipoprotein measures with worsening of glycemia and incident type 2 diabetes in 6607 non-diabetic Finnish men. Atherosclerosis. 2015;240:272–7.
Abbasi A, Corpeleijn E, Gansevoort RT, Gans ROB, Hillege HL, Stolk RP, et al. Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 diabetes in the general population: the PREVEND study. J Clin Endocrinol Metab. 2013;98:E1352-1359.
Sasongko MB, Wong TY, Nguyen TT, Kawasaki R, Jenkins A, Shaw J, et al. Serum apolipoprotein AI and B are stronger biomarkers of diabetic retinopathy than traditional lipids. Diabetes Care. 2011;34:474–9.
van den Broek I, Sobhani K, Van Eyk JE. Advances in quantifying apolipoproteins using LC-MS/MS technology: implications for the clinic. Expert Rev Proteomics. 2017;14:869–80.
Blanchard V, Garçon D, Jaunet C, Chemello K, Billon-Crossouard S, Aguesse A, et al. A high-throughput mass spectrometry-based assay for large-scale profiling of circulating human apolipoproteins. J Lipid Res. 2020;61(7):1128–39.
Wargny M, Smati S, Pichelin M, Bigot-Corbel E, Authier C, Dierry V, et al. Fatty liver index is a strong predictor of changes in glycemic status in people with prediabetes: the IT-DIAB study. PLoS ONE. 2019;14:e0221524.
Ramin-Mangata S, Wargny M, Pichelin M, Le May C, Thédrez A, Blanchard V, et al. Circulating PCSK9 levels are not associated with the conversion to type 2 diabetes. Atherosclerosis. 2020;293:49–56.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Blanchard V, Ramin-Mangata S, Billon-Crossouard S, Aguesse A, Durand M, Chemello K, et al. Kinetics of plasma apolipoprotein E isoforms by LC-MS/MS: a pilot study. J Lipid Res. 2018;59:892–900.
R: The R Project for Statistical Computing [Internet]. Accessed 17 Dec 2020. https://www.r-project.org/
Li L, Li P, Yang J, Huang X, Bao H, Zhang C, et al. Lipid levels and new-onset diabetes in a hypertensive population: the China Stroke Primary Prevention Trial. Sci Rep. 2017;7:7014.
Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, et al. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet Lond Engl. 2008;371:1927–35.
Zanetti D, Gustafsson S, Assimes TL, Ingelsson E. Comprehensive investigation of circulating biomarkers and their causal role in atherosclerosis-related risk factors and clinical events. Circ Genomic Precis Med. 2020;13:e002996.
Digenio A, Dunbar RL, Alexander VJ, Hompesch M, Morrow L, Lee RG, et al. Antisense-mediated lowering of plasma apolipoprotein C-III by volanesorsen improves dyslipidemia and insulin sensitivity in type 2 diabetes. Diabetes Care. 2016;39:1408–15.
Marais AD. Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology. 2019;51:165–76.
Liu Y, Morton RE. Apolipoprotein F—a natural inhibitor of CETP and key regulator of lipoprotein metabolism. Curr Opin Lipidol. 2020;31:194–9.
Schwartz GG, Leiter LA, Ballantyne CM, Barter PJ, Black DM, Kallend D, et al. Dalcetrapib reduces risk of new-onset diabetes in patients with coronary heart disease. Diabetes Care. 2020;43:1077–84.
Barter PJ, Cochran BJ, Rye K-A. CETP inhibition, statins and diabetes. Atherosclerosis. 2018;278:143–6.
Seo JA, Kang M-C, Yang W-M, Hwang WM, Kim SS, Hong SH, et al. Apolipoprotein J is a hepatokine regulating muscle glucose metabolism and insulin sensitivity. Nat Commun. 2020;11:2024.
Shim Y-J, Kang B-H, Jeon H-S, Park I-S, Lee K-U, Lee I-K, et al. Clusterin induces matrix metalloproteinase-9 expression via ERK1/2 and PI3K/Akt/NF-κB pathways in monocytes/macrophages. J Leukoc Biol. 2011;90:761–9.
Kwon MJ, Ju T-J, Heo J-Y, Kim Y-W, Kim J-Y, Won K-C, et al. Deficiency of clusterin exacerbates high-fat diet-induced insulin resistance in male mice. Endocrinology. 2014;155:2089–101.
Flehmig G, Scholz M, Klöting N, Fasshauer M, Tönjes A, Stumvoll M, et al. Identification of adipokine clusters related to parameters of fat mass, insulin sensitivity and inflammation. PLoS ONE. 2014;9:e99785.
Daimon M, Oizumi T, Karasawa S, Kaino W, Takase K, Tada K, et al. Association of the clusterin gene polymorphisms with type 2 diabetes mellitus. Metabolism. 2011;60:815–22.
Nishimura K, Murakami T, Sakurai T, Miyoshi M, Kurahashi K, Kishi S, et al. Circulating apolipoprotein L1 is associated with insulin resistance-induced abnormal lipid metabolism. Sci Rep. 2019;9:14869.
Duchateau PN, Movsesyan I, Yamashita S, Sakai N, Hirano K, Schoenhaus SA, et al. Plasma apolipoprotein L concentrations correlate with plasma triglycerides and cholesterol levels in normolipidemic, hyperlipidemic, and diabetic subjects. J Lipid Res. 2000;41:1231–6.
Bach-Ngohou K, Ouguerram K, Nazih H, Maugère P, Ripolles-Piquer B, Zaïr Y, et al. Apolipoprotein E kinetics: influence of insulin resistance and type 2 diabetes. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 2002;26:1451–8.
Anthopoulos PG, Hamodrakas SJ, Bagos PG. Apolipoprotein E polymorphisms and type 2 diabetes: a meta-analysis of 30 studies including 5423 cases and 8197 controls. Mol Genet Metab. 2010;100:283–91.
Chaudhary R, Likidlilid A, Peerapatdit T, Tresukosol D, Srisuma S, Ratanamaneechat S, et al. Apolipoprotein E gene polymorphism: effects on plasma lipids and risk of type 2 diabetes and coronary artery disease. Cardiovasc Diabetol. 2012;11:36.
Chen DW, Shi JK, Li Y, Yang Y, Ren SP. Association between ApoE polymorphism and type 2 diabetes: a meta-analysis of 59 studies. Biomed Environ Sci BES. 2019;32:823–38.
Huth C, von Toerne C, Schederecker F, de Las Heras Gala T, Herder C, Kronenberg F, et al. Protein markers and risk of type 2 diabetes and prediabetes: a targeted proteomics approach in the KORA F4/FF4 study. Eur J Epidemiol. 2019;34:409–22.
Elhadad MA, Wilson R, Zaghlool SB, Huth C, Gieger C, Grallert H, et al. Metabolic syndrome and the plasma proteome: from association to causation. Cardiovasc Diabetol. 2021;20:111.
Acknowledgements
We are grateful to the subjects for their participation in the IT-DIAB cohort. We thank Stéphanie Billon-Crossouard for technical assistance with biochemical determinations as well as Violette Dierry and Jeremy Thureau for their help with data collection. We also thank the Biological Resource Center for Biobanking (CHU Nantes, Hôtel Dieu, Centre de ressources biologiques (CRB), Nantes, F-44093, France (BRIF: BB-0033-00040).
Funding
We are grateful to the “Biogenouest Corsaire” core facility for financial support. KC received a scholarship from the European Union (European Regional Development Fund INTERREG V) and the Region Reunion (Saint-Denis, Reunion, France). IT-DIAB was funded by an ISI grant from OSEO-API, France. This work was also supported by a grant from Société Francophone du Diabète (SFD) and by an internal Grant of CHU Nantes (RC17_0470).
Author information
Authors and Affiliations
Contributions
MC, MW, and BC conceived and designed the research; MC, KC, CC, VB and EBC performed the experiments and laboratory analyses; MC, MW and BC analyzed the data and drafted the first version of the manuscript; GL, CLM and SH revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The IT-DIAB (Innovation Thérapeutique-Diabète) study is registered as NCT01218061 and NCT01432509. The institutional ethics committee approved the protocol, and all of the reported investigations have been carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008. All participants provided their written informed consent for research use of the anonymized data collected according to local regulation.
Consent for publication
All authors consent to this publication.
Competing interests
There are no competing interests related to this work to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional Tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Croyal, M., Wargny, M., Chemello, K. et al. Plasma apolipoprotein concentrations and incident diabetes in subjects with prediabetes. Cardiovasc Diabetol 21, 21 (2022). https://doi.org/10.1186/s12933-022-01452-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01452-5