Abstract
Background
The treatment effects on hospitalization for heart failure (hHF) from sodium-glucose cotransporter 2 (SGLT2) inhibitors may vary among type 2 diabetes (T2D) patients depending on whether or not they have established atherosclerotic cardiovascular diseases (ASCVD). We aimed to examine differences in hHF outcomes after dapagliflozin or empagliflozin use between T2D patients with and without a history of established ASCVD.
Methods
We conducted a retrospective multi-institutional cohort study in Taiwan. We included T2D patients newly receiving dapagliflozin or empagliflozin during 2016–2019, and followed them up until December 31, 2020. We implemented 1:1 propensity score matching to create homogenous groups for comparisons. We generated Cox proportional hazard models to compare the risk of hHF between dapagliflozin and empagliflozin (reference group). We included interaction terms of SGLT2 inhibitor and ASCVD history in the regression models to examine effect modification by ASCVD.
Results
We included a total cohort of 9,586 dapagliflozin new users and 9,586 matched empagliflozin new users. The overall hHF risks were similar for dapagliflozin and empagliflozin (HR: 0.90, 95% CI 0.74–1.09). However, differential hHF risks between dapagliflozin and empagliflozin were observed only in the subgroup without ASCVD (HR: 0.67, 95% CI 0.49–0.90), while not in the subgroup with ASCVD (HR: 1.12, 95% 0.87–1.45), and the p-value for examining interaction was 0.0097.
Conclusion
In this study, history of established ASCVD was associated with different hHF risks among SGLT2 inhibitors. For T2D patients without ASCVD, dapagliflozin may offer a more favorable hHF reduction effect, compared to empagliflozin, in clinical practice. Future prospective studies should be conducted to validate our findings.
Similar content being viewed by others
Introduction
Sodium glucose co-transporter 2 (SGLT2) inhibitors, the second-line treatment for type 2 diabetes (T2D) with their pleiotropic metabolic effects [1,2,3,4,5], offer robust benefits with an approximately 30% reduction in risk of hospitalization for heart failure (hHF) in patients with type 2 diabetes (T2D) [6,7,8,9,10,11,12,13,14,15,16]. However, it is noteworthy that some studies have provided evidence implying the magnitude of hHF risk reduction from the treatment with SGLT2 inhibitors may vary between patients with established atherosclerotic cardiovascular disease (ASCVD) and those without. For example, compared to placebo, the DECLARE-TIMI 58 trial has found that dapagliflozin reduces the risk of hHF in T2D patients without established ASCVD by 36%, but only 22% in those with established ASCVD [17]. A recent study by Patorno et al. using real-world data found T2D patients receiving SGLT2 inhibitors had lower risk of hHF, compared to those receiving DPP4 inhibitors, but the magnitudes of the risk differences varied between patients with established ASCVD (44%) and those without established ASCVD (54%) [18]. Similar findings came from another study using Asian population data [19]. However, a major issue with these studies was the lack of statistical examinations to test for effect modification by ASCVD on hHF risk.
Recently, Shao et al. analyzing Taiwan’s multi-institutional electronic medical records (EMR) database found that the use of dapagliflozin may have more favorable outcomes on heart failure, compared to the use of empagliflozin [20]. However, the study only focused on T2D patients without a history of established ASCVD; it was unclear whether SGLT2 inhibitors produced different hHF risk profiles in patients with ASCVD. The probable effect modification of hHF outcomes through history of established ASCVD has not been well evaluated. Therefore, this study aims to compare the risk of hHF outcomes among T2D patients receiving dapagliflozin or empagliflozin. We stratified by patients’ baseline history of ASCVD and examined whether the hHF outcomes were modified by ASCVD history. We hypothesized that the hHF outcomes after SGLT2 inhibitor treatment would differ between T2D patients with established ASCVD and those without. Understanding the association between the history of established ASCVD and outcomes of hHF risk can help clinical physicians to select the most appropriate drug.
Methods
Data source
This retrospective cohort study is based on Taiwan’s extensive, multi-institutional Chang Gung Research Database (CGRD). CGRD comprises seven Chang Gung Memorial Hospitals located throughout Northern and Southern Taiwan, and contains the EMR of 1.3 million patients (6% of Taiwan’s population). Disease identification in the CGRD follows the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) prior to 2016, and ICD-10-CM thereafter. For the purpose of assuring patients’ baseline status in regard to cardio-metabolic and cardiovascular conditions, the database also includes laboratory examination data. Details of data structures and representativeness of the CGRD are described elsewhere [21], and many of the diagnostic codes used in CGRD have been validated [22,23,24]. The Institutional Review Board of Chang Gung Medical Foundation approved this study under ID# 201801493B0.
Study population
For the analysis we adopted a new-user design with an active-comparator to minimize potential selection bias and other unmeasured confounding factors [25]. We included adult patients with T2D newly receiving at least two prescriptions of dapagliflozin or empagliflozin from 2016 to 2019. We considered all SGLT2 inhibitor use to be new use since SGLT2 inhibitors were only approved in Taiwan from 2016 onwards. The index date was defined as the first dispensing date of dapagliflozin or empagliflozin. We excluded patients with no clinical visit before the index date to ensure that we had sufficient data for baseline evaluation. We also excluded patients without a laboratory examination, including glycated hemoglobin A1c (HbA1c), estimated glomerular filtration rate (eGFR), systolic blood pressure (SBP) and diastolic blood pressure (DBP) at baseline, to ensure the study patients were under the routine care of the study hospitals. We excluded patients with baseline eGFR less than 30 ml/min/1.73 m2 because, following the drug label information, SGLT2 inhibitor use was not suggested for them [26].
Outcomes definition and follow-up
The study outcome was hHF, as determined by the clinical diagnosis (ICD-10-CM codes: I50) at any point in the hospital discharge records. The diagnosis codes for heart failure in CGRD have been validated by previous study with positive predictive value of 89.8% [20]. We followed up patients from the index date to December 31, 2020, the occurrence of hHF, patients’ death or their last clinical visit based on intention-to-treat method [27]. Additional file 1: Figure S1 presents the details of the study design.
Covariates
We determined potential confounding covariates on the basis of expert opinion and previous studies [12,13,14, 28,29,30,31]. We retrieved the data from a year prior to the index date for patients’ baseline co-morbidities including the history of established ASCVD (i.e., coronary heart disease, ischemic stroke and periphery artery diseases), cardiovascular diseases (i.e., hypertension, hyperlipidemia, atrial fibrillation and heart failure), diabetic complications (i.e., diabetic nephropathy, neuropathy and retinopathy) and other chronic diseases (i.e., liver disease, chronic obstructive pulmonary disease (COPD), schizophrenia and cancer). Charlson comorbidity index (CCI) was included as the composite score for patients’ disease burdens [32]. We retrieved data of the 3 months prior to the index date for concomitant anti-hyperglycemic agents and other co-medications, including statins, anti-platelets and anti-hypertensives. We also included indicators for diabetes (i.e., HbA1c), renal functions (i.e., eGFR) and some risk factors for heart failure (i.e., SBP and DBP), based on the most recent laboratory and examination data before the index date [33]. Other covariates related to medical care utilization (i.e. hospital levels and specialties of the prescriber) on the index date were also included. The details of co-morbidity and concomitant medications are presented in Additional file 1: Table S1 and Table S2.
Statistical analyses
To afford more homogeneous comparisons, we used the propensity score method to generate comparable groups [34, 35]. We estimated the propensity scores for the new dapagliflozin or empagliflozin users by using multivariable logistic regression models based on all baseline characteristics listed in Table 1. We implemented a nearest neighbor matching algorithm to minimize distance within matched sets on the propensity score scale with 8 → 1 greedy matching [36]. One user of empagliflozin was selected for each dapagliflozin user, based on propensity score matching. We calculated absolute standardized mean differences (ASMD) to evaluate the differences between the two comparison groups. An ASMD < 0.1 indicates a negligible difference between the two treatment groups [37]. We performed Cox proportional hazards regression modeling after propensity score matching to estimate hazard ratios (HR) and 95% confidence intervals (CI) on the risk of hHF. Empagliflozin users were considered the reference group because empagliflozin was the most frequently prescribed SGLT2 inhibitor in Taiwan during the study period. Specifically, we have included interaction terms in the regression models to examine effect modification by the history of established ASCVD (with vs. without). We considered results with a 2-sided P < 0.05 statistically significant. Statistical analyses were performed using SAS Enterprise Guide (Version 7.1; SAS Institute Inc., Cary, NC, USA).
Sensitivity analyses
We conducted several sensitivity analyses to examine the robustness of our results. First, to ensure the outcome validity, we re-defined hHF to include only diagnosis from specialized cardiologists at discharge. Second, we used heart failure diagnosis codes at discharge plus the serum B-type Natriuretic Peptide (BNP) levels at two cutoffs of 100 pg/ml and 400 pg/ml during hospitalization to redefine heart failure. BNP levels appear useful for the detection of heart failure, with a cutoff < 100 pg/ml excluding heart failure and > 400 pg/ml a possible diagnosis of heart failure [38]. Third, to reduce the impact of patients’ non-adherence to treatment or loss to follow-up, we performed on-treatment analysis and censored patients exceeding 90 days without a prescription refill, or who switched to another SGLT2 inhibitor or other index medication. Finally, we excluded patients if hHF occurred within 90 days or 180 days after the index date to avoid confounding since hHF that occurs in such a short period of time is likely to be caused by patients’ underlying diseases rather than being the outcome of medications [39].
E-value analyses
We calculated E-value on the basis of our study outcomes. E-value was developed by Vanderweele et al. to estimate the minimum strength of association that an unmeasured confounder, conditional on the measured covariates, would require to fully explain away the observed associations [40]. For example, E-value 2.0 indicates that an unmeasured confounder associated with both exposure and outcome by a hazard ratio of at least 2 times would eliminate the observed associations.
Result
Study population
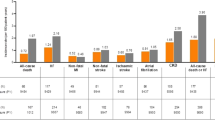
We identified a total of 10,274 dapagliflozin and 12,030 empagliflozin new users after applying study inclusion and exclusion criteria (Additional file 1: Figure S2). Their baseline characteristics are presented in Additional file 1: Table S3. After 1:1 propensity score matching, we included 9586 dapagliflozin and 9586 matched empagliflozin new users with well-balanced baseline characteristics (all ASMDs < 0.1) (Table 1). For example, for dapagliflozin new users the mean age, HbA1c and eGFR values were 59.9 (SD 11.6) years, 8.7 (SD 1.6) % and 92.8 (SD 27.1) ml/min/1.73 m2, respectively. This was similar to empagliflozin new users with a mean age of 60.0 (SD 12.0) years, HbA1c of 8.7 (SD 1.7) % and eGFR of 92.3 (SD 31.6) ml/min/1.73 m2, respectively. At baseline, 25.1% of dapagliflozin new users and 25.4% of empagliflozin new users had a history of ASCVD. The mean follow-up periods were similar for dapagliflozin (2.7 years) and empagliflozin (2.7 years) new users. Without stratification by history of ASCVD, there was no significant difference in hHF incidence between dapagliflozin (7.56/1000 person-years) and empagliflozin new users (8.39/1000 person-years) (Additional file 1: Table S4) with an HR of 0.90 (95% CI 0.74–1.09; E-value: 1.46) (Fig. 1a).
Examination of interaction for history of established ASCVD
Among patients without a history of established ASCVD, and newly receiving dapagliflozin (3.62/1000 person-years), lower hHF risks were observed, compared to those receiving empagliflozin (5.42/1000 person-years), with an HR of 0.67 (95% CI 0.49–0.90; E-value: 2.35) (Fig. 1b). However, among patients with a history of established ASCVD, we found similar hHF risks (HR: 1.12; 95% CI 0.87–1.45; E-value: 1.49) for dapagliflozin users (19.86/1000 person-years) and empagliflozin users (17.62/1000 person-years) (Fig. 1c). Significant effect modification for the association between ASCVD history and hHF risk was found (P value for interaction: 0.0097, Table 2). We present the number of patients at risk, patients with hHF outcomes and patients censored in Additional file 1: Table S5.
Sensitivity analyses
The sensitivity analyses showed the results were robust. Specifically, when we identified hHF using stricter definitions, the results remained consistent with the main analyses. The on-treatment analyses also showed similar results when we stratified patients by their history of established ASCVD (Fig. 2).
Discussion
The study results indicated the use of dapagliflozin and empagliflozin had a similar effect on hHF outcomes in all T2D patients, of whom approximately 75% were without history of established ASCVD. Notably, when we stratified patients into those with and without a history of established ASCVD, we found the hHF effects of dapagliflozin and empagliflozin to be different. That is, compared to empagliflozin, dapagliflozin was associated with a greater effect of reducing hHF in patients without a history of ASCVD, but this association was not observed in patients with a history of ASCVD. This may reflect the fact that having a history of ASCVD acted as an effect modifier when using SGLT2 inhibitors. These findings highlight the need to consider patients’ history of ASCVD when selecting SGLT2 inhibitors.
RCTs and real-world cohort studies have demonstrated that the use of SGLT2 inhibitors reduces the incidence of hHF in T2D patients. Specifically, the DECLARE-TIMI 58 trial found that dapagliflozin offers more hHF risk reduction in T2D patients without established ASCVD, compared to those with established ASCVD [17]. In addition, in a previous real-world study which focused on T2D patients without established ASCVD, dapagliflozin was proven to offer more reduction of heart failure risk than empagliflozin [20]. Our study further evaluated possible effect modifications among different SGLT2 inhibitors, based on the patients’ baseline history of ASCVD. We found the use of dapagliflozin was associated with a lower risk of hHF in patients without established ASCVD, while this association was not observed in patients with established ASCVD. A plausible explanation may be found in the different pharmacokinetics and differences in SGLT2 and SGLT1 receptor selectivity. A recent study by Lin et al. found that compared to empagliflozin, dapagliflozin may have better glucose-lowering effects, and also lower the risk of renal function decline in T2D patients in clinical practice [41], and that improvement in these conditions was associated with lower risk of hHF. Several studies have also indicated that dapagliflozin, having longer pharmacological effects, compared to empagliflozin, may lead to less variability in SBP and glycemic levels, and thus may be associated with reduced risk of heart failure [42,43,44,45,46,47]. In particular, recent meta-regression analyses have indicated better risk reduction for hHF events with decreasing SGLT2 receptor selectivity [48]. The SGLT2:SGLT1 receptor selectivity ratio of dapagliflozin is 1200:1, lower than that of empagliflozin (2500:1) [49], and this relatively higher selectivity of SGLT1 receptors in the case of dapagliflozin may decrease variations in postprandial blood glucose levels, since SGLT1 receptors can be found predominantly in the human intestine. This may also result in additional benefits with regard to heart failure risk reduction [50,51,52]. Recent studies have reported that inhibition of SGLT1 receptors in the heart may decrease hyperglycemia-induced generation of reactive oxygen species, leading to potential prevention of heart failure [53, 54]. In addition, compared to empagliflozin, dapagliflozin did not increase plasma aldosterone and noradrenaline levels, which may also prove beneficial for the prevention of hHF [55].
In this study, we used standard statistical approaches to confirm effect modification through the history of established ASCVD. It is noteworthy that the superior effects of dapagliflozin over empagliflozin as regards the risk of hHF were only observed in patients without a history of established ASCVD. The mechanisms behind this effect modification remain unclear. One possible explanation is that patients with established ASCVD may develop ischemic cardiomyopathy associated with lower cardiac functions [56], since the diuretic and natriuretic effects of the SGLT2 inhibitor that improve preload and afterload have become relatively restricted. That is, the history of established ASCVD may be a crucial predisposing factor leading to hHF. This may also explain the observed 3 to 5-fold higher incidence rates of hHF in patients with a history of established ASCVD, compared to those without a history of established ASCVD, in our study. Since there was no significant difference in hHF between dapagliflozin and empagliflozin in T2D patients with a history of established ASCVD, the selection of SGLT2 inhibitors in this population should be based on other clinical factors.
This presented study has several strengths. First, this study was based on a large multi-institutional EMR database, covering about 1.3 million individuals, to provide sufficient sample size and statistical power for the analyses. Second, many clinically relevant data related to the disease severity were considered in this study, such as laboratory parameters for patients’ baseline blood glucose levels, renal functions and blood pressure. However, several limitations should be noted due to the nature of the observational study design. First, although the diagnosis codes for heart failure have been validated in our dataset [20], possible misclassification bias cannot be ignored. We conducted two sensitivity analyses applying narrower criteria for case ascertainments, including diagnosis by cardiologists only, and the use of BNP-level data to define heart failure [38], whereby the results were consistent with the main analyses. Second, although we used propensity score methods to minimize the differences in baseline covariates between empagliflozin and dapagliflozin, some unmeasured confounders remained unresolved [57]. However, we considered the new user design with active comparator approach should minimize these biases since the impact of unmeasured confounders should be non-differential between the two treatment groups [58, 59]. For example, the study did not consider patients’ lifestyle modifications after SGLT2 inhibitor initiation. We assumed that lifestyle changes were similar between the two SGLT2 inhibitor groups, and that any differences would be of no influence. Furthermore, while the smoking status is not routinely recorded in CGRD, those demographics (e.g., age and sex) and medical disorders (e.g., COPD) associated with smoking behaviors were similar for the two SGLT2 inhibitor groups [60]. To address potential effects from unmeasured confounders, we applied the E-value approach [40, 61], and found it unlikely that an unmeasured confounder having a substantial association with the prescribing choice between dapagliflozin and empagliflozin would have a relative risk exceeding 2.35. Third, the study may be subject to incomplete follow-up data as a result of patients being transferred to other hospitals. To evaluate the effects due to loss of follow-up, we conducted on-treatment analyses, whereby the findings were consistent with the main analyses. Finally, we may have failed to capture conditions with mild to moderate symptoms of HF without hospitalization.
Conclusion
This real-world study reported the difference in hHF risk after treatment with dapagliflozin or empagliflozin, between those with and those without a history of established ASCVD. Compared to empagliflozin, the use of dapagliflozin was associated with a lower risk of hHF in patients without established ASCVD, while this association was not observed in patients with established ASCVD. This study lays the groundwork for future prospective study to support our findings.
Availability of data and materials
Data sharing is not applicable to this study as data management and analysis were performed on a statistics server through remote access in Chang Gung Medical Foundation in Taiwan, out of privacy and safety concerns.
Abbreviations
- ASCVD:
-
Atherosclerotic cardiovascular disease
- ASMD:
-
Absolute standardized mean differences
- BNP:
-
B-type Natriuretic Peptide
- CCI:
-
Charlson comorbidity index
- CI:
-
Confidence intervals
- eGFR:
-
Estimated glomerular filtration rate
- EMR:
-
Electronic medical records
- HbA1c:
-
Glycated hemoglobin A1c
- hHF:
-
Hospitalization for heart failure
- HR:
-
Hazard ratios
- ICD-9-CM:
-
International Classification of Diseases, Ninth Revision, Clinical Modification
- ICD-10-CM:
-
International Classification of Diseases, Tenth Revision, Clinical Modification
- RCTs:
-
Randomized controlled trials
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- SGLT2:
-
Sodium-glucose co-transporter 2
- T2D:
-
Type 2 diabetes
References
Shao SC, Chang KC, Lin SJ, Chien RN, Hung MJ, Chan YY, et al. Favorable pleiotropic effects of sodium glucose cotransporter 2 inhibitors: head-to-head comparisons with dipeptidyl peptidase-4 inhibitors in type 2 diabetes patients. Cardiovasc Diabetol. 2020;19(1):17.
Zheng H, Liu M, Li S, Shi Q, Zhang S, Zhou Y, et al. Sodium-glucose co-transporter-2 inhibitors in non-diabetic adults with overweight or obesity: a systematic review and meta-analysis. Front Endocrinol. 2021;12:706914.
Pharmacologic Approaches to Glycemic Treatment. Standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S111–24.
Shao SC, Kuo LT, Chien RN, Hung MJ, Lai EC. SGLT2 inhibitors in patients with type 2 diabetes with non-alcoholic fatty liver diseases: an umbrella review of systematic reviews. BMJ Open Diabetes Res Care. 2020;8(2):e001956.
Shao SC, Chang KC, Chien RN, Lin SJ, Hung MJ, Chan YY, et al. Effects of sodium-glucose co-transporter-2 inhibitors on serum alanine aminotransferase levels in people with type 2 diabetes: a multi-institutional cohort study. Diabetes Obes Metab. 2020;22(1):128–34.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Wiviott SD, Raz I, Bonaca MP, Kato ET, Cahn A, Silverman MG, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9.
Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573.
Tsapas A, Avgerinos I, Karagiannis T, Malandris K, Manolopoulos A, Andreadis P, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020;173(4):278–86.
Filion KB, Lix LM, Yu OH, Dell’Aniello S, Douros A, Shah BR, et al. Sodium glucose cotransporter 2 inhibitors and risk of major adverse cardiovascular events: multi-database retrospective cohort study. BMJ. 2020;370:m3342.
Birkeland KI, Bodegard J, Banerjee A, Kim DJ, Norhammar A, Eriksson JW, et al. Lower cardiorenal risk with sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes without cardiovascular and renal diseases: a large multinational observational study. Diabetes Obes Metab. 2021;23(1):75–85.
Kohsaka S, Lam CSP, Kim DJ, Cavender MA, Norhammar A, Jørgensen ME, et al. Risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors compared with DPP-4 inhibitors: an analysis from the CVD-REAL 2 multinational cohort study. Lancet Diabetes Endocrinol. 2020;8(7):606–15.
Varzideh F, Kansakar U, Santulli G. SGLT2 inhibitors in cardiovascular medicine. Eur Heart J Cardiovasc Pharmacother. 2021;7(4):e67–8.
Qiu M, Ding LL, Zhan ZL, Zhou HR. Commentary: sodium glucose cotransporter 2 inhibitors reduce the risk of heart failure hospitalization in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Front Endocrinol. 2021;12:664502.
McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6(2):148–58.
Patorno E, Pawar A, Franklin JM, Najafzadeh M, Déruaz-Luyet A, Brodovicz KG, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation. 2019;139(25):2822–30.
Seino Y, Kim DJ, Yabe D, Tan EC, Chung WJ, Ha KH, et al. Cardiovascular and renal effectiveness of empagliflozin in routine care in East Asia: Results from the EMPRISE East Asia study. Endocrinol Diabetes Metab. 2021;4(1):e00183.
Shao SC, Chang KC, Hung MJ, Yang NI, Chan YY, Chen HY, et al. Comparative risk evaluation for cardiovascular events associated with dapagliflozin vs. empagliflozin in real-world type 2 diabetes patients: a multi-institutional cohort study. Cardiovasc Diabetol. 2019;18(1):120.
Shao SC, Chan YY, Kao Yang YH, Lin SJ, Hung MJ, Chien RN, et al. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. 2019;28(5):593–600.
Chang SL, Huang YL, Lee MC, Hu S, Hsiao YC, Chang SW, et al. Association of varicose veins with incident venous thromboembolism and peripheral artery disease. JAMA. 2018;319(8):807–17.
Lin YS, Chen TH, Chi CC, Lin MS, Tung TH, Liu CH, et al. Different implications of heart failure, ischemic stroke, and mortality between nonvalvular atrial fibrillation and atrial flutter-a view from a national cohort study. J Am Heart Assoc. 2017;6(7):e006406.
Chan YH, Yeh YH, See LC, Wang CL, Chang SH, Lee HF, et al. Acute kidney injury in Asians with atrial fibrillation treated with dabigatran or warfarin. J Am Coll Cardiol. 2016;68(21):2272–83.
Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–8.
Das SR, Everett BM, Birtcher KK, Brown JM, Januzzi JL Jr, Kalyani RR, et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117–45.
Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319(7211):670–4.
Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation. 2017;136(3):249–59.
Birkeland KI, Jorgensen ME, Carstensen B, Persson F, Gulseth HL, Thuresson M, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5(9):709–17.
Gautam S, Agiro A, Barron J, Power T, Weisman H, White J. Heart failure hospitalization risk associated with use of two classes of oral antidiabetic medications: an observational, real-world analysis. Cardiovasc Diabetol. 2017;16(1):93.
Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71(23):2628–39.
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275(20):1557–62.
Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314(15):1637–8.
Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27(12):2037–49.
Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 2):69–80.
Stuart EA, Lee BK, Leacy FP. Prognostic score-based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66(8 Suppl):S84–90.
Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, et al. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation. 2017;135(22):e1054–91.
Faillie JL. Indication bias or protopathic bias? Br J Clin Pharmacol. 2015;80(4):779–80.
VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–74.
Lin YH, Huang YY, Hsieh SH, Sun JH, Chen ST, Lin CH. Renal and glucose-lowering effects of empagliflozin and dapagliflozin in different chronic kidney disease stages. Front Endocrinol. 2019;10:820.
Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4(3):211–20.
Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, Woerle HJ, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420–8.
Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med. 2015;163(5):329–38.
Chowdhury EK, Owen A, Krum H, Wing LM, Nelson MR, Reid CM. Systolic blood pressure variability is an important predictor of cardiovascular outcomes in elderly hypertensive patients. J Hypertens. 2014;32(3):525–33.
Vianna AGD, Lacerda CS, Pechmann LM, Polesel MG, Marino EC, Scharf M, et al. Improved glycaemic variability and time in range with dapagliflozin versus gliclazide modified release among adults with type 2 diabetes, evaluated by continuous glucose monitoring: a 12-week randomized controlled trial. Diabetes Obes Metab. 2020;22(4):501–11.
Yokota S, Tanaka H, Mochizuki Y, Soga F, Yamashita K, Tanaka Y, et al. Association of glycemic variability with left ventricular diastolic function in type 2 diabetes mellitus. Cardiovasc Diabetol. 2019;18(1):166.
Täger T, Frankenstein L, Atar D, Agewall S, Frey N, Grundtvig M, et al. Influence of receptor selectivity on benefits from SGLT2 inhibitors in patients with heart failure: a systematic review and head-to-head comparative efficacy network meta-analysis. Clin Res Cardiol. 2021. https://doi.org/10.1007/s00392-021-01913-z (Epub ahead of print).
Anker SD, Butler J. Empagliflozin, calcium, and SGLT1/2 receptor affinity: another piece of the puzzle. ESC Heart Fail. 2018;5:549–51.
Musso G, Gambino R, Cassader M, Paschetta E. Efficacy and safety of dual SGLT 1/2 inhibitor sotagliflozin in type 1 diabetes: meta-analysis of randomised controlled trials. BMJ. 2019;365:l1328.
Sha S, Polidori D, Farrell K, Natarajan J, Vaccaro N, Pinheiro J, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab. 2015;17:188–97.
Yokota S, Tanaka H, Mochizuki Y, Soga F, Yamashita K, Tanaka Y, et al. Association Of Glycemic Variability With Left Ventricular Diastolic Function In Type 2 Diabetes Mellitus. J Am Coll Cardiol. 2019;73(9 Supplement 1):764.
Sayour AA, Oláh A, Ruppert M, Barta BA, Horváth EM, Benke K, et al. Characterization of left ventricular myocardial sodium-glucose cotransporter 1 expression in patients with end-stage heart failure. Cardiovasc Diabetol. 2020;19(1):159.
Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020;5(6):632–44.
Nakagaito M, Joho S, Ushijima R, Nakamura M, Kinugawa K. Comparison of canagliflozin, dapagliflozin and empagliflozin added to heart failure treatment in decompensated heart failure patients with type 2 diabetes mellitus. Circ Rep. 2019;1(10):405–13.
Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus—mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459–502.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424.
Oshima M, Neuen BL, Jardine MJ, Bakris G, Edwards R, Levin A, et al. Effects of canagliflozin on anaemia in patients with type 2 diabetes and chronic kidney disease: a post-hoc analysis from the CREDENCE trial. Lancet Diabetes Endocrinol. 2020;8(11):903–14.
Prada-Ramallal G, Takkouche B, Figueiras A. Bias in pharmacoepidemiologic studies using secondary health care databases: a scoping review. BMC Med Res Methodol. 2019;19(1):53.
Chung WS, Kung PT, Chang HY, Tsai WC. Demographics and medical disorders associated with smoking: a population-based study. BMC Public Health. 2020;20(1):702.
Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602–3.
Acknowledgements
We thank Chang Gung Memorial Hospitals for making the Chang Gung Research Database available for our research. The result interpretation and study conclusion do not reflect the position of Chang Gung Memorial Hospitals.
Funding
This study received a grant from Chang Gung Medical Foundation (ID: CMRPG3H1553) and Ministry of Science and Technology of Taiwan (107-2320-B-006-070-MY3), which had no role in design, analysis, interpretation, reporting of results or the decision to develop this manuscript.
Author information
Authors and Affiliations
Contributions
Author contributions to the present study were as follows: Study concept and design: SCS and ECCL; Acquisition of participants and/or data: YYC; Analysis and interpretation of data: SCS, KCC and ECCL; Preparation of manuscript: all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional review board at Chang Gung Medical Foundation (No. 201801493B0), and the need for informed consent was waived.
Consent for publication
Not applicable.
Competing interests
We report no potential conflict of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Overview of study design. Figure S2. Study patient cohort assembly flowchart. Table S1. Diagnosis codes for study outcome and co-morbidity. Table S2. Individual drug for study co-medication. Table S3. Baseline characteristics before 1:1 propensity score matching (original cohort). Table S4. Results from the Cox regression model after 1:1 propensity score matching Table S5. The number of patients at risk, patients with hHF outcomes, and patients censored.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shao, SC., Chang, KC., Lin, SJ. et al. Differences in outcomes of hospitalizations for heart failure after SGLT2 inhibitor treatment: effect modification by atherosclerotic cardiovascular disease. Cardiovasc Diabetol 20, 213 (2021). https://doi.org/10.1186/s12933-021-01406-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-021-01406-3