Abstract
Background
The global burden of atrial fibrillation (AF) and diabetes mellitus (DM) is constantly rising, leading to an increasing healthcare burden of stroke. AF often remains undiagnosed due to the occurrence in an asymptomatic, silent form, i.e., silent AF (SAF). The study aims to evaluate the relationships between DM and AF prevalence using a mobile long-term continuous ECG telemonitoring vest in a representative Polish and European population ≥ 65 years for detection of AF, symptomatic or silent.
Methods
A representative sample of 3014 participants from the cross-sectional NOMED-AF study was enrolled in the analyses (mean age 77.5, 49.1% female): 881 (29.2%) were diagnosed with DM. AF was screened using a telemonitoring vest for a mean of 21.9 ± 9.1days.
Results
Overall, AF was reported in 680 (22.6%) of the whole study population. AF prevalence was higher among subjects with concomitant DM (DM+) versus those without DM (DM−) [25%, 95% CI 22.5-27.8% vs 17%; 95% CI 15.4–18.5% respectively, p < 0.001]. DM patients were commonly associated with SAF [9%; 95% CI 7.9–11.4 vs 7%; 95% CI 5.6–7.5 respectively, p < 0.001], and persistent/permanent AF [12.2%; 95% CI 10.3–14.3 vs 6.9%; 95% CI 5.9–8.1 respectively, p < 0.001] compared to subjects without DM. The prolonged screening was associated with a higher percentage of newly established AF diagnosis in DM+ vs DM− patients (5% vs 4.5% respectively, p < 0.001). In addition to shared risk factors, DM+ subjects were associated with different AF and SAF independent risk factors compared to DM− individuals, including thyroid disease, peripheral/systemic thromboembolism, hypertension, physical activity and prior percutaneous coronary intervention/coronary artery bypass graft surgery.
Conclusions
AF affects 1 out of 4 subjects with concomitant DM. The higher prevalence of AF and SAF among DM subjects than those without DM highlights the necessity of active AF screening specific AF risk factors assessment amongst the diabetic population.
Trial registration: NCT03243474
Similar content being viewed by others
Background
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia worldwide [1,2,3]. AF is associated with substantially impaired quality of life by increasing the risk of stroke, thromboembolism, heart failure, dementia and all-cause mortality, thus constituting the public health priority of utmost importance [2, 4,5,6,7]. The global burden of this arrhythmia is rapidly increasing due to the widespread ageing of the population and related occurrence of numerous comorbidities.
Nonetheless, the AF prevalence in many prior studies is likely to be underestimated due to many patients with silent AF (SAF), an asymptomatic form of arrhythmia, which remains undiagnosed. For that reason, although comprehensive and holistic AF management [8] is crucial, the active screening and early detection of the arrhythmia poses a real challenge.
The likelihood of establishing the diagnosis increases along with the monitoring timespan [9]. These emphasize the necessity of actively searching for AF, preferably taking advantage of non-invasive wearable ECG-monitoring devices, to maintain the balance between efficiency and compliance in predicting rhythm disturbances [10, 11]. In a cross-sectional epidemiological study, the Non-invasive Monitoring for Early Detection of Atrial Fibrillation (NOMED-AF), we investigated the AF prevalence in the European population aged ≥ 65 using long-term continuous monitoring [12].
One of the common aetiological factors for incident AF is diabetes mellitus (DM) [13]. Indeed, DM is the most predominant metabolic disorder in the general population, affecting 451 million people in 2017 with an increase projected to 693 million by 2045 [14]. Not only is diabetes a well-known major risk factor for AF development, but it is also associated with stroke, thromboembolism and is a cornerstone of metabolic syndrome, which has an adverse impact on the overall prognosis [15, 16]. Multiple studies have reported the association between DM and substantially increased risk of AF incidence [17,18,19,20,21,22].
In this ancillary analysis to NOMED-AF, we aimed to evaluate the relationships between DM and AF prevalence using a mobile long-term continuous ECG telemonitoring vest in a representative Polish population ≥ 65 years for detection of both symptomatic or silent AF.
Methods
The study was conducted as a sub-analysis of Non-invasive Monitoring for Early Detection of Atrial Fibrillation (NOMED-AF) study, a cross-sectional observational study aiming to evaluate the AF prevalence and its associated comorbidities in the Polish population. The detailed study protocol has been previously described [12]. The study used a long-term wearable non-invasive ECG monitoring system linked with an online platform for data analysis and storage.
The enrolment period was between March 15th, 2017 and March 10th, 2018. The trial schedule comprises multistage, stratified and clustered population sampling, during which the representative Polish population ≥ 65 was stratified by province and place of residence. The procedure is thoroughly described in the Additional file 1: appendix. Briefly, the whole country territory was stratified into 59 geographical strata. After that, the regions from each stratum (villages, towns, cities) were randomly selected by the proportional probability, the study participants from the previously chosen areas were also selected at random manner, based on the personal identity number. A similar number of men and women in each 5-year age group were designated. Therefore, we achieved the oversampling of older age groups. We did the process to assure that the size of the final subsample of the oldest subjects will be enough for separate analyses. The oversampling was corrected at the stage of statistical analysis with weights to get population estimates. For each of the 3000 participants, another 9 subjects living in the same cluster were drawn. These “spare” addresses were used in a predefined random order only if the address of the primarily chosen subject was incorrect or a subject refused to take part in the study.
Data collection
Each of the study participants was interviewed at home by a trained nurse, using a standardized questionnaire. Relevant questions for the current analysis are as follows: previously diagnosed AF, symptoms and signs related to AF, symptoms of other cardiovascular diseases, presence of concomitant diabetes mellitus or chronic kidney disease. There were also collected data needed to calculate CHA2DS2-VASc score.
Moreover, height and weight were taken from each participant. Blood pressure was measured during two separate visits at home, using validated automated oscillometric devices. Urine and fasting blood samples were collected and processed in the central laboratory.
Long term ECG monitoring
Thirty-day, surface, 2-lead ECG recording was attempted in each of the study subjects, including respondents with already established AF diagnosis. The dedicated ECG monitoring system was developed and manufactured by Comarch Healthcare (Krakow, Poland) specifically for the purpose of the current study. The system consisted of a vest equipped with ECG leads, two exchangeable recorders and a docking station allowing to charge recorders and transmit data, while another recorder was at the same time connected to vest and recording. ECG data was transmitted to a central database with the use of GSM technology.
The ECG recording was screened automatically for AF and atrial flutter episodes lasting longer than 30 s, using software developed and validated, especially for the purpose of the study. Episodes of atrial fibrillation/atrial flutter lasting longer than 30 s were automatically detected by AF detection algorithms of the analytical platform. Finally, each of the automatically detected episodes was reviewed by trained cardiologists.
Outcomes
The presence of AF was established based on the patient’s medical records assessed for all subjects by the qualified study nurse on-site, confirmed by ECG record/monitoring (all participants had long term ECG monitoring). All of the newly diagnosed AF cases (not previously detected) were established based on up to 30 days of surface ECG monitoring for episodes of AF lasting 30 s or longer. Newly diagnosed AF was defined as AF found in patients without previous history of this arrhythmia in available medical documentation. In this paper, the term AF refers both to atrial fibrillation and/or atrial flutter.
Patients were diagnosed with paroxysmal AF if the duration of the recorded longest arrhythmia event was shorter than 7 days. All other cases were considered as persistent/permanent. Because it is not always possible to distinguish precisely between persistent and permanent AF using patients’ medical documentation, we analyzed both as one group.
DM type 2 diagnosis was established in line with the American Diabetes Association [23] and European Association for the Study of Diabetes [24]. Guidelines if the haemoglobin A1c (HbA1c) measured by HPLC was ≥ 6.5% or if the patient was aware of diabetes and a glucose-lowering treatment was applied. Physical activity threshold was defined as exercise at least > 30 min ≥ 3 times a week.
The studied cohort was divided into two study groups based on DM presence: DM (+) group—participants with concomitant DM; and DM (−) group—subjects without DM. AF prevalence was also analyzed in correlation to age and gender. The detailed baseline characteristics were described for both—NOMED and Polish population, while all other analyses were weighted and reported only for the Polish population. None of the study participants was reported with DM type 1, hence the analyses comprise only individuals diagnosed with type 2 diabetes.
Signed, informed consent was obtained from each eligible participant of the trial in accordance with protocol regulations approved by the local review boards governing research involving human subjects and local bioethical committee (26/2015), and the Declaration of Helsinki. The trial was registered on clinicaltrials.gov (NCT03243474).
Statistical analysis
Continuous variables were presented as mean and standard deviation (SD). Categorical variables were depicted as counts and percentages, analyzed by chi-squared test. National estimation, i.e., the frequency of comorbidities prevalence, average values for age, BMI etc., were analyzed on weighted data. The estimations were calculated so that the sample proportions were stratified by sex, age and city class were the same as in the Polish population. 95% Confidence intervals were determined, including the complex sampling scheme and were used to express the significance of differences between specific categories. Fisher’s exact test was performed to compare differences between individual age categories. A logistic regression analysis was conducted to obtain the risk changes relative to age and sex. A multiple logistic regression analysis was conducted to obtain independent risk factors of AF and SAF in DM+ and DM- populations. The independent variable was 5-year age groups and gender. A two-sided p-value < 0.05 was considered to be statistically significant.
Oversampling of elderly age groups was addressed by using weights, which corrected the age and sex structure of the sample to the structure of the Polish population. Statistical analyses accounted for complex survey design. Prevalence and 95% confidence intervals (95% CI) were reported.
Finally, for each patient with paroxysmal AF, the number of hours of ECG monitoring before the first recorded AF event (lasting at least 30 s) was assessed. Based on these data, the relationship between the duration of ECG monitoring and the number of AF cases was examined.
Results
A representative sample of 3014 participants of the NOMED-AF study was eligible and enrolled in the analysis. From the study population, 881 (29.2%) people were diagnosed with DM (DM+ group). Subjects with concomitant diabetes were less likely to be female (57%; 95% CI 53.5–60.4 vs 62%; 95% CI 60.3–63.9%, p = 0.009, respectively), had higher BMI index (30.35 ± 4.98 vs 27.5 ± 4.63, p < 0.001, respectively) and more comorbidities including hypertension, heart failure, chronic kidney disease, coronary artery disease and stroke (all p < 0.001). Moreover, DM subjects had higher stroke risk according to the CHA2DS2-VASc score (5.4 ± 1.3 vs 3.8 ± 1.3 pt.; p < 0.001, respectively). Detailed baseline characteristics of the analyzed population are reported in Table 1.
The mean ECG monitoring interval in the studied population was 20.07 ± 8.98 days, while the mean monitoring timespan among the DM+ group was 20.31 ± 8.97 days and in DM− group, 19.97 ± 9.01 days. The mean time to detect any first AF episode was 7.25 ± 7.79 days, and for the first episode of SAF, 8.48 ± 8.29 days.
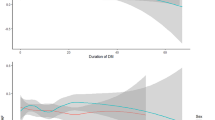
Overall, AF was identified in 22.6% (n = 680) of the overall study population. The analyses, conducted with the use of data weighted for the Polish population, indicated significantly greater AF prevalence among participants diagnosed with DM compared to those without DM (25%; 95% CI 22.5–27.8% vs 17%; 95% CI 15.4–18.5% respectively, p < 0.001) (Fig. 1). DM patients were commonly associated with SAF (9%; 95% CI 7.9–11.4 vs 7%; 95% CI 5.6–7.5 respectively, p < 0.001) (Fig. 2).
Prolonged screening for AF was associated with more newly established AF diagnoses in participants with concomitant DM compared to those without DM (5% vs 4.5% respectively, p < 0.001). Also, DM+ patients had a greater prevalence of persistent or sustained AF than those in the DM− group (12.2%; 95% CI 10.3–14.3 vs 6.9%; 95% CI 5.9–8.1 respectively, p < 0.001). The arrhythmia classification is described in Table 2.
Age and sex
There was increasing arrhythmia prevalence along with age noticeable in both women and men. Similar trends of rising prevalence along with age are described in SAF. Arrhythmia prevalence in 5-year age ranges is summarised in Table 3. In the general population, the AF prevalence was relevantly higher in men vs women (OR 2.56, 95% CI 1.68–3.90 p < 0.001), while in the DM+ group analysis there were no significant differences in AF prevalence between males and females (OR 1.78, 95% CI 0.75–4.32, p = 0.19). Detailed analysis of odds ratios in correlation to age and gender also indicated no differences between AF prevalence in men and women with concomitant diabetes in equivalent age groups (Additional file 2: Table S2).
Comorbidities
When compared to those without DM, participants with AF and DM+ had a higher prevalence of following comorbidities: acute coronary syndrome (24% ± 3% vs 14% ± 2%, p = 0.003), peripheral arterial disease (PAD) (20% ± 3% vs 14% ± 2%, p = 0.048) and hypertension (97% ± 1% vs 81% ± 2%, p < 0.001). Furthermore, they were less physically active (29% ± 3% vs 44% ± 3%, p < 0.001) and significantly more obese (55% ± 3% vs 31% ± 2%, p < 0.001). A comparison of concomitant diseases between participants with AF from DM+ and DM- study groups is shown in Additional file 2: Table S1.
Multivariate analyses
Multivariate regression analysis demonstrated that independent risk factors for AF differed between patients with and without diabetes. Apart from shared risk factors in both groups, thyroid disease (OR 1.99, 95% CI 1.38–2.87, p < 0.001), peripheral or systemic thromboembolism (OR 1.92, 95% CI 1.28–2.87, p = 0.002), prior percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) (OR 0.23, 95% CI 0.15–0.35, p < 0.001), hypertension (OR 2.16, 95% CI 1.27–3.68, p = 0.005), physical activity (OR 0.74, 95% CI 0.57–0.96, p = 0.021) were independently associated with prevalent AF in the DM+ population, unlike in DM− group.
Similar analysis showed that thyroid disease (OR 2.22, 95% CI 1.30–3.78, p = 0.004), peripheral or systemic thromboembolism (OR 1.89, 95% CI 1.13–3.15, p = 0.015), prior PCI or CABG (OR 0.31, 95% CI 0.17–0.58, p < 0.001), hypertension (OR 2.84, 95% CI 1.26–6.38, p = 0.012) and BMI > 30 (OR 1.49, 95% CI 1.03–2.17, p = 0.036) were independent risk factors for SAF in diabetic population (Table 4).
Discussion
In this prospective cross-sectional observational study, our principal findings are as follows: (i) we found a higher AF prevalence when diabetes was present; (ii) subjects with DM are more likely to have silent, asymptomatic AF; and (iii) DM patients were more commonly associated with persistent and permanent AF, and (iv) independent risk factors for AF incidence may vary in patients with concomitant DM comparing to the general population.
To the best of our knowledge, this is the first prospective study on AF prevalence in patients with DM, which, based on a comprehensive epidemiological methodology, was conducted on a randomly selected cohort. Unlike prior surveys based mainly on registries or cohort studies, the current study was based on prolonged non-invasive continuous ECG monitoring with a mean monitoring time span of almost 22 days. The data were transmitted remotely to the cardiovascular centres and analyzed by qualified medical professionals, resulting in a more accurate investigation. Hence, our novel finding is that 1 out of 4 Polish subjects aged ≥ 65 years with concomitant diabetes has AF. Also, diabetic patients are at a substantially higher risk of AF comparing to non-DM subjects.
AF prevalence has been reported in around 1–4% of the general European population [25]. The intimate association between AF and DM has been previously reported. The Framingham Heart Study demonstrated a 40% increase in the AF incidence among patients with concomitant DM [26]. A study of nearly 846 thousand patients from Veterans Health Administration Hospitals revealed a significantly higher AF prevalence in DM patients vs the control group without this metabolic disorder (14.9% vs 10.3%, p < 0.001) [15]. Similar results were also obtained by Huxley et al. in a case-control study on a cohort of over 100 thousand subjects [17]. Finally, a systematic review based on 32 studies and over 10 million participants found a 28% higher risk of developing AF among patients with diabetes [27]. Many of these studies have been based on ‘one off’ ECG recordings, and few studies have used prolonged ECG monitoring.
Furthermore, 9% of the Polish population with coexisting DM was diagnosed with asymptomatic AF. Even short runs of SAF may increase the risk of stroke and should not be ignored [28, 29]. Indeed, the vast majority of diabetes patients aged ≥ 65 would benefit from oral anticoagulation, and Chao et al. reported that the age threshold for initiating oral anticoagulation was 50 years in an AF patient with diabetes as a single risk factor [30]. Hence, long-term monitoring plays a pivotal role in stroke prevention, which is often the first arrhythmia symptom, and the whole population age ≥ 50 with concomitant DM should be actively screened for AF, even opportunistically when they attend clinic check-ups.
Nonetheless, the associations between DM and AF have been subject to debate and controversy [31]. Although the precise pathophysiological and clinical mechanisms are still not completely understood, there seems to be a multifactorial and bidirectional influence, including atrial structural and electrical remodelling as well as autonomic regulation [32].
The Danish population-based registry studies have either pointed out that the DM occurrence did not elevate the risk of AF incidence or that the association between AF and DM was only evident among the obese [33, 34]. Furthermore, the impact of sex on incident AF also seems to be unclear [19, 35]. In our study among DM patients, there was no significant influence of sex on AF prevalence.
The current study confirms prior observations referring to a higher number of comorbidities in the AF population with diabetes versus those without. Although there are multiple reports investigating AF risk factors in the general population, analyses evaluating independent AF risk factors in diabetic patients are lacking. Hence, we conducted a multivariate analysis, which indicated that the risk factors for the arrhythmia incidence might differ in subjects with concomitant DM compared to the general population. In contrast to the entire population, in individuals burdened by DM, comorbidities such as hypertension, PAD, obesity, or thromboembolism seem to play a pivotal role in AF development. The results are compliant with the Swedish National Diabetes Register report, which emphasized the independent association of elevated blood pressure, increased BMI, and heart failure in AF development [36]. These outcomes underline that DM should not be treated as a separate disease entity but need to be considered a complex syndrome including hypertension, dyslipidaemia or thromboembolic complications. Therefore, relevant efforts should be undertaken in the holistic management of AF patients with DM.
Strengths and limitations
As far as we are aware, this is the first observational and epidemiological study evaluating the AF prevalence in patients with concomitant DM using a nationwide, representative population sample. Furthermore, all visits and procedures conducted during the study were taken at the subject’s home; hence, even disabled and critically ill individuals were eligible to take part. Our study is also one of the few surveys using long term ECG monitoring and the first-ever, which enrolled randomly selected participants from the general population. These facts contribute significantly to objectivity and reduce possible bias. Furthermore, we analyzed independent AF risk factors in the diabetic population, which is novel and seems to be relevant in the holistic management of diabetic subjects in everyday clinical practice.
However, the study also has some limitations. Although the participants' selection was at random manner, the response rate was modest, which could possibly influence a selection bias. Nonetheless, due to the fact that presumably healthier subjects are more likely not to respond, the response rates in the study probably might be underestimated than overestimate AF prevalence. Moreover, the limited number of elderly participants with concomitant DM and AF might impact a possible bias in the analyses. Each of the study participants had blood tests taken, including fasting plasma glucose and HbA1c levels, so it is less unlikely that the population may have included a few participants with DM who remain undiagnosed. Finally, the current study is based on a nationwide representative sample from the Polish population. Therefore, the results reflect this particular population and can be directly applied only to Polish inhabitants, mainly Caucasians, who were ethnically homogenous, with universal access to healthcare.
Conclusions
AF affects 1 out of 4 subjects with concomitant DM. The higher prevalence of AF and SAF among DM subjects compared to those without DM highlights the necessity of active AF screening and evaluation of specific AF risk factors amongst the diabetic population.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AF:
-
Atrial fibrillation
- BMI:
-
Body mass index
- CABG:
-
Coronary artery bypass graft surgery
- CHA2DS2-VASc:
-
Clinical prediction score for stroke risk evaluation in patients with atrial fibrillation (congestive heart failure, hypertension, age≥75, diabetes mellitus, prior stroke or thromboembolism, vascular disease, age 65–74, sex category)
- DM:
-
Diabetes mellitus
- ECG:
-
Electrocardiography
- HbA1c:
-
Haemoglobin A1c
- PAD:
-
Peripheral arterial disease
- PCI:
-
Percutaneous coronary intervention
- SAF:
-
Silent atrial fibrillation
- SD:
-
Standard deviation
- 95% CI:
-
95% confidence interval
References
Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–47.
Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–20.
Krijthe BP, Kunst A, Benjamin EJ, Lip GYH, Franco OH, Hofman A, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–51. https://doi.org/10.1093/eurheartj/eht280.
Andersson T, Magnuson A, Bryngelsson I-L, Frøbert O, Henriksson KM, Edvardsson N, et al. All-cause mortality in 272 186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long-term case–control study. Eur Heart J. 2013;34:1061–7.
Thrall G, Lane D, Carroll D, Lip GYH. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119:448.
Thrall G, Lip GYH, Carroll D, Lane D. Depression, anxiety, and quality of life in patients with atrial fibrillation. Chest. 2007;132:1259–64.
Charitakis E, Barmano N, Walfridsson U, Walfridsson H. Factors predicting arrhythmia-related symptoms and health-related quality of life in patients referred for radiofrequency ablation of atrial fibrillation. JACC Clin Electrophysiol. 2017;3:494–502.
Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14:627–8.
Sposato LA, Cipriano LE, Saposnik G, Vargas ER, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14:377–87.
Guo Y, Lane DA, Wang L, Chen Y, Lip GYH, Eckstein J, et al. Mobile Health (mHealth) technology for improved screening, patient involvement and optimising integrated care in atrial fibrillation: the mAFA (mAF-App) II randomised trial. Int J Clin Pract. 2019;73:e13352.
Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019;381:1909–17.
Kalarus Z, Balsam P, Bandosz P, Grodzicki T, Kazmierczak J, Kiedrowicz R, et al. NOninvasive monitoring for early detection of atrial fibrillation: rationale and design of the NOMED-AF study. Kardiol Pol. 2018;76:1482–5.
Allan V, Honarbakhsh S, Casas J-P, Wallace J, Hunter R, Schilling R, et al. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? Thromb Haemost. 2017;117:837–50. https://doi.org/10.1160/TH16-11-0825.
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. https://doi.org/10.1016/j.diabres.2018.02.023.
Movahed MR, Hashemzadeh M, Mazen Jamal M. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105:315–8.
Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonso A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;159:850–6.
Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62.
Huxley RR, Alonso A, Lopez FL, Filion KB, Agarwal SK, Loehr LR, et al. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart. 2012;98:133–8.
Nichols GA, Reinier K, Chugh SS. Independent contribution of diabetes to increased prevalence and incidence of atrial fibrillation. Diabetes Care. 2009;32:1851–6.
Chatterjee NA, Chae CU, Kim E, Moorthy MV, Conen D, Sandhu RK, et al. Modifiable risk factors for incident heart failure in atrial fibrillation. JACC Heart Fail. 2017;5:552–60.
Sun G, Ma M, Ye N, Wang J, Chen Y, Dai D, et al. Diabetes mellitus is an independent risk factor for atrial fibrillation in a general Chinese population. J Diabetes Investig. 2016;7:791–6.
Schoen T, Pradhan AD, Albert CM, Conen D. Type 2 diabetes mellitus and risk of incident atrial fibrillation in women. J Am Coll Cardiol. 2012;60:1421–8.
Association American Diabetes. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetesd2019. Diabetes Care. 2019;42:S13-28.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323.
Rahman F, Kwan GF, Benjamin EJ. Erratum: Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2016;13:501–501.
Kannel W, Wolf P, Benjamin E, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N-9N.
Aune D, Feng T, Schlesinger S, Janszky I, Norat T, Riboli E. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: A systematic review and meta-analysis of cohort studies. J Diabetes Complicat. 2018;32:501–11.
Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–86. https://doi.org/10.1056/NEJMoa1313600.
Gladstone DJ, Dorian P, Spring M, Panzov V, Mamdani M, Healey JS, et al. Atrial premature beats predict atrial fibrillation in cryptogenic stroke. Stroke. 2015;46:936–41.
Chao T-F, Lip GYH, Lin Y-J, Chang S-L, Lo L-W, Hu Y-F, et al. Age threshold for the use of non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with atrial fibrillation: insights into the optimal assessment of age and incident comorbidities. Eur Heart J. 2019;40:1504–14.
Şerban RC, Scridon A. Data linking diabetes mellitus and atrial fibrillation—How strong is the evidence? From epidemiology and pathophysiology to therapeutic implications. Can J Cardiol. 2018;34:1492–502.
Tadic M, Cuspidi C. Type 2 diabetes mellitus and atrial fibrillation: From mechanisms to clinical practice. Arch Cardiovasc Dis. 2015;108:269–76.
Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: The Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–95.
Aune D, Sen A, Schlesinger S, Norat T, Janszky I, Romundstad P, et al. Body mass index, abdominal fatness, fat mass and the risk of atrial fibrillation: a systematic review and dose–response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32:181–92.
Méndez-Bailón M, Muñoz-Rivas N, Jiménez-García R, Esteban-Hernández J, Hernández-Barrera V, de Miguel-Yanes JM, et al. Impact of type 2 diabetes mellitus in hospitalizations for atrial fibrillation in Spain (2004–2013). Int J Cardiol. 2016;221:688–94.
Zethelius B, Gudbjörnsdottir S, Eliasson B, Eeg-Olofsson K, Svensson AM, Cederholm J. Risk factors for atrial fibrillation in type 2 diabetes: report from the Swedish National Diabetes Register (NDR). Diabetologia. 2015;58:2259–68. https://doi.org/10.1007/s00125-015-3666-9.
Acknowledgements
J Gumprecht was supported by the Polish Cardiac Society Club 30 Specialized Research Fellowship Grant for Early Career Researchers and Polpharma Scientific Foundation.
Funding
The research has received funding from the National Centre for Research and Development under grant agreement (STRATEGMED2/269343/18/NCBR/2016).
Author information
Authors and Affiliations
Contributions
JG: substantial contribution to the conception, design and interpretation of data; has drafted the manuscript, GYHL: substantial contribution to the conception, analysis and manuscript revision. AS, KM, ŁW, AR, MR, TZ, TG, JK, GO: contribution in data interpretation. BŚ: contribution to the conception. JS: substantial contribution to the analysis and manuscript revision. ZK: substantial contribution to the conception, design and manuscript revision. All authors have approved the submitted version and have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Signed, informed consent was obtained from each eligible participant of the trial in accordance with protocol regulations approved by the local review boards governing research involving human subjects and local bioethical committee (26/2015), and the Declaration of Helsinki. The trial was registered on clinicaltrials.gov (NCT03243474).
Consent for publication
Not applicable.
Competing interests
Gregory Y. H. Lip – Consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally. Other authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
NOMED-AF – study profile.
Additional file 2: Table S1.
Comparison of AF- patients’ comorbidities between DM- and DM+ groups. Table S2. Odds ratio of atrial fibrillation prevalence in Polish population with concomitant diabetes mellitus in correlation to age and gender.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gumprecht, J., Lip, G.Y.H., Sokal, A. et al. Relationship between diabetes mellitus and atrial fibrillation prevalence in the Polish population: a report from the Non-invasive Monitoring for Early Detection of Atrial Fibrillation (NOMED-AF) prospective cross-sectional observational study. Cardiovasc Diabetol 20, 128 (2021). https://doi.org/10.1186/s12933-021-01318-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-021-01318-2