Abstract
Background
Given the rising prevalence of dysglycemia and disparities in heart failure (HF) burden, we determined race- and sex-specific lifetime risk of HF across the spectrum of fasting plasma glucose (FPG).
Methods
Individual-level data from adults without baseline HF was pooled from 6 population-based cohorts. Modified Kaplan–Meier analysis, Cox models adjusted for the competing risk of death, and Irwin’s restricted mean were used to estimate the lifetime risk, adjusted hazard ratio (aHR), and years lived free from HF in middle-aged (40–59 years) and older (60–79 years) adults with FPG < 100 mg/dL, prediabetes (FPG 100–125 mg/dL) and diabetes (FPG ≥ 126 mg/dL or on antihyperglycemic agents) across race-sex groups.
Results
In 40,117 participants with 638,910 person-years of follow-up, 4846 cases of incident HF occurred. The lifetime risk of HF was significantly higher among middle-aged White adults and Black women with prediabetes (range: 6.1% [95% CI 4.8%, 7.4%] to 10.8% [95% CI 8.3%, 13.4%]) compared with normoglycemic adults (range: 3.5% [95% CI 3.0%, 4.1%] to 6.5% [95% CI 4.9%, 8.1%]). Middle-aged Black women with diabetes had the highest lifetime risk (32.4% [95% CI 26.0%, 38.7%]) and aHR (4.0 [95% CI 3.0, 5.4]) for HF across race-sex groups. Middle-aged adults with prediabetes and diabetes lived on average 0.9–1.6 and 4.1–6.0 fewer years free from HF, respectively. Findings were similar in older adults except older Black women with prediabetes did not have a higher lifetime risk of HF.
Conclusions
Prediabetes was associated with higher lifetime risk of HF in middle-aged White adults and Black women, with the association attenuating in older Black women. Black women with diabetes had the highest lifetime risk of HF compared with other race-sex groups.
Similar content being viewed by others
Introduction
Diabetes is a major risk factor for heart failure (HF) with multiple observational studies demonstrating a two- to fourfold greater risk in middle-aged and older adults [1,2,3,4,5,6]. In contrast, risk of HF in adults with prediabetes has not been firmly established as some studies have shown an increased risk while other studies in older and Black adults have not [7,8,9,10,11,12]. A better understanding of HF risk across the spectrum of dysglycemia is needed given the link between the rising prevalence of prediabetes and diabetes and the growing burden of HF. As of 2018, approximately 88 million US adults had prediabetes and 34 million US adults had diabetes[13]; while more than 8 million US adults are expected to develop HF by 2030 [14].
Comprehensive risk assessment that includes lifetime risk and years lived free from HF would enhance communication about the overall risk and impact of prevention to patients, physicians, and public health officials [15, 16]. Furthermore, incorporating the competing risk of death from non-HF causes is essential when estimating lifetime risk of HF because both prediabetes and diabetes are associated with other life-limiting conditions; not accounting for this competing risk results in systematic overestimation of risk [17]. Lifetime risk may also provide a more accurate risk estimate as prediabetes represents an early stage of insulin resistance and clinical cardiac effects may only manifest after a prolonged period of follow-up. Importantly, there are also notable race-sex disparities in HF incidence and outcomes, which may be influenced by diabetes status [18,19,20]. Age is another important factor in determining risk as evidence suggests that older adults are more likely to revert to normoglycemia [21]. Therefore, a comprehensive assessment of HF risk across the spectrum of dysglycemia stratified by age, sex, and race can help understand potential disparities and inform effective implementation of HF prevention strategies such as aggressive risk factor modification and use of sodium-glucose transporter 2 (SGLT2) inhibitors [22].
We pooled and harmonized data from 6 contemporary population-based cohorts in the Lifetime Risk Pooling Project (LRPP) to estimate: (1) lifetime risks of HF in adults with normal fasting plasma glucose (FPG), prediabetes, and diabetes according to race and sex, after adjusting for the competing risk of non-HF death, (2) years lived free of and with HF in the context of overall survival, and (3) competing adjusted HRs for HF and non-HF death. Analyses were performed in both middle-aged and older adults given the growing prevalence of prediabetes and diabetes with age [13].
Methods
Study cohorts
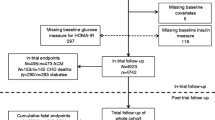
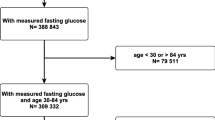
The LRPP dataset has been previously described in detail [23]. Briefly, it consists of pooled individual-level data from multiple population-based cohorts in the US. We included 40,117 White and Black participants free from baseline HF or atherosclerotic cardiovascular disease (CVD) from the following 6 cohorts: Atherosclerosis Risk in Communities Study, Cardiovascular Health Study, Coronary Artery Risk Development in Young Adults Study, Framingham Heart Study starting from 1985, Framingham Offspring Cohort Study starting from 1985, and Multi-Ethnic Study of Atherosclerosis. These cohorts represent contemporary longitudinal studies with available measures of fasting plasma glucose (FPG), other major cardiovascular risk factors, adjudication of HF, and essentially complete follow up for vital status. All data were de-identified, and all study protocols and procedures were approved by the Institutional Review Board at Northwestern University with a waiver for informed consent.
Demographics and cardiovascular risk factor ascertainment
We categorized participants with baseline FPG levels according to two index age groups—middle age adults between ages 40 and 59 years and older adults between ages 60 and 79 years. We also stratified participants by self-reported race/sex groups for White and Black men and women; we excluded participants of non-Black and non-White self-reported race due to small sample sizes. FPG was measured using validated methods specific to each cohort, as has been previously described [24]. Normal FPG was defined as < 100 mg/dL. Prediabetes was defined as FPG of 100–125 mg/dL and diabetes was defined as FPG of ≥ 126 mg/dL, self-reported physician diagnosis of diabetes or use of anti-hyperglycemic agents. Demographics including age, sex, and race were self-reported. Blood pressure, weight, and height were measured by trained clinical staff and a fasting lipid profile was obtained. The use of medications and smoking status were self-reported. Current smokers were defined as those who had smoked cigarettes within a year of the examination. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or use of antihypertensive agents. Hyperlipidemia was defined as total cholesterol ≥ 200 mg/dL or use of lipid-lowering agents.
HF definition and adjudication
Adjudication criteria for incident HF were specific to each cohort and detailed descriptions are provided in Additional file 1: Additional methods. For death events, some cohorts used linkage to the National Death Index to determine the underlying cause of death from death certificate data while other cohorts adjudicated the cause of death by reviewing medical records and autopsy data, when available. Vital status was known for 98% of the pooled cohort.
Statistical analysis
Lifetime risk of HF was estimated using a modified Kaplan–Meier analysis, which accounts for the competing risk of non-HF related death. In this analysis, fatal events before incident HF were treated as a separate endpoint rather than a censoring event, which reduces overestimation of risk. Lifetime risk of HF for each race-sex group was calculated to age 80 years in the middle-aged index group and to age 90 years in the older index group. We calculated the competing cumulative incidences of HF events compared with non-HF death as the first event during follow-up. The Fine and Gray method was used to estimate the competing hazards for incident HF and non-HF death by FPG status for each race-sex group [25]. Adults with normoglycemia were used as the reference group and the analysis was adjusted for age, BMI, hypertension, smoking status, and hyperlipidemia. The mean survival time or years lived free from HF, with HF, and overall were estimated using the Irwin restricted mean to compare differences in compression of morbidity by FPG status in each race-sex group [16, 26].
All statistical analyses were performed with SAS version 9.2 (SAS Institute) and R version 3.1.2 (The R Foundation).
Results
Baseline characteristics
The pooled cohort was stratified by race, sex, and index age. Baseline characteristics of middle-aged (index age 40–59) and older adults (index age 60–79) are described in Table 1 and Additional file 1: Table S1, respectively. A total of 638,910 person-years of follow-up was included in this study. Among middle-aged adults, the prevalence of prediabetes was lowest in White women (20%) and highest in White men (34%). The prevalence of diabetes was higher in Black men (12.0%) and women (11.9%) compared with White men (5.8%) and women (4.3%). Black men and women also had a higher prevalence of hypertension (40% and 44%, respectively) compared with White men and women (24% and 22%, respectively). Similar trends for diabetes and other cardiovascular risk factors were observed in older adults across the race-sex groups.
Lifetime risk of heart failure by FPG status
During the follow up period, there were a total of 1507 and 3339 cases of incident HF in middle-aged and older adults, respectively. The unadjusted HF event rates were higher in middle-aged Black men and women compared with middle-aged White men and women (Additional file 1: Table S2). As expected, unadjusted HF event rates were higher in older adults compared with middle-aged adults.
The lifetime risk of HF after adjusting for the competing risk of non-HF death stratified by FPG status across race-sex groups is shown in Fig. 1 for middle-aged adults and Additional file 1: Figure S1 for older adults. In middle-aged adults, lifetime risk of HF with prediabetes compared with normal FPG was significantly higher in White men ([7.9%, 95% CI 6.2, 9.7] vs. [4.5%, 95% CI 3.7, 5.3]), White women ([6.1%, 95% CI 4.8, 7.4] vs. [3.5%, 95% CI 3.0, 4.1]), and Black women ([10.8%, 95% CI 8.3, 13.4] vs. [6.5%, 95% CI 4.9, 8.1]). In middle-aged Black men, the lifetime risk with prediabetes compared with normal FPG was not significantly higher ([14.4%, 95% CI 9.6, 19.2] vs. [11.7%, 95% CI 9.1, 14.3]). In older adults, lifetime risk of HF with prediabetes compared with normal FPG was significantly higher in White men ([13.5%, 95% CI 12.0, 14.9] vs. [10.4%, 95% CI 9.2%, 11.6%]) and women ([12.2%, 95% CI 10.8%, 13.6%] vs. [7.8%, 95% CI 6.9%, 8.7%]) but not in Black men ([12.2%, 95% CI 9.0%, 15.3%] vs. [14.6%, 95% CI 11.6%, 17.6%]) or women ([13.5%, 95% CI 10.1%, 16.8%] vs [11.7%, 95% CI 9.4%, 14.0%]). The lifetime risk of HF with diabetes compared with prediabetes or normal FPG was significantly higher in all race-sex groups regardless of age (Additional file 1: Table S3).
Lifetime risk of heart failure among middle-aged adults (index age 40–59 years) across fasting plasma glucose categories stratified by race and sex. Lifetime risk estimates for heart failure (HF) after adjusting for competing risk of non-heart failure death in middle-aged (index age, 40–59 years) Black and White men (a, b) and women (c, d) stratified by fasting plasma glucose (FPG) categories. Lifetime risk for HF was greater in participants with prediabetes or diabetes than those with normal FPG in all groups
When comparing between race-sex groups, the lifetime risk of HF with prediabetes was significantly higher in middle-aged Black men and women compared with middle-aged White men and women, respectively. But there were no significant race-sex disparities in the lifetime risk of HF in older adults with prediabetes. The lifetime risk of HF with diabetes was significantly higher in middle-aged Black women (32.4%, 95% CI 26.0%, 38.7%) than middle-aged White women (16.2%, 95% CI 11.3%, 21.0%). In contrast, the lifetime risk of HF in middle-aged men with diabetes was not different between Black and White men.
Years lived free of heart failure
Mean years lived free from and with HF for middle-aged adults in each race-sex group stratified by FPG status are provided in Fig. 2 and Additional file 1: Table S4. White men and women with prediabetes lived on average 1.2 and 1.6 fewer years free from HF than White men and women with normal FPG. Black women with prediabetes lived on average 1.4 fewer years free from HF than those with normal FPG. Although not meeting significance, Black men with prediabetes lived on average 0.9 fewer years free from HF than those with normal FPG. In contrast, years lived free from HF were not significantly lower in older adults with prediabetes than normal FPG (Additional file 1: Figure S2 and Table S5). This was the case across all race-sex groups except in White women with prediabetes, who lived on average 0.8 fewer years free from HF than those with normal FPG. Adults with diabetes lived significantly fewer years free from HF, ranging from 4.1 to 6.0 years across race-sex groups, compared with adults with normal FPG. Similar trends were observed in the older adults with diabetes.
Years lived free from and with heart failure among middle-aged adults (index age 40–59 years) stratified by fasting plasma glucose status. Mean years lived free from and with heart failure (HF) in middle-aged (index age, 40–59 years) Black and White men (a, b) and women (c, d) across categories of fasting plasma glucose (FPG). Prediabetes was defined as FPG of 100–125 mg/dL and diabetes defined FPG ≥ 126 mg/dL. Participants with normal FPG (< 100 mg/dL) lived more years free from HF compared with participants with prediabetes or diabetes. Years lived with HF was greater in participants with diabetes than those with normal FPG
Competing risks of heart failure and non-heart failure mortality by FPG status
We next determined competing hazard ratios for incident HF and non-HF death in adults with prediabetes and diabetes for each race-sex group after adjusting for other risk factors (Table 2). Given that non-HF death encompasses non-HF CVD death and non-CVD death, we provided hazard ratios for both of those competing risks. Adults with normal FPG served as the reference group. In middle-aged adults with prediabetes, the HR of HF was significantly higher in White men (HR 1.39, 95% CI 1.15, 1.69) and women (HR 1.29, 95% CI 1.04, 1.59) as well as Black women (HR 1.59, 95% CI 1.19, 2.13) compared with normal FPG. While older White men and women with prediabetes also had a significantly greater HR of HF, older Black women with prediabetes did not (Additional file 1: Table S6). Black men with prediabetes did not have a significantly greater HR of HF in middle-age (HR 1.15, 95% CI 0.84, 1.57) or older age (HR 0.89, 95% CI 0.65, 1.21). The competing risk-adjusted HR for incident HF in adults with diabetes was significantly higher than those with normal FPG across all race-sex groups. Similar patterns were observed in older adults with diabetes. Finally, we also evaluated the cumulative incidence of HF as the first event (vs. non-HF death). The cumulative incidence of HF was highest in middle-aged Black women with prediabetes (19.2%) and diabetes (44.8%). The cumulative incidence of HF as the first event was greater with worsening dysglycemia across all race-sex groups (Additional file 1: Table S7).
Discussion
In an analysis of 40,117 participants across six cohorts, we quantified lifetime risk of HF in White and Black middle-aged and older adults across the clinical spectrum of glycemia, including prediabetes. We observed (1) higher lifetime risk of HF in middle-aged White adults and Black women with prediabetes but the risk attenuated in older Black women with prediabetes and (2) significant disparities in the risk of HF by race and sex, specifically a disproportionate burden on middle-aged Black women with diabetes. These findings have important implications given the growing prevalence of prediabetes and diabetes as well as a recent rise in HF-related mortality with significant race-sex differences in all three outcomes.
Prior studies have primarily focused on short-term risk of incident HF in adults with prediabetes and have yielded inconsistent results across age and race [7,8,9,10,11,12]. Our large, pooled cohort allowed us to quantify the association with HF over a longer time horizon and stratify by age, race, and sex. We observed that middle-aged adults with prediabetes had a higher lifetime risk of incident HF and lived fewer years free of HF, on average, than normoglycemic adults. This difference was observed in all race-sex groups except in middle-aged Black men with prediabetes, where the difference did not meet significance, but the trend was similar. Our findings can likely be explained by two, not mutually exclusive, mechanisms. First, cumulative exposure to glucose levels in the prediabetes range during middle-age and beyond may contribute to cardiac dysfunction and development of HF. This explanation is supported by mechanistic and clinical studies demonstrating direct and indirect myocardial effects of insulin resistance and hyperglycemia on myocardial energetics, fibrosis, and subclinical cardiac dysfunction [27,28,29]. Second, middle-aged adults with prediabetes are more likely to go on to develop diabetes later in life, which results in greater lifetime risk of HF [30]. This contrasts with our findings in older Black women with prediabetes, who did not have a significantly higher lifetime risk of HF. These findings suggest that onset of prediabetes earlier in life is an important HF risk factor in this group that may reflect significant underlying insulin resistance whereas onset of prediabetes later in life may be a more benign manifestation of aging that does not increase the risk of HF in the setting of other competing risks. Further studies are needed to evaluate trends of FPG over time and the association with HF.
These results have significant public health importance given that 88 million US adults in 2018 had prediabetes, with over 63 million of them being under the age of 65 years [13]. Currently the recommendations for therapeutic intervention with metformin in adults with prediabetes are limited to those with additional risk factors (e.g. age less than 60 years, morbid obesity or history of gestational diabetes) [31]. Despite these recommendations, less than 1% of US adults with prediabetes are on metformin [32]. Our results suggest that targeted efforts on HF prevention should be an important aspect of management in adults with prediabetes. Studies are needed to determine if therapies such as SGLT2 inhibitors and intensive blood pressure reduction that have been successful in preventing HF in adults with diabetes will provide a similar benefit in prediabetes [22, 33].
Importantly, our findings can also help convey the importance to patients of early intervention in prediabetes and prevention of diabetes. Both lifetime risk and years lived free from HF are impactful and easy to understand metrics that can help empower patients. It shifts the focus to HF prevention and allows physicians to effectively communicate the benefit of maintaining normal FPG in the context of how many more healthy years the patient may live. Patient and community engagement will be essential in improving healthy life expectancy. Evidence-based lifestyle interventions focused on diet and exercise, such as the Diabetes Prevention Program (DPP) have been shown to reduce the incidence of diabetes in clinical trials and have become the basis of a national partnership of public and private organizations that provide this resource to adults across the country [34, 35]. Expanding access to programs such as DPP with in-person and virtual platforms focused on primordial prevention of diabetes will be key in preventing HF and improving overall cardiovascular health in the US.
Our results also underscore the disparities observed in the context of the relationship between dysglycemia and HF, as middle-aged Black women with diabetes had a relatively higher lifetime risk of HF compared with other groups. There is growing appreciation that this discrepancy is driven, in large part, by underlying social determinants of health, which also contribute to other shared co-morbidities. Black women face racial and gender-based discrimination, socioeconomic adversity, and are at higher risk for developing prediabetes and diabetes after gestational diabetes [36, 37]. These avoidable inequities fuel cardiovascular health disparities by limiting access to health care and creating an environment of chronic mental and physical stress [38]. Underestimation of CVD risk in younger Black women also contributes to disparities, further emphasizing the importance of providing race and sex specific risk estimates [39]. Thus, programs focused on prevention must be sensitive to the barriers and needs within the community and be able to tailor their content and services accordingly. A novel example of this was the blood pressure reduction program focused on Black men throughout barbershops across Los Angeles County [40]. Similar innovative programs are needed to slow the rising burden of HF in Black women with prediabetes and diabetes.
Study limitations
Our study has potential limitations. A single measure of FPG was used as the primary criteria for determining diabetes status, which may result in misclassification, as participants may change categories of glycemic control during follow-up and this may be differ by race and sex. However, if present this bias would likely result in attenuation of the association and prior investigation has demonstrated good correlation between FPG and hemoglobin A1c in these cohorts [24]. While pooling multiple cohorts provides a larger sample size and improves generalizability, there can be potential for heterogeneity and cohort effects. Although treatment practices and prevalence of prediabetes and diabetes may differ between cohorts and over time, the association with incident HF was similar across cohorts and time periods. The differences between incident HF risk by glycemic status may be driven by other cardiometabolic risk factors such as BMI. Thus, the analyses were adjusted for age, BMI, HTN, HLD, and smoking status.
Conclusions
We pooled six longitudinal cohort studies to provide race- and sex-specific estimates of the lifetime risk of HF across different categories of FPG in middle-aged and older adults. Our results highlight the role of prediabetes as an independent risk factor for lifetime risk of HF, specifically in middle-aged adults. We also demonstrated important race-sex disparities, as middle-aged Black women with diabetes had the highest lifetime risk, cumulative incidence, and adjusted HR for HF. Given the emergence of novel pharmacological diabetes therapies, such as SGLT2 inhibitors, our findings support future studies examining the benefit of SGLT2 inhibitors earlier in the dysglycemia spectrum for the prevention of HF. Mitigation of lifetime burden of HF will require innovative and culturally tailored prevention programs to target those at greatest risk with impaired FPG.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HF:
-
Heart failure
- LRPP:
-
Lifetime Risk Pooling Project
- HR:
-
Hazard ratio
- CVD:
-
Cardiovascular disease
- FPG:
-
Fasting plasma glucose
- SGLT2:
-
Sodium-glucose transporter 2
- CI:
-
Confidence interval
- DPP:
-
Diabetes prevention program
References
Barzilay JI, Kronmal RA, Gottdiener JS, Smith NL, Burke GL, Tracy R, et al. The association of fasting glucose levels with congestive heart failure in diabetic adults > or =65 years: the cardiovascular health study. J Am Coll Cardiol. 2004;43(12):2236–41. https://doi.org/10.1016/j.jacc.2003.10.074.
Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159(11):1197–204. https://doi.org/10.1001/archinte.159.11.1197.
Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27(8):1879–84. https://doi.org/10.2337/diacare.27.8.1879.
Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241(19):2035–8. https://doi.org/10.1001/jama.241.19.2035.
He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996–1002. https://doi.org/10.1001/archinte.161.7.996.
Ahmad FS, Ning H, Rich JD, Yancy CW, Lloyd-Jones DM, Wilkins JT. Hypertension, obesity, diabetes, and heart failure-free survival: the cardiovascular disease lifetime risk pooling project. JACC Heart Fail. 2016;4(12):911–9. https://doi.org/10.1016/j.jchf.2016.08.001.
Held C, Gerstein HC, Yusuf S, Zhao F, Hilbrich L, Anderson C, et al. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation. 2007;115(11):1371–5. https://doi.org/10.1161/circulationaha.106.661405.
Matsushita K, Blecker S, Pazin-Filho A, Bertoni A, Chang PP, Coresh J, et al. The association of hemoglobin a1c with incident heart failure among people without diabetes: the atherosclerosis risk in communities study. Diabetes. 2010;59(8):2020–6. https://doi.org/10.2337/db10-0165.
Nielson C, Lange T. Blood glucose and heart failure in nondiabetic patients. Diabetes Care. 2005;28(3):607–11. https://doi.org/10.2337/diacare.28.3.607.
Deedwania P, Patel K, Fonarow GC, Desai RV, Zhang Y, Feller MA, et al. Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: findings from a population-based cohort study. Int J Cardiol. 2013;168(4):3616–22. https://doi.org/10.1016/j.ijcard.2013.05.038.
Hubbard D, Colantonio LD, Tanner RM, Carson AP, Sakhuja S, Jaeger BC, et al. Prediabetes and risk for cardiovascular disease by hypertension status in black adults: the jackson heart study. Diabetes Care. 2019;42(12):2322–9. https://doi.org/10.2337/dc19-1074.
Banerjee D, Biggs ML, Mercer L, Mukamal K, Kaplan R, Barzilay J, et al. Insulin resistance and risk of incident heart failure: cardiovascular health study. Circ Heart Fail. 2013;6(3):364–70. https://doi.org/10.1161/circheartfailure.112.000022.
Prevention. CfDCa. National diabetes statistics report: estimates of diabetes and its burden in the United States. 2020.
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56–528. https://doi.org/10.1161/cir.0000000000000659.
Karmali KN, Lloyd-Jones DM. Adding a life-course perspective to cardiovascular-risk communication. Nat Rev Cardiol. 2013;10(2):111–5. https://doi.org/10.1038/nrcardio.2012.185.
Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308(17):1795–801. https://doi.org/10.1001/jama.2012.14312.
Abdel-Qadir H, Fang J, Lee DS, Tu JV, Amir E, Austin PC, et al. Importance of considering competing risks in time-to-event analyses: application to stroke risk in a retrospective cohort study of elderly patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2018;11(7):e004580. https://doi.org/10.1161/circoutcomes.118.004580.
Glynn P, Lloyd-Jones DM, Feinstein MJ, Carnethon M, Khan SS. Disparities in cardiovascular mortality related to heart failure in the United States. J Am Coll Cardiol. 2019;73(18):2354–5. https://doi.org/10.1016/j.jacc.2019.02.042.
Chang PP, Wruck LM, Shahar E, Rossi JS, Loehr LR, Russell SD, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005–2014): ARIC study community surveillance. Circulation. 2018;138(1):12–24. https://doi.org/10.1161/CIRCULATIONAHA.117.027551.
Zareini B, Blanche P, D’Souza M, Elmegaard Malik M, Nørgaard CH, Selmer C, et al. Type 2 diabetes mellitus and impact of heart failure on prognosis compared to other cardiovascular diseases: a nationwide study. Circ Cardiovasc Qual Outcomes. 2020. https://doi.org/10.1161/circoutcomes.119.006260.
Shang Y, Marseglia A, Fratiglioni L, Welmer A-K, Wang R, Wang H-X, et al. Natural history of prediabetes in older adults from a population-based longitudinal study. J Intern Med. 2019;286(3):326–40. https://doi.org/10.1111/joim.12920.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9. https://doi.org/10.1016/s0140-6736(18)32590-x.
Wilkins JT, Karmali KN, Huffman MD, Allen NB, Ning H, Berry JD, et al. Data resource profile: the cardiovascular disease lifetime risk pooling project. Int J Epidemiol. 2015;44(5):1557–64. https://doi.org/10.1093/ije/dyv150.
Bancks MP, Ning H, Allen NB, Bertoni AG, Carnethon MR, Correa A, et al. Long-term absolute risk for cardiovascular disease stratified by fasting glucose level. Diabetes Care. 2019;42(3):457–65. https://doi.org/10.2337/dc18-1773.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. https://doi.org/10.1080/01621459.1999.10474144.
Karrison TG. Use of Irwin’s restricted mean as an index for comparing survival in different treatment groups—interpretation and power considerations. Control Clin Trials. 1997;18(2):151–67. https://doi.org/10.1016/s0197-2456(96)00089-x.
Arnlöv J, Lind L, Zethelius B, Andrén B, Hales CN, Vessby B, et al. Several factors associated with the insulin resistance syndrome are predictors of left ventricular systolic dysfunction in a male population after 20 years of follow-up. Am Heart J. 2001;142(4):720–4. https://doi.org/10.1067/mhj.2001.116957.
Arnlöv J, Lind L, Sundström J, Andrén B, Vessby B, Lithell H. Insulin resistance, dietary fat intake and blood pressure predict left ventricular diastolic function 20 years later. Nutr Metab Cardiovasc Dis. 2005;15(4):242–9. https://doi.org/10.1016/j.numecd.2004.10.002.
Fang ZY, Yuda S, Anderson V, Short L, Case C, Marwick TH. Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol. 2003;41(4):611–7. https://doi.org/10.1016/s0735-1097(02)02869-3.
Tseng E, Yeh HC, Maruthur NM. Metformin use in prediabetes among US adults, 2005–2012. Diabetes Care. 2017;40(7):887–93. https://doi.org/10.2337/dc16-1509.
American Diabetes Association. 3. Prevention or delay of type 2 diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S29–33. https://doi.org/10.2337/dc19-S003.
Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–90. https://doi.org/10.1016/s0140-6736(12)60283-9.
Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–16. https://doi.org/10.1056/NEJMoa1511939.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. https://doi.org/10.1056/NEJMoa012512.
Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the diabetes prevention program outcomes study. Lancet Diabetes Endocrinol. 2015;3(11):866–75. https://doi.org/10.1016/s2213-8587(15)00291-0.
Kalinowski J, Taylor JY, Spruill TM. Why are young black women at high risk for cardiovascular disease? Circulation. 2019;139(8):1003–4. https://doi.org/10.1161/circulationaha.118.037689.
Xiang AH, Li BH, Black MH, Sacks DA, Buchanan TA, Jacobsen SJ, et al. Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia. 2011;54(12):3016–21. https://doi.org/10.1007/s00125-011-2330-2.
American Psychological Association AWGoSaHD. Stress and health disparities: contexts, mechanisms, and interventions among racial/ethnic minority and low-socioeconomic status populations. 2017.
McSweeney JC, Rosenfeld AG, Abel WM, Braun LT, Burke LE, Daugherty SL, et al. Preventing and experiencing ischemic heart disease as a woman: state of the science: a scientific statement from the American heart association. Circulation. 2016;133(13):1302–31. https://doi.org/10.1161/cir.0000000000000381.
Victor RG, Blyler CA, Li N, Lynch K, Moy NB, Rashid M, et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139(1):10–9. https://doi.org/10.1161/circulationaha.118.038165.
Acknowledgements
The authors thank the investigators of all the cohort studies included in this analysis for their hard work and dedication in collecting the underlying data, and the study participants for their time and commitment.
Funding
Dr. Arjun Sinha is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number T32HL069771. This research was supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute (KL2TR001424) and the American Heart Association (AHA#19TPA34890060) to Dr. Sadiya Khan. The Lifetime Risk Pooling Project was supported in its inception by the National Institutes of Health/National Heart, Lung, and Blood Institute (R21 HL085375).
Author information
Authors and Affiliations
Contributions
AS designed the research study and wrote the manuscript. HN performed the analyses. FS, MB, MC, MO, NA, JW, DLJ contributed to the discussion and edited the manuscript. SK designed the research study and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All data were de-identified, and all study protocols and procedures were approved by the Institutional Review Board at Northwestern University with a waiver for informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional methods
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sinha, A., Ning, H., Ahmad, F.S. et al. Association of fasting glucose with lifetime risk of incident heart failure: the Lifetime Risk Pooling Project. Cardiovasc Diabetol 20, 66 (2021). https://doi.org/10.1186/s12933-021-01265-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-021-01265-y