Abstract
Background
Controversies exist regarding the optimal blood pressure (BP) level that is safe and provides cardiovascular protection in patients with type 2 diabetes mellitus (T2DM) and coexistent coronary artery disease. Several new glucose-lowering agents have been found to lower BP as well, making the interaction between BP and T2DM even more complex.
Methods
With the reference to recent literature, this review article describes the potential mechanisms of increased risk of hypertension in T2DM and outlines the possible optimal BP levels based upon recommendations on the management of hypertension by the current guidelines, in combination with our research findings, for type 2 diabetic patients with coronary artery disease.
Results
The development of hypertension in T2DM involves multiple processes, including enhanced sympathetic output, inappropriate activation of renin-angiotensin- aldosterone system, endothelial dysfunction induced through insulin resistance, and abnormal sodium handling by the kidney. Both AGE-RAGE axis and adipokine dysregulation activate intracellular signaling pathways, increase oxidative stress, and aggravate vascular inflammation. Pancreatic β-cell specific microRNAs are implicated in gene expression and diabetic complications. Non-pharmacological intervention with lifestyle changes improves BP control, and anti-hypertensive medications with ACEI/ARB, calcium antagonists, β-blockers, diuretics and new hypoglycemic agent SGLT2 inhibitors are effective to decrease mortality and prevent major adverse cardiovascular events. For hypertensive patients with T2DM and stable coronary artery disease, control of BP < 130/80 mmHg but not < 120/70 mmHg is reasonable, whereas for those with chronic total occlusion or acute coronary syndromes, an ideal BP target may be somewhat higher (< 140/90 mmHg). Caution is advised with aggressive lowering of diastolic BP to a critical threshold (< 60 mmHg).
Conclusions
Hypertension and T2DM share certain similar aspects of pathophysiology, and BP control should be individualized to minimize adverse events and maximize benefits especially for patients with T2DM and coronary artery disease.
Similar content being viewed by others
Background
Epidemiologic studies have shown that hypertension and type 2 diabetes mellitus (T2DM) are global public health issues and become the major cause of disease burden and mortality [1, 2]. The World Health Organization estimated that 40% of adults worldwide have hypertension (about 90% are classified with essential hypertension) [3], and approximately 422 million adults were living with diabetes(more than 90% are T2DM) [4]. In addition, hypertension is present in more than half of type 2 diabetic patients and contributes significantly to macro- and micro-vascular complications [5]. The development of T2DM is often asymptomatic and subclinical for a long period, and before diagnosis of T2DM, individuals can reside in the high-risk state of prediabetes, defined as impaired fasting glucose or impaired glucose tolerance [6, 7]. Recently, the prevalence of hypertension and T2DM is increasing in many Asian countries, with a number of countries with blood pressure (BP) and glucose above the global average [7,8,9,10,11,12]. The Chinese National Report of Cardiovascular Disease 2018 pointed out that the prevalence of hypertension and diabetes reaches 23.2% and 10.9%, respectively, leading to an estimate of about 290 million of adult people suffering from cardiovascular disease in China [9]. The major goal for cardiovascular care is to prevent morbidity and mortality by controlling glucose, normalizing BP, and reducing other cardiovascular risk factors. Data frequently suggest an existence of the relationship between BP and cardiovascular risks as low as 110–115 mmHg for systolic BP and 70–75 mmHg for diastolic BP. Every 20 mmHg systolic and 10 mmHg diastolic BP increase above the threshold has shown to double the risk of mortality from ischemic heart disease and stroke [10]. For decades, clinical practice guidelines vary in determining the optimal BP target in patients with T2DM. Whereas several guidelines recommend a BP goal of < 140/90 mmHg [13, 14], some recommend a lower target of systolic and diastolic BP in certain diabetic population [15, 16]. The newly released American College of Cardiology (ACC)/American Heart Association (AHA) Guideline for the Prevention, Detection, Evaluation, and Management of High BP in adults supports a more aggressive diagnostic and treatment approach, recommending hypertensive patients to maintain their BP < 130/80 mmHg [17]. Although the adoption of new guideline is expected to increase the prevalence of hypertension, endorsing the aggressive approach including lifestyle change and medical treatment would lead to reduced risk of major adverse cardiac events and improvement in overall clinical outcome [10, 17]. However, controversies exist regarding the optimal level of BP attained with therapeutic interventions that is safe and provides cardiovascular protection, especially in patients with T2DM and coexistent coronary artery disease [18, 19]. Furthermore, the class of drugs most appropriate for the treatment of hypertensive diabetics is also unclear and different guidelines emphasize use of different classes for anti-hypertensive treatment in type 2 diabetic patients [16]. Particularly, several new glucose-lowering agents for the treatment of diabetes have been found to lower BP as well, making the interaction between BP and T2DM even more complex [20]. In this review, we will outline the possible optimal BP levels based upon recommendations on the management of hypertension by the current guidelines, in combination with our research findings, for type 2 diabetic patients with coronary artery disease.
Mechanisms of increased risk of hypertension in type 2 diabetes
Obviously, the actual mechanism of hypertension in T2DM is complex and multi-factorial,including enhanced sympathetic output, inappropriate activation of the renin-angiotensin-aldosterone system (RAAS), oxidative stress, inflammation, insulin resistance-mediated endothelial dysfunction, and abnormal sodium handling by the kidney (Fig. 1). Nevertheless, both T2DM and hypertension share certain similar aspects of pathophysiology [21, 22].
Insulin resistance/hyperinsulinemia
Approximately 50% of hypertensive patients manifest systemic insulin resistance or hyperinsulinemia, which plays an important role in the development of both T2DM and hypertension [23]. Loss of sensitivity to insulin action principally affects glucose and lipid metabolism, e.g., sparing insulin’s action to retain sodium in the distal tubule [16, 24]. When insulin-mediated glucose uptake is reduced, the secretion of insulin is increased to maintain homeostasis. Insulin resistance or hyperinsulinemia is frequently associated with a low-grade inflammation of endothelial and smooth muscle cells in the vascular wall, which induces endothelial dysfunction, vascular stiffness, hypertrophy, fibrosis and remodeling [25]. It is also found that insulin resistance or hyperinsulinemia enhances sympathetic output and disrupts the intricate physiological balance in vascular tone and vessel growth, leading to reduced arterial compliance, a characteristic phenotype in hypertension [26]. Abundant evidence suggests that impaired endothelium-dependent vasodilation may in turn contribute to or exacerbate insulin resistance by limiting the delivery of substrate (glucose) to key target tissue [21,22,23,24,25].
Obesity/adipokines
Obesity (particularly increased visceral adiposity) is a key pathogenic factor behind the coexistence of both T2DM and hypertension [27]. There is increasing evidence that increased afferent traffic from and efferent activity to the kidney promotes the development of hypertension associated with obesity and insulin resistance [23]. Chronic low-grade inflammation and oxidative stress in the adipose tissue contributes to systemic elevation in BP, in part, through local production of components of the RAAS. Activation of angiotensin II type 1 receptor in non-adrenal tissues causes multiple intracellular events, including production of reactive oxygen species (ROS), reduced insulin metabolic signaling, and increased proliferative and inflammatory vascular responses [28]. Adipose tissue is known to produce a lipid-soluble factor that stimulates aldosterone production from the adrenal zona glomerulosa [29, 30]. Aldosterone activation of the mineralocorticoid receptor in the renal distal tubule and collecting duct increases sodium retention, leading to expansion of plasma volume and increased BP [31]. In addition, aldosterone exerts non-genomic actions likely via mineralocorticoid receptor activation, which contribute to hypertension by altering cellular redox state, signaling and endothelial-mediated vascular relaxation [30, 31].
Dysregulation of adipose tissue-derived adipokines is involved in the development of proliferative and inflammatory vascular diseases, including hypertension. Adiponectin, a protein widely implicated in the pathogenesis of insulin resistance, has a profound effect on metabolism and vasculature and conveys anti-hypertensive properties [32, 33]. In several population-based studies, levels of circulating adiponectin have been shown to be inversely proportional to adiposity (body mass index) and burden of hypertension and T2DM [34]. Mechanisms underlying BP lowering effects of adiponectin remains unclear but expression of vascular endothelial nitric oxide (NO) synthase (eNOS) and prostaglandin I2 synthase may play a role [35]. Adiponectin has been suggested to have sympatho-inhibitory action and may also protect against incident hypertension through its anti-inflammatory effects [36]. Leptin, an adipokine elevated in obese individuals, increases sympathetic output likely through a central nervous system effect involving leptin receptor activation [26]. The C1q/TNF-related protein 1 (CTRP1) is expressed at high levels in adipose tissue by proinflammatory cytokines, and increased levels of CTRP1 are associated with the extent of coronary atherosclerosis [37] and reduced collateral formation in patients with chronic coronary total occlusion (CTO) [38, 39]. It was also revealed that CTRP1 is expressed in glomerulosa of the adrenal cortex and stimulates production of aldosterone, suggesting that angiotensin II-induced aldosterone production is, at least in part, mediated by the stimulation of CTRP1 secretion [40]. In addition, circulating levels of CTRP1 were significantly up-regulated in obese subjects as well as hypertensive patients [40,41,42]. Taken together, these observations support a notion that CTRP1 may be a newly identified molecular link between obesity and hypertension.
AGEs-RAGE axis
Chronic hyperglycemia and altered redox state in T2DM increase the formation and accumulation of advanced glycation end-products (AGE). Binding of AGE to receptor for AGE (RAGE) triggers several intracellular signaling pathways and increases the expression and release of inflammatory cytokines, generation of ROS, and activates nuclear factor kappa-B. These alterations might produce contraction of the arterial wall [43], which could reduce arterial pliability and increase vascular stiffness, particularly leading to a rise in the systolic BP and a widening of pulse pressure [44]. In patients with hypertension, there is a positive correlation between plasma levels of AGE and arterial stiffness, and an inverse association between arterial stiffness and serum levels of soluble RAGE (sRAGE) and endogenous secretory RAGE (esRAGE) [43]. The properties of collagen and elastin are altered through AGE–RAGE intermolecular covalent bond or cross-linking [45], which make them less susceptible to hydrolytic turn-over and more reduced elasticity of the arterial wall. Besides structural changes in the artery (increased collagen and decreased elastin), interaction of AGE with RAGE may also induce hypertension through production of ROS, such as superoxide anion, hydrogen peroxide and hydroxyl radicals irrespective of arterial stiffness [43]. Diabetic dyslipidemia (elevated concentration of small dense low-density lipoprotein cholesterol, high concentration of triglycerides, triglyceride-rich remnants, very low-density lipoprotein cholesterol and apoprotein B, usually in combination with low levels of high-density lipoprotein [HDL] cholesterol) will cause endovascular toxicity. Previous studies have shown that the clearance of AGE-modified low-density lipoprotein was reduced in patients with T2DM, causing a significant increase in oxide low-density lipoprotein (oxLDL) [46, 47]. At the same time, HDL can also be glycated, which decrease its ability of reverse transportation of cholesterol and increase the formation of foam cells after enhanced uptake of oxLDL by mononuclear cells. We found that glycated HDL decreases the activity of paraoxonase (PON) [48]. Patients with T2DM and coronary artery disease had elevated plasma levels of glycated apoprotein A-I and A-IV and decreased PON-1 and -3. These biochemical changes are strongly associated with the severity and progression of coronary artery disease [48,49,50] and reduced coronary collateralization [51].
Diabetic nephropathy
It is well recognized that kidney and cardiovascular system are inextricably interlinked as determinants of ambient BP levels in both normal and diseased conditions. The complex interplay between renal disease and hypertension appears to be especially evident in patients with T2DM, who are inherently at high risk for progressive glomerular damage via vascular fibrosis, calcification, prothrombotic effects, and vascular damage [22]. It has been reported that almost 40% of patients with T2DM (especially for elderly) are already hypertensive at diagnosis, and nephropathy is one of the major microvascular complications of T2DM. In fact, approximately 85% of patients with overt diabetic nephropathy have hypertension [21]. In a bi-directional manner, the incidence and severity of hypertension increases with the emergence and progression of nephropathy.
MicroRNA
Recent studies showed that a number of pancreatic β-cell specific microRNAs, a group of noncoding RNAs that are multifunctional, are implicated in the gene expression and various disease processes. For example, diabetic vascular complications are associated with increased levels of miR-223, miR-320, miR-501, miR-504 and miR-1 and decreased levels of miR-16, miR-133, miR-492 and miR-373 [5, 52].
BP levels in diabetic patients with stable coronary artery disease
The beneficial effects of anti-hypertensive drugs on clinical and cardiovascular outcomes are well established [53], and strict BP control is strongly recommended by most previous guidelines for general patients [13,14,15, 17]. However, this therapeutic strategy has been challenged in hypertensive patients with T2DM [16, 18, 19], especially for those with coronary artery disease [54, 55].
Systolic BP
Data from several landmark trials and meta-analyses demonstrate benefit of decreased systolic BP with intensive BP control in reducing the risk of ischemic as well as hemorrhagic stroke for patients with T2DM and hypertension [56,57,58]. Recently, the SPRINT (Systolic Blood Pressure Intervention Trial) reported that for non-diabetic patients with increased cardiovascular risk, intensive BP control (target systolic BP < 120 mmHg) was associated with 25% lower rate of primary composite outcome after 3.26 years of follow-up compared with standard BP goal (target systolic BP < 140 mmHg) [59]. However, these beneficial effects of intensive BP lowering seen in non-diabetic patients have not been demonstrated in patients with T2DM [54, 60,61,62,63,64]. In fact, the results of prospective ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial showed no differences in composite outcome of cardiovascular death, non-fatal myocardial infarction and non-fatal stroke between intensive and standard BP control [60, 61]. An observational analysis of the INVEST (International Verapamil SR-Trandolapril) study revealed that all-cause mortality was increased in diabetic patients with systolic BP < 115 mmHg [62]. A subgroup analysis of the INVEST study involving 6400 patients who were at least 50 years old and had diabetes and coexistent coronary artery disease showed that tight control of systolic BP was not associated with improved cardiovascular outcomes compared with usual BP control [63]. In an international, prospective, longitudinal registry including 22,672 patients with stable coronary artery disease and treated for hypertension, systolic and diastolic BP before each event were averaged and categorized into 10 mmHg increments. After a median follow-up of 5 years, systolic BP > 140 mmHg or < 120 mmHg was correlated with increased risks of cardiovascular mortality, myocardial infarction, or stroke [54]. Recently, Bohm et al. reported the results of the secondary analyses of ONTARGET (Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) and TRANSCEND (Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with CV Disease). These trials aimed to assess the risk in patients with and without diabetes over the whole spectrum of achieved systolic and diastolic BP. The results have shown that mean achieved in-trial systolic BP < 120 mmHg was associated with 1.53-fold increased risk for combined outcome in patients with diabetes [64]. The overall findings thereby underscore the need for caution when aggressive lowering BP therapy is applied, and further question the concept of ‘lower BP is better’ for hypertensive patients with T2DM and coronary artery disease.
Diastolic BP
Since physiological coronary blood flow predominantly occurs during diastole, diastolic BP would be expected to have greater clinical relevance. The INVEST study showed that cardiovascular risk was reduced for type 2 diabetic patients with a diastolic BP < 90 mmHg but was increased for those with a diastolic BP < 70 mmHg [62]. Similarly, the results of the secondary analyses of ONTARGET and TRANSCEND trials also showed that a diastolic BP < 70 mmHg was associated with increased risk for the combined outcome in diabetic and non-diabetic patients, and also for all other endpoints except stroke [64]. These data suggest that cardiovascular risk may be defined by diastolic BP levels, despite optimally achieved systolic BP. In patients with hypertension, the Framingham Heart Study also showed that the same cutoff point of diastolic BP was associated with increased cardiovascular events. Furthermore, the risk was increased among those with both low diastolic BP and a wide pulse pressure [65].
The importance of optimal diastolic BP levels in determining clinical outcomes for patients with coronary artery disease was further substantiated by several recent studies. Peri-Okonny et al. assessed the relationship between reduced diastolic BP and occurrence of angina in a cohort of 1259 patients with stable coronary artery disease (more than one-third of them had diabetes). In the unadjusted model, diastolic BP was associated with angina with a J-shaped relationship (p for nonlinearity = 0.027), with a progressive increase in odds of angina as diastolic BP below 70–80 mmHg. Patients with a diastolic BP of 60 mmHg had 1.37-fold increased risk of angina compared with those having a diastolic BP of 80 mmHg. This association remained significant after adjustment for demographics, comorbidities, heart rate, systolic BP, and anti-angina and anti-hypertensive medications [66].
Angiographically-documented CTO occurs in around 20–30% of type 2 diabetic patients with or without hypertension, especially for those with multi-vessel disease [67]. Percutaneous coronary intervention(PCI)of chronic totally occluded lesions with drug-eluting stent implantation as a part of complete revascularization has become a routine clinical practice [68]. We classified 431 type 2 diabetic and 287 non-diabetic patients with stable angina and angiographic total occlusion of at least one major coronary artery according to 10 mmHg increments of diastolic BP from < 60 to ≥ 100 mmHg and systolic BP from < 100 to ≥ 180 mmHg. The results showed that diastolic BP was related to the degree of coronary collateral formation in a U-shaped pattern, with the lowest risk of poor collateralization at diastolic BP 80–89 mmHg for patients with T2DM and at 90–99 mmHg for non-diabetic counterparts, respectively [69]. In an additional study, we assessed the interactive effects of predominant collateral donor artery (PCDA) stenosis and BP on coronary collateral flow to the chronically occluded bed in 200 type 2 diabetic patients and 200 age- and sex- matched non-diabetic controls. Collateral flow index (CFI) was determined by simultaneous recording of central aortic pressure and intracoronary pressure distal to the occluded segment during PCI. The study demonstrated that when the PCDA was mildly stenotic, CFI was gradually increased along with a reduction in aortic diastolic BP, but it was decreased when diastolic BP was below 60 mmHg in type 2 diabetic patients, with a relative reduction of 32.1% compared with non-diabetic controls. In the presence of moderate PCDA stenosis, with decreasing diastolic BP, the difference of CFI between type 2 diabetic patients and non-diabetic controls was gradually increased. When diastolic BP was below 80 mmHg, patients with T2DM had a significantly lower CFI compared to non-diabetic controls, with a relative reduction of 19.8% at diastolic BP 70–79 mmHg, 28.2% at 60–69 mmHg and 38.2% below 60 mmHg, respectively. A severe stenotic lesion in the PCDA always led to a more pronounced decrease in CFI, with a relative reduction of 37.3% for type 2 diabetic patients compared to non-diabetic controls when diastolic BP was below 60 mmHg. Thus, presence of PCDA stenosis confers greater risk for reduced coronary collateral flow when diastolic BP is decreased. For patients with T2DM, even a moderate stenosis in the PCDA is associated with more pronounced collateral flow reduction as diastolic BP decreases below 80 mmHg compared with non-diabetic patients [70]. These different effects of the severity of PCDA stenosis on collateral flow relative to BP between type 2 diabetic and non-diabetic patients remains unclear, but a likely explanation is the presence of more diffuse coronary atherosclerosis and microvascular disease and various influence of coronary vascular tone in patients with T2DM [71, 72]. Nevertheless, these observations are consistent with the J-curve phenomenon relating the overly reduced or elevated diastolic BP to adverse outcomes [73], and substantiate the concept that coronary autoregulation may be exhausted with low diastolic BP in the setting of atherosclerotic narrowing of the epicardial coronary arteries.
BP levels in diabetic patients with acute coronary syndrome
Recently, White et al. evaluated the relationships between achieved clinician-measured BP and cardiovascular outcomes in 5380 patients with T2DM and recent acute coronary syndromes of the EXAMINE (Examination of Cardiovascular Outcomes With Alogliptin Versus Standard of Care) trial. Risks of major adverse cardiac events and cardiovascular death or heart failure were analyzed using a Cox proportional hazard model with adjustment for baseline covariates in 10 mmHg increments of diastolic BP from ≤ 60 to > 100 mmHg and systolic BP from ≤ 100 to > 160 mmHg during 2-year follow-up. Systolic BP of 131 to 140 mmHg and diastolic BP of 81 to 90 mmHg were used as reference groups. They observed a U-shaped relationship between cardiovascular outcome and BP. Importantly, average follow-up BP < 130/80 mmHg was associated with worsened cardiovascular outcomes, and the degree of risk was notably greater for those who had achieved average follow-up BP of < 120/70 mmHg [19].
BP management for type 2 diabetic patients with coronary artery disease
The anti-hypertensive strategies most appropriate to type 2 diabetic patients with coronary artery disease have been widely studied.
General considerations
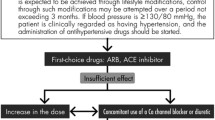
It becomes increasingly important to individualize BP treatment to minimize adverse events and maximize benefits. Non-pharmacological modalities include weight loss, increased potassium-based diet, reduced total intake of sodium and fat (especially saturated fat), and regular physical activity and exercise. Although the cardiovascular benefits of lifestyle changes were not evaluated in type 2 diabetic patients, their implementation seems reasonable as these measures could favorably affect glycemia, lipid profile and BP level [74]. Certainly, pharmacological therapy is effective to decrease mortality, prevent major adverse cardiovascular events including non-fatal myocardial infarction, stroke and heart failure, and slow the progression of pre-existing kidney disease in patients with T2DM. Based on available evidence, type 2 diabetic patients with persistent BP > 140/90 mmHg should be started on anti-hypertensive drug therapy [13, 14]. Notably, the anti-hypertensive strategy (including the choice of BP lowering agents) for type 2 diabetic patients with coronary artery disease should be individualized according to the clinical conditions of the patients. It is important to keep in mind that the degree of BP reduction per se is the major determinant of reduction in cardiovascular risk, superseding the choice of anti-hypertensive drugs; a dictum that is valid in patients with T2DM and coronary artery disease. Monotherapy with angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) can attain BP target in certain type 2 diabetic patients with coronary artery disease, especially when BP is only modestly elevated. However, combination therapy is eventually required in many individuals with T2DM and coronary artery disease, and most guidelines recommend adding a calcium antagonist or diuretic to RAAS inhibitors as add-on therapy [4, 6, 8, 13,14,15]. The superiority of a calcium channel blocker over a thiazide as an addition to ACEI or ARB was shown in terms of reduction of cardiovascular events, renal protection and improvement in insulin resistance [75]. In obese patients or when volume overload is present, diuretics may be used as well, and sometimes the escalation of double-drug treatment to triple-drug therapy is required to improve BP control in type 2 diabetic patients with hypertension [16]. Fixed-dose combinations in a single pill may increase compliance compared with corresponding free-drug components given separately, as it simplifies treatment and thereby can improve adherence on the part of the patients [76].
Not so infrequently, elderly patients with T2DM with or without coronary artery disease may experience a high systolic BP in the presence of a low diastolic BP, reflecting increased aortic stiffness. In this circumstance, the lowering of systolic BP (< 140 mmHg) is clearly beneficial even at the price of further lowering diastolic BP. However, for patients with coronary artery disease and diastolic BP below 60 mmHg, caution is advised during treatment. Alternative medications for angina (e.g., ivabradine or isosorbide) and revascularization or other non-pharmacological interventions may be more beneficial as opposed to further titration of anti-hypertensive medications.
Anti-hypertensive medications
Several classes of anti-hypertensive agents have been used in the treatment of patients with T2DM and coronary artery disease.
RAAS inhibitors
ACEI/ARB are the first-line anti-hypertensive drugs in type 2 diabetic patients with coronary artery disease because they have at least similar [77] or even greater cardiovascular protection and more effectively reduce risk of mortality, myocardial infarction, heart failure and stroke than other anti-hypertensive agents, particularly for high-risk patients [78, 79]. In addition, available literature demonstrates that blockade of the RAAS also has potential benefits beyond BP lowering effects, including renal protection, improvements in insulin resistance, inflammation, oxidative stress, and endothelial function and decrease in activation of matrix metalloproteinases, along with amelioration of vascular function and ventricular remodeling [80]. However, combined use of both ACEI and ARB does not yield additional benefits and is, in fact, not recommended. Aldosterone antagonists such as spironolactone or eplerenone may be considered in type 2 diabetic patients with resistant hypertension as long as careful monitoring of renal function and serum potassium is made [81].
Calcium channel blockers
Calcium antagonists are commonly used for treating hypertension in type 2 diabetic patients with or without coronary artery disease, particularly in the elderly with isolated systolic hypertension [82]. In type 2 diabetic patients who require more than one drug for BP control, a combination of an ACEI or ARB and a dihydropyridine calcium channel blocker (such as amlodipine) is appropriate [83]. Calcium channel blockers were superior to thiazide diuretics in reducing cardiovascular events, with no disadvantages of worsening lipid and glucose uptake.
Diuretics
Despite some concern about the increased risk for metabolic and electrolytic disturbance, diuretics are effective for the treatment of hypertension in type 2 diabetic patients. In post hoc analyses of patients with hypertension and T2DM, thiazide resulted in a significant reduction in cardiovascular events, all-cause mortality, and hospitalization for heart failure compared to placebo, and generally was shown to be non-inferior to other antihypertensive agents. Benefits attributed to thiazide diuretics in terms of cardiovascular event reduction outweigh the risk of worsening glucose control in type 2 diabetic patients [84]. Low dose thiazides in combination with ACEI/ARB may minimize or prevent some of metabolic and electrolytic disturbance associated with diuretic therapy. Thiazide-like diuretics chlortalidone and indapamide were found to be less markedly associated metabolic abnormalities than hydrochlorothiazide but were as good as amlodipine or lisinopril in preventing fatal or non-fatal coronary artery disease and was more effective in hypertensive patients with T2DM [85]. However, the risk of worsening glucose control in type 2 diabetes and of new-onset diabetes in non-diabetic patients correlate with thiazide treatment due to its potential to negatively influence insulin resistance [86]. Thus, glucose and electrolytes should be monitored when initiating therapy.
β-adrenergic blockers
Hypertension is underpinned by high sympathetic nerve activity especially in younger or middle-age subjects. β-blockers reduce heart rate, decrease catecholamine-induced inflammatory reaction, and improve endothelial shear stress,which exert a beneficial effect on coronary blood flow and clinical outcomes [87]. β-blockers are frequently used as add-on treatment in hypertensive patients with coronary artery disease, heart failure or atrial fibrillation with rapid ventricular response [13]. Caution should be made when they were used in patients with T2DM due to its potential adverse metabolic effects, including an increase in triglyceride levels, a decrease in HDL cholesterol levels, weight gain, masking the worsening symptoms of hypoglycemia and aggravating insulin resistance [88]. Cavedilol with combined non-selective β and ɑ1 adrenergic antagonist actions improves survival in patients with heart failure and could not be as deleterious for glucose control [89].
New hypoglycemic agents
The role of certain new anti-diabetic medications including dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon like peptide 1 (GLP-1)agonists, and sodium-glucose cotransporter 2 (SGLT 2) inhibitors, in BP control besides their glucose lowering effects in diabetic individuals has been investigated.
DPP-4 inhibitors and GLP-1 agonists
DPP-4 inhibitors increase endogenous GLP-1 by inhibiting the endogenous substance responsible for its degradation [16]. Several studies assessing the effect of treatment with DPP-4 inhibitors yielded conflicting results in terms of BP changes, with some showing a modest decrease in BP [90] and others revealing an increase in BP [91] or a counteraction against the hypotensive effects of ACEI [92]. GLP-1 agonists produce a mild BP reduction in clinical trials using office BP measurements [93, 94] but have no BP-lowering effect when using 24-h ambulatory BP monitoring [95]. On the other hand, GLP-1 agonists have been reported to increase heart rate via activation of the sympathetic nervous system [96]. Overall, both DPP-4 inhibitors and GLP-1 agonists appear to exert a neutral effect on BP and thus should not serve as an alternative to anti-hypertensive treatment in type 2 diabetic patients [16].
The CAROLINA (Cardiovascular Outcome Study of Linagliptin vs. Glimepiride in Type 2 Diabetes) randomized clinical trial examined the effect of treatment with the DPP-4 inhibitor linagliptin vs. the commonly used sulfonylurea glimepiride on cardiovascular safety in patients with relatively early T2DM and cardiovascular risk factors or established atherosclerotic cardiovascular disease. The results showed that the use of linagliptin compared with glimepiride over a median 6.3 years led to a noninferior risk of composite cardiovascular outcome [97]. Likewise, studies of the effects of GLP-1 agonists on clinical outcome have shown mixed results [98,99,100]. Although the EXSCEL (Exenatide Study of Cardiovascular Events Lowering) study has reported a potential cardiovascular benefit of once-weekly extended-release exenatide, the primary endpoint did not reach statistical significance [101]. These observations suggest that DPP-4 inhibitors and GLP-1 agonists may not improve clinical outcome for patients with T2DM.
SGLT2 inhibitors
These agents belong to a new class of unique oral glucose-lowering drugs and at the same time pertain multifaceted effects on hemodynamic and metabolic parameters beyond glycemic control [102, 103]. Recently, Mazidi et al. undertook a systematic review and meta-analysis of 43 randomized control trials (dapagliflozin: 22 trials; canagliflozin: 14 trials; empagliflozin: 4 trials; remogliflozin: 2 trials; pragliflozin: 1 trial) to determine the effect of SGLT2 inhibitors on BP among individuals with T2DM [104]. They found that the pooled estimate of the effect of SGLT2 on systolic BP levels was − 2.46 mmHg across all studies, − 2.23 mmHg across studies using canagliflozin, − 1.03 mmHg across studies using dapagliflozin, and − 2.59 mmHg across studies using empagliflozin. Likewise, the pooled estimate of the effect of SGLT2 inhibitors on diastolic BP levels was − 1.46 mmHg across all studies, − 2.23 mmHg across studies using canagliflozin, − 1.09 mmHg across studies using dapagliflozin, and − 2.59 mmHg across studies using empagliflozin. Further analyses revealed that the effect of SGLT2 inhibitors on systolic and diastolic BP was not influenced by length of follow-up, and remained similar across all studies and their subgroups [104]. These findings are consistent with previous reports and indicate that treatment with SGLT2 inhibitors either as monotherapy or add-on therapy with other drugs (such as ACEI/ARB), is associated with a small but significant reduction in systolic and diastolic BP measured in-office as well as by 24-h ambulatory BP monitoring, without increase in orthostatic hypotension [105,106,107].
The use of SGLT2 inhibitors also impacts favorably clinical outcome especially in patients with T2DM and cardiovascular disease [108]. In the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial and the CANVAS (CANagliflozin cardioVascular Assessment Study) program, there was a reduction in primary composite cardiovascular endpoints with empagliflozin and canagliflozin, respectively, in high-risk patients with T2DM [109, 110]. The results of real-world observational CVD-REAL (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors) study support the cardiovascular benefits seen in the randomized trials [111]. Compared with DPP-4 inhibitors, some SGLT2 inhibitors have shown greater glycosylated hemoglobin (HbA1c) lowering in individuals with high baseline HbA1c levels [103, 112], greater weight loss, as well as greater BP lowering and satisfaction of renal function [103, 109, 110, 113]. Likewise, these agents may be also superior to DPP-4 inhibitors in terms of cardiovascular protection [114]. A pooled analysis of data from empagliflozin and canagliflozin trials supports a direct renal effect of SGLT2 inhibitors, including those receiving concomitant RAAS blockers [115, 116]. Notably, the risk of dehydration and urinary tract and genital infection is higher with SGLT2 inhibitors [109, 117].
The mechanism underlying the BP decrease by SGLT2 inhibitors remains unclear, and may include diuresis due to their chronic natriuretic and osmotic diuretic effects, weight loss, nephron remodeling, decrease in sympathetic overactivity and arterial stiffness, and increase in HDL cholesterol levels [118,119,120]. Therefore, SGLT2 inhibitors may play a significant role in reducing cardiovascular risk factors in people with T2DM. Canagliflozin and dapagliflozin inhibit SGLT2 activity in the proximal tubule, blocking the reabsorption of glucose back into the bloodstream. Furthermore, canagliflozin also blocks intestinal SGLT1, thereby reducing glucose absorption [118].
BP control during PCI for CTO in type 2 diabetic patients
It is generally accepted that coronary revascularization with either drug-eluting stent-based PCI or coronary artery bypass grafting confers a substantial benefit to long-term outcome [67, 121]. In type 2 diabetic patients with multivessel disease especially at the presence of CTO, hypotension (especially low diastolic BP) should be avoided during PCI procedures, which could exacerbate myocardial ischemia. Similarly, if the vasodilatory reserve of the arterioles in the vascular bed supplied by a chronically occluded coronary artery is completely exhausted, whereas that of the PCDA is still preserved, coronary (collateral) steal may result. This phenomenon has been reported to occur in a very high proportion of well collateralized myocardial beds [122] and is most likely to occur in patients with moderate or severe stenosis of the PCDA, as vasodilator-induced increase in flow could cause a pressure drop across the stenotic lesions, thereby lowering collateral perfusion. Overall, multiple aspects should be taken into consideration when planning PCI procedure on patients with multi-vessel disease, including characteristics of totally occluded lesion, severity of PCDA stenosis, quality of collaterals, and clinical status of patients (diabetes and BP level). In type 2 diabetic patients with moderate PDCA stenosis, the use of fractional flow reserve to reveal ischemia may help in clinical decision-making [123], and warrants further investigation.
Clinical perspective
The available literature and results of recent clinical studies and meta-analyses suggest that the primary BP goal in patients with established coronary artery disease is below 140/90 mmHg. Control of BP < 130/80 mmHg but not < 120/70 mmHg is reasonable for hypertensive and type 2 diabetic patients with stable coronary artery disease, whereas for those with CTO or acute coronary syndromes, an ideal BP target may be somewhat higher (< 140/90 mmHg) as recommended by the current guidelines [4, 13,14,15, 17]. Caution is advised with aggressive lowering of diastolic BP to a critical threshold (< 60 mmHg) which may result in no benefits but rather harmful particularly for hypertensive patients with T2DM and coronary artery disease [66, 124]. Likewise, in type 2 diabetic patients undergoing PCI for CTO, any excessive decrease in BP (especially low diastolic BP) before restoring anterograde flow of a chronic totally occluded lesion should be avoided during the procedure, because it may compromise collateral perfusion and exacerbate ischemia in the presence of at least moderate stenosis of the PCDA [69, 70]. In contrast, lower BP targets might be appropriate in those patients at higher risk of stroke and other micro-vascular complications such as chronic kidney disease, but this issue requires further study [125].
Most previous studies concerning the BP management of type 2 diabetic patients with coronary artery disease rely heavily on in-office BP measurement, however, ambulatory BP monitoring certainly improves baseline BP assessment and risk stratification after anti-hypertensive treatment [57, 126, 127]. Recent guidelines strongly recommended the use of ambulatory BP recording for accurate diagnosis of hypertension and individualized BP target in the treatment of patients with hypertension [13,14,15, 17]. Notably, concurrent masked hypertension and blunt response to nocturnal hypotension are not uncommon in patients with T2DM, which increases the risk of cardiovascular disease [128,129,130]. A large body of clinical evidence supports that BP lowering reduces macro- and micro-vascular complications in patients with T2DM. In future, it remains important to educate type 2 diabetic patients with coronary artery disease for increasing their treatment compliance and guideline adherence and improving the control rate of BP goal [131]. In addition, observational studies have demonstrated that there is often poor control of other cardiovascular risk factors in patients with T2DM [132]. Thus, to achieve the greatest risk reduction for the incidence of cardiovascular disease, the ultimate goal of treatment should be to achieve target control of glucose, BP, and lipids [133].
Conclusions
The mechanism of hypertension in T2DM is complex, involving multiple neuro-humoral and metabolic factors, and both conditions share several similar aspects of pathophysiology. For type 2 diabetic patients with coronary artery disease, the optimal BP targets (especially for diastolic BP) vary based on clinical status in a given individual, and the strategy of BP control should be individualized to minimize adverse events and maximize benefits. Novel information as such is useful for clinicians and researchers to further add new knowledge on pathophysiology and therapeutic goal in diabetes and hypertension.
Available of data and materials
Not applicable. No new datasets were generated for this manuscript.
Abbreviations
- ACC:
-
American College of Cardiology
- ACEI:
-
angiotensin-converting enzyme inhibitor
- AGE:
-
advanced glycation end products
- AHA:
-
American Heart Association
- ARB:
-
angiotensin receptor blocker
- BP:
-
blood pressure
- CFI:
-
collateral flow index
- CTO:
-
chronic total occlusion
- CTRP:
-
C1q tumor necrosis factor related protein
- DDP4:
-
dipeptidyl peptidase 4
- eNOS:
-
endothelial nitric oxide synthase
- esRAGE:
-
endogenous secretory RAGE
- GLP-1:
-
glucagon-like peptide 1
- HDL:
-
high-density lipoprotein
- NO:
-
nitric oxide
- oxLDL:
-
oxidized low-density lipoprotein
- PCDA:
-
predominant collateral donor artery
- PCI:
-
percutaneous coronary intervention
- PON:
-
peroxidase
- RAAS:
-
renin–angiotensin–aldosterone system
- RAGE:
-
receptor for advanced glycation end products
- ROS:
-
reactive oxide spices
- SGLT2:
-
sodium-glucose cotransporter 2
- sRAGE:
-
soluble RAGE
References
GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioral, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1345–422.
Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mmHg, 1990–2015. JAMA. 2017;317(2):165–82.
World Health Organization. Global report on diabetes. Geneva: World Health Organization; 2016.
American Diabetes Association. 9. Cardiovascular disease and risk management: standards of medical care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S86–104.
Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–84.
Venuraju SM, Lahiri A, Jeevarethinam A, Cohen M, Darko D, Nair D, Rosenthal M, Rakhit RD. Duration of type 2 diabetes mellitus and systolic blood pressure as determinants of severity of coronary stenosis and adverse events in an asymptomatic diabetic population: PROCEED study. Cardiovasc Diabetol. 2019;18(1):51.
Wang T, Lu J, Su Q, Chen Y, Bi Y, Mu Y, Chen L, Hu R, Tang X, Yu X, et al. Ideal cardiovascular health metrics and major cardiovascular events in patients with prediabetes and diabetes. JAMA Cardiol. 2019. https://doi.org/10.1001/jamacardio.2019.2499.
Angeli F, Reboldi G, Trapasso M, Aita A, Verdecchia P. Managing hypertension in 2018: which guideline to follow? Heart Asia. 2019;11(1):e011127.
Hu SS, Gao RL, Liu LS, Zhu ML, Wang W, Wang YJ, Wu ZS, Li HJ, Gu DF, Yang YJ, et al. Overview of the report of cardiovascular disease in China 2018. Chin Circ J. 2019;34(3):209–20.
Lee JH, Kim SH, Kang SH, Cho JH, Cho Y, Oh IY, Yoon CH, Lee HY, Youn TJ, Chae IH, et al. Blood pressure control and cardiovascular outcomes: real-world implications of the 2017 ACC/AHA Hypertension Guideline. Sci Rep. 2018;8(1):13155.
Ihm SH, Bakris G, Sakuma I, Sohn IS, Koh KK. Controversies in the 2017 ACC/AHA Hypertension Guidelines: who can be eligible for treatments under the new guidelines? An asian perspective. Circ J. 2019;83(3):504–10.
International Diabetes Federation. IDF diabetes atlas: 8th edition. http://www.diabetesatlas.org/. Accessed 31 Oct 2018.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104.
Leung AA, Daskalopoulou SS, Dasgupta K, McBrien K, Butalia S, Zarnke KB, Nerenberg K, Harris KC, Nakhla M, Cloutier L, et al. Hypertension Canada’s 2016 Canadian hypertension education program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32(5):569–88.
Grossman A, Grossman E. Blood pressure control in type 2 diabetic patients. Cardiovasc Diabetol. 2017;16(1):3.
Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, Miller EPR 3rd, Polonsky T, Thompson-Paul AM, Vupputuri S. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. 2018;71(6):e116–35.
Deedwania P. The ongoing saga of optimal blood pressure level in patients with diabetes mellitus and coronary artery disease. J Am Heart Assoc. 2018;7(20):e010752.
White WB, Jalil F, Cushman WC, Bakris GL, Bergenstal R, Heller SR, Liu Y, Mehta C, Zannad F, Cannon CP. Average clinician-measured blood pressures and cardiovascular outcomes in patients with type 2 diabetes mellitus and ischemic heart disease in the EXAMINE trial. J Am Heart Assoc. 2018;7(20):e009114.
Pfeifer M, Townsend RR, Davies MJ, Vijapurkar U, Ren J. Effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: a post hoc analysis. Cardiovasc Diabetol. 2017;16(1):29.
Lastra G, Syed S, Kurukulasuriya LR, Manrique C, Sowers JR. Type 2 diabetes mellitus and hypertension: an update. Endocrinol Metab Clin North Am. 2014;43(1):103–22.
Rizvi AA. Addressing hypertension in the patient with type 2 diabetes mellitus: pathogenesis, goals, and therapeutic approach. Eur Med J Diabetes. 2017;5(1):84–92.
Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61(5):943–7.
Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S. Insulin resistance in essential hypertension. N Engl J Med. 1987;317(6):350–7.
Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(15):1888–904.
Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why). J Hypertens. 2001;19(3 Pt 2):523–8.
Grootveld LR, Van Valkengoed IG, Peters RJ, Ujcic-Voortman JK, Brewster LM, Stronks K, Snijder MB. The role of body weight, fat distribution and weight change in ethnic differences in the 9-year incidence of hypertension. J Hypertens. 2014;32(5):990–6 (discussion 996–7).
Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292(1):C82–97.
Whaley-Connell A, Johnson MS, Sowers JR. Aldosterone: role in the cardiometabolic syndrome and resistant hypertension. Prog Cardiovasc Dis. 2010;52(5):401–9.
Caprio M, Fève B, Claës A, Viengchareun S, Lombès M, Zennaro MC. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J. 2007;21(9):2185–94.
Williams JS, Williams GH. 50th anniversary of aldosterone. J Clin Endocrinol Metab. 2003;88(6):2364–72.
Peri-Okonny PA, Ayers C, Maalouf N, Das SR, de Lemos JA, Berry JD, Turer AT, Neeland IJ, Scherer PE, Vongpatanasin W. Adiponectin protects against incident hypertension independent of body fat distribution: observations from the Dallas heart study. Diabetes Metab Res Rev. 2017. https://doi.org/10.1002/dmrr.2840.
Kim DH, Kim C, Ding EL, Townsend MK, Lipsitz LA. Adiponectin levels and the risk of hypertension: a systematic review and meta-analysis. Hypertension. 2013;62(1):27–32.
Chow WS, Cheung BM, Tso AW, Xu A, Wat NM, Fong CH, Ong LH, Tam S, Tan KC, Janus ED, et al. Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. Hypertension. 2007;49(6):1455–61.
Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47(6):1108–16.
Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51(1):8–14.
Lu L, Zhang RY, Wang XQ, Liu ZH, Shen Y, Ding FH, Meng H, Wang LJ, Yan XX, Yang K, et al. C1q/TNF-related protein-1: an adipokine marking and promoting atherosclerosis. Eur Heart J. 2016;37(22):1762–71.
Shen Y, Lu L, Liu ZH, Wu F, Zhu JZ, Sun Z, Zhang RY, Zhang Q, Hu J, Chen QJ, et al. Increased serum level of CTRP1 is associated with low coronary collateralization in stable angina patients with chronic total occlusion. Int J Cardiol. 2014;174(1):203–6.
Shen Y, Ding FH, Dai Y, Wang XQ, Zhang RY, Lu L, Shen WF. Reduced coronary collateralization in type 2 diabetic patients with chronic total occlusion. Cardiovasc Diabetol. 2018;17(1):26.
Han S, Yang Y. A novel blood pressure modulator C1q/TNF-α-related protein 1 (CTRP1). BMB Rep. 2018;51(12):611–2.
Pan X, Lu T, Wu F, Jin L, Zhang Y, Shi L, Li X, Lin Z. Circulating complement-C1q TNF-related protein 1 levels are increased in patients with type 2 diabetes and are associated with insulin sensitivity in Chinese subjects. PLoS ONE. 2014;9(5):e94478.
Han S, Jeong AL, Lee S, Park JS, Buyanravjikh S, Kang W, Choi S, Park C, Han J, Son WC, et al. C1q/TNF-α-related protein 1 (CTRP1) maintains blood pressure under dehydration conditions. Circ Res. 2018;123(5):e5–19.
Prasad K, Mishra M. Do advanced glycation end products and its receptor play a role in pathophysiology of hypertension? Int J Angiol. 2017;26(1):1–11.
Semba RD, Sun K, Schwartz AV, Varadhan R, Harris TB, Satterfield S, Garcia M, Ferrucci L, Newman AB, Health ABC Study. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with arterial stiffness in older adults. J Hypertens. 2015;33(4):797–803.
Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Investig. 1995;96(3):1395–403.
Rodriguez V, Newman JD, Schwartzbard AZ. Towards more specific treatment for diabetic dyslipidemia. Curr Opin Lipidol. 2018;29(4):307–12.
Kuwano T, Miura SI, Norimatsu K, Arimura T, Shiga Y, Tomita S, Nakayama A, Matsuo Y, Imaizumi S, Saku K. Advanced glycation of high-density lipoprotein and the functionality of aldosterone release in type 2 diabetes. Hypertens Res. 2017;40(3):271–6.
Shen Y, Ding FH, Sun JT, Pu LJ, Zhang RY, Zhang Q, Chen QJ, Shen WF, Lu L. Association of elevated apoA-I glycation and reduced HDL-associated paraoxonase1, 3 activity, and their interaction with angiographic severity of coronary artery disease in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2015;14:52.
Dai Y, Shen Y, Li QR, Ding FH, Wang XQ, Liu HJ, Yan XX, Wang LJ, Yang K, Wang HB, et al. Glycated apolipoprotein A-IV induces atherogenesis in patients with CAD in type 2 diabetes. J Am Coll Cardiol. 2017;70(16):2006–19.
Pu LJ, Lu L, Zhang RY, Du R, Shen Y, Zhang Q, Yang ZK, Chen QJ, Shen WF. Glycation of apoprotein A-I is associated with coronary artery plaque progression in type 2 diabetic patients. Diabetes Care. 2013;36(5):1312–20.
Shen Y, Lu L, Ding FH, Sun Z, Zhang RY, Zhang Q, Yang ZK, Hu J, Chen QJ, Shen WF. Association of increased serum glycated albumin levels with low coronary collateralization in type 2 diabetic patients with stable angina and chronic total occlusion. Cardiovasc Diabetol. 2013;12:165.
Zhang Y, Sun X, Icli B, Feinberg MW. Emerging roles for microRNAs in diabetic microvascular disease: novel targets for therapy. Endocr Rev. 2017;38(2):145–68.
Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, He H, Chen J, Whelton PK, He J. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2(7):775–81.
Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG, CLARIFY Investigators. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016;388(10056):2142–52.
ACCORD Study Group, Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–85.
UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–13.
Arima H, Chalmers J, Woodward M, Anderson C, Rodgers A, Davis S, Macmahon S, Neal B, PROGRESS Collaborative Group. Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. J Hypertens. 2006;24(6):1201–8.
Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123(24):2799–810.
SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–16.
ACCORD Study Group. Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 2016;39(5):701–8.
Margolis KL, O’Connor PJ, Morgan TM, Buse JB, Cohen RM, Cushman WC, Cutler JA, Evans GW, Gerstein HC, Grimm RH Jr, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37(6):1721–8.
Winchester DE, Cooper-Dehoff RM, Gong Y, Handberg EM, Pepine CJ, INVEST Investigators. Mortality implications of angina and blood pressure in hypertensive patients with coronary artery disease: new data from extended follow-up of the International verapamil/trandolapril study (INVEST). Clin Cardiol. 2013;36(8):442–7.
Cooper-DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, Pepine CJ. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304(1):61–8.
Böhm M, Schumacher H, Teo KK, Lonn EM, Mahfoud F, Mann JFE, Mancia G, Redon J, Schmieder RE, Marx N, et al. Cardiovascular outcomes and achieved blood pressure in patients with and without diabetes at high cardiovascular risk. Eur Heart J. 2019;40(25):2032–43.
Franklin SS, Gokhale SS, Chow VH, Larson MG, Levy D, Vasan RS, Mitchell GF, Wong ND. Does low diastolic blood pressure contribute to the risk of recurrent hypertensive cardiovascular disease events? The Framingham Heart Study. Hypertension. 2015;65(2):299–305.
Peri-Okonny PA, Patel KK, Jones PG, Breeding T, Gosch KL, Spertus JA, Arnold SV. Low diastolic blood pressure is associated with angina in patients with chronic coronary artery disease. J Am Coll Cardiol. 2018;72(11):1227–32.
Fefer P, Kundtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, Gannot S, Samuel M, Weisbrod M, Bierstone D, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusion Registry. J Am Coll Cardiol. 2012;59(11):991–7.
Khan MF, Wendel CS, Thai HM, Movahed MR. Effects of percutaneous revascularization of chronic total occlusions on clinical outcomes: a meta-analysis comparing successful versus failed percutaneous intervention for chronic total occlusion. Catheter Cardiovasc Interv. 2013;82(1):95–107.
Shen Y, Ding FH, Wu F, Lu L, Zhang RY, Zhang Q, Wu ZG, Shen WF. Association of blood pressure and coronary collateralization in type 2 diabetic and nondiabetic patients with stable angina and chronic total occlusion. J Hypertens. 2015;33(3):621–6.
Shen Y, Yang ZK, Hu J, Wang XQ, Dai Y, Zhang S, Zhang RY, Lu L, Ding FH, Shen WF. Donor artery stenosis interactions with diastolic blood pressure on coronary collateral flow in type 2 diabetic patients with chronic total occlusion. Cardiovasc Diabetol. 2018;17(1):76.
Kennedy MW, Fabris E, Suryapranata H, Kedhi E. Is ischemia the only factor predicting cardiovascular outcomes in all diabetes mellitus patients? Cardiovasc Diabetol. 2017;16(1):51.
Hinkel R, Howe A, Renner S, et al. Diabetes mellitus-induced microvascular destabilization in the myocardium. J Am Coll Cardiol. 2017;69(2):131–43.
Vamos EP, Harris M, Millett C, Pape UJ, Khunti K, Curcin V, Molokhia M, Majeed A. Association of systolic and diastolic blood pressure and all-cause mortality in people with newly diagnosed type 2 diabetes: retrospective cohort study. BMJ. 2012;345:e5567.
Noda K, Zhang B, Iwata A, Nishikawa H, Ogawa M, Nomiyama T, Miura S, Sako H, Matsuo K, Yahiro E, et al. Lifestyle changes through the use of delivered meals and dietary counseling in a single-blind study. The STYLIST study. Circ J. 2012;76(6):1335–44.
Weber MA, Bakris GL, Jamerson K, Weir M, Kjeldsen SE, Devereux RB, Velazquez EJ, Dahlöf B, Kelly RY, Hua TA, et al. Cardiovascular events during differing hypertension therapies in patients with diabetes. J Am Coll Cardiol. 2010;56(1):77–85.
Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55(2):399–407.
Fretheim A. VALUE: analysis of results. Lancet. 2004;364(9438):934–5.
Daly CA, Fox KM, Remme WJ, Bertrand ME, Ferrari R, Simoons ML, EUROPA Investigators. The effect of perindopril on cardiovascular morbidity and mortality in patients with diabetes in the EUROPA study: results from the PERSUADE substudy. Eur Heart J. 2005;26(14):1369–78.
Ogihara T, Nakao K, Fukui T, Fukiyama K, Ueshima K, Oba K, Sato T, Saruta T, Candesartan Antihypertensive Survival Evaluation in Japan Trial Group. Effects of candesartan compared with amlodipine in hypertensive patients with high cardiovascular risks: candesartan antihypertensive survival evaluation in Japan trial. Hypertension. 2008;51(2):393–8.
Lastra-Lastra G, Sowers JR, Restrepo-Erazo K, Manrique-Acevedo C, Lastra-González G. Role of aldosterone and angiotensin II in insulin resistance: an update. Clin Endocrinol. 2009;71(1):1–6.
Takahashi S, Katada J, Daida H, Kitamura F, Yokoyama K. Effects of mineralocorticoid receptor antagonists in patients with hypertension and diabetes mellitus: a systematic review and meta-analysis. J Hum Hypertens. 2016;30(9):534–42.
Tuomilehto J, Rastenyte D, Birkenhäger WH, Thijs L, Antikainen R, Bulpitt CJ, Fletcher AE, Forette F, Goldhaber A, Palatini P, et al. Effects of calcium-channel blockade in older patients with diabetes and systolic hypertension. Systolic Hypertension in Europe Trial Investigators. N Engl J Med. 1999;340(9):677–84.
Gradman AH, Basile JN, Carter BL, Bakris GL, Materson BJ, Black HR, Izzo JL Jr, Oparil S, Weber MA. Combination therapy in hypertension. J Am Soc Hypertens. 2010;4(2):90–8.
Scheen AJ. Type 2 diabetes and thiazide diuretics. Curr Diab Rep. 2018;18(2):6.
Whelton PK, Barzilay J, Cushman WC, et al. Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). Arch Intern Med. 2005;165(12):1401–9.
Lin JJ, Chang HC, Ku CT, Chen HY. Hydrochlorothiazide hypertension treatment induced metabolic effects in type 2 diabetes: a meta-analysis of parallel-design RCTs. Eur Rev Med Pharmacol Sci. 2016;20(13):2926–46.
Cruickshank JM. The role of beta-blockers in the treatment of hypertension. Adv Exp Med Biol. 2017;956:149–66.
Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomized controlled trial. Lancet. 2005;366(9489):895–906.
Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, Raskin P, Wright JT Jr, Oakes R, Lukas MA, et al. Metabolic effects of carvedilol vs. metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA. 2004;292(18):2227–36.
Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, Pu Y, Zhu Z, Xu A, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60(3):833–41.
Jackson EK, Dubinion JH, Mi Z. Effects of dipeptidyl peptidase iv inhibition on arterial blood pressure. Clin Exp Pharmacol Physiol. 2008;35(1):29–34.
Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension. 2010;56(4):728–33.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Effects of the glucagon-like peptide-1 receptor agonist liraglutide on 24-h ambulatory blood pressure in patients with type 2 diabetes and stable coronary artery disease: a randomized, double-blind, placebo-controlled, crossover study. J Hypertens. 2017;35(5):1070–8.
Smits MM, Muskiet MH, Tonneijck L, Hoekstra T, Kramer MH, Diamant M, van Raalte DH. Exenatide acutely increases heart rate in parallel with augmented sympathetic nervous system activation in healthy overweight males. Br J Clin Pharmacol. 2016;81(4):613–20.
Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, Pfarr E, Keller A, Mattheus M, Baanstra D, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes. The CAROLINA randomized clinical trial. JAMA. 2019. https://doi.org/10.1001/jama.2019.13772.
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patietns with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nanck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Marso SP, Bain SC, Cousoli A, Eliaschewits FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44.
Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects on once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–39.
Tanaka A, Node K. Emerging roles of sodium-glucose cotransporter 2 inhibitors in cardiology. J Cardiol. 2017;69(3):501–7.
Nauck MA. Update on development with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014;8:1335–80.
Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium–glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc. 2017. https://doi.org/10.1161/JAHA.116.004007.
Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WR. Effects of sodium–glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8(262–275):e269.
Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium–glucose co-transporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:457–66.
Baker WL, Buckley LF, Kelly MS, Bucheit JD, Parod ED, Brown R, Carbone S, Abbate A, Dixon DL. Effects of sodium–glucose cotransporter 2 inhibitors on 24-hour ambulatory blood Pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2017. https://doi.org/10.1161/JAHA.117.005686.
d’Emden M, Amerena J, Deed G, Pollock C, Cooper ME. SGLT2 inhibitors with cardiovascular benefits: transforming clinical care in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;136:23–31.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, Birkeland KI, Jørgensen ME, Thuresson M, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 Inhibitors versus other glucose-lowering drugs: the CVD-REAL Study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation. 2017;136(3):249–59.
Min SH, Yoon JH, Hahn S, Cho YM. Comparison between SGLT2 inhibitors and DPP4 inhibitors added to insulin therapy in type 2 diabetes: a systematic review with indirect comparison meta-analysis. Diabetes Metab Res Rev. 2017;33(1):e2818.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34.
Persson F, Nyström T, Jørgensen ME, Carstensen B, Gulseth HL, Thuresson M, Fenici P, Nathanson D, Eriksson JW, Norhammar A, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab. 2018;20(2):344–51.
Cherney D, Lund SS, Perkins BA, Groop PH, Cooper ME, Kaspers S, Pfarr E, Woerle HJ, von Eynatten M. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860–70.
Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18(6):590–7.
Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27(5):479–84.
Sternlicht H, Bakris GL. Blood pressure lowering and sodium-glucose co-transporter 2 Inhibitors (SGLT2is): more than osmotic diuresis. Curr Hypertens Rep. 2019;21(2):12.
Tikanen I, Chilton R, Johansen OE. Potential role of sodium glucose cotransporter 2 inhibitors in the treatment of hypertension. Curr Opin Nephrol Hypertens. 2016;25(2):81–6.
Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018;71(5):471–6.
Blundhun PK, Wu ZJ, Chen MH. Coronary artery bypass surgery compared with percutaneous coronary interventions in patients with insulin-treated type 2 diabetes mellitus: a systemic review and meta-analysis of 6 randomized controlled trials. Cardiovasc Diabetol. 2016;15:2.
Zimarino M, D’Andreamatteo M, Waksman R, Epstein SE, De Caterina R. The dynamics of the coronary collateral circulation. Nat Rev Cardiol. 2014;11(4):191–7.
Kennedy MW, Kaplan E, Hermanides RS, Fabris E, Hemradj V, Koopmans PC, Dambrink JH, Marcel Gosselink AT, Van’t Hof AW, Ottervanger JP, et al. Clinical outcomes of deferred revascularisation using fractional flow reserve in patients with and without diabetes mellitus. Cardiovasc Diabetol. 2016;15:100.
Armario P, Waeber B. Therapeutic strategies to improve control of hypertension. J Hypertens. 2013;31(Suppl 1):S9–12.
Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, Woodward M, MacMahon S, Turnbull F, Hillis GS, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387(10017):435–43.
Mancia G, Verdecchia P. Clinical value of ambulatory blood pressure: evidence and limits. Circ Res. 2015;116(6):1034–45.
Salles GF, Reboldi G, Fagard RH, Cardoso CR, Pierdomenico SD, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polonia J, et al. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the ambulatory blood pressure collaboration in patients with hypertension (ABC-H) meta-analysis. Hypertension. 2016;67(4):693–700.
Mancia G, Bombelli M, Cuspidi C, Facchetti R, Grassi G. Cardiovascular risk associated with white-coat hypertension: pro side of the argument. Hypertension. 2017;70(4):668–75.
Mancia G. Clinical significance of white-coat hypertension. J Hypertens. 2016;34(4):623–6.
Zhao H, Zeng F, Wang X, Wang L. Prevalence, risk factors, and prognostic significance of masked hypertension in diabetic patients. Medicine. 2017;96(43):e8363.
Lu J, Lu Y, Wang X, Li X, Linderman GC, Wu C, Cheng X, Mu L, Zhang H, Liu J, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1.7 million adults in a population-based screening study (China PEACE Million Persons Project). Lancet. 2017;390(10112):2549–58.
Mazidi M, Karimi E, Rezaie P, Ferns GA. Treatment with GLP1 receptor agonists reduces serum CRP concentrations in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Complications. 2016;8:77–83.
Campbell DJ, Tam-Tham H, Dhaliwal KK, Manns BJ, Hemmelgarn BR, Sanmartin C, King-Shier K. Use of mixed methods research in research on coronary artery disease, diabetes mellitus, and hypertension: a scoping review. Circ Cardiovasc Qual Outcomes. 2017. https://doi.org/10.1161/CIRCOUTCOMES.116.003310.
Acknowledgements
Not applicable.
Funding
This study was supported in part by the Research Foundation of Chinese National Natural Science (81770447, 81970362, 81970293, 81870357), Shanghai Science & Technology Committee (14ZR1425800), Medico-engineering Project (GY2016MS66), Talent Young Investigators (17XJ11009) of Shanghai Jiao Tong University School of Medicine and Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20181801).
Author information
Authors and Affiliations
Contributions
YS, YD, FHD wrote the article, substantially contributed to discussion of the content, and edited the manuscript. YD, XQW researched data for the article. RYZ, LL substantially contributed to discussion of the content and reviewed the manuscript. WFS reviewed the manuscript before submission. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics committee of Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shen, Y., Dai, Y., Wang, X.Q. et al. Searching for optimal blood pressure targets in type 2 diabetic patients with coronary artery disease. Cardiovasc Diabetol 18, 160 (2019). https://doi.org/10.1186/s12933-019-0959-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-019-0959-1