Abstract
Background
Daily glucose variability may contribute to vascular complication development irrespective of mean glucose values. The incremental glucose peak (IGP) during an oral glucose tolerance test (OGTT) can be used as a proxy of glucose variability. We investigated the association of IGP with arterial stiffness, arterial remodeling, and microvascular function, independent of HbA1c and other confounders.
Methods
IGP was calculated as the peak minus baseline plasma glucose value during a seven-point OGTT in 2758 participants (age: 60 ± 8 years; 48% women) of The Maastricht Study, an observational population-based cohort. We assessed the cross-sectional associations between IGP and arterial stiffness (carotid-femoral pulse wave velocity [cf-PWV], carotid distensibility coefficient [carDC]), arterial remodeling (carotid intima-media thickness [cIMT]; mean [CWSmean] and pulsatile [CWSpuls] circumferential wall stress), and microvascular function (retinal arteriolar average dilatation; heat-induced skin hyperemia) via multiple linear regression with adjustment for age, sex, HbA1c, cardiovascular risk factors, lifestyle factors, and medication use.
Results
Higher IGP was independently associated with higher cf-PWV (regression coefficient [B]: 0.054 m/s [0.020; 0.089]) and with higher CWSmean (B: 0.227 kPa [0.008; 0.446]). IGP was not independently associated with carDC (B: − 0.026 10−3/kPa [− 0.112; 0.060]), cIMT (B: − 2.745 µm [− 5.736; 0.245]), CWSpuls (B: 0.108 kPa [− 0.054; 0.270]), retinal arteriolar average dilatation (B: − 0.022% [− 0.087; 0.043]), or heat-induced skin hyperemia (B: − 1.380% [− 22.273; 19.513]).
Conclusions
IGP was independently associated with aortic stiffness and maladaptive carotid remodeling, but not with carotid stiffness, cIMT, and microvascular function measures. Future studies should investigate whether glucose variability is associated with cardiovascular disease.
Similar content being viewed by others
Background
Chronic hyperglycemia is a key factor in the development of type 2 diabetes-related macrovascular and microvascular complications [1, 2]. In the macrovasculature, elevated mean blood glucose levels contribute to arterial stiffening [3, 4], atherosclerosis [1], and large artery endothelial dysfunction [5]. In the microvasculature, hyperglycemia and endothelial dysfunction are considered to be bidirectionally related, potentially entering a vicious cycle that could lead to microvascular complications [6]. Of note, these pathophysiologic processes have been shown to already occur in the prediabetic state [7, 8].
Importantly, chronic hyperglycemia per se does not fully explain the incidence of complications [9]. Daily glucose variability could play a role in vascular complication development irrespective of mean glucose values [10]. While relatively small observational studies have found conflicting results regarding the association between glucose variability and classic diabetic complications [11,12,13], experimental studies have shown that greater glucose variability can be harmful independent of mean glucose values [14, 15].
Continuous glucose monitoring, the gold standard for glucose variability assessment [16], is a challenging technology to use in a large epidemiological setting. The incremental glucose peak (IGP), i.e. the glucose increase from baseline during an oral glucose tolerance test (OGTT), can be used as an index of glucose variability [17]. In view of the aforementioned, we investigated, in a large population-based cohort, whether IGP is associated with arterial stiffness, arterial remodeling, and microvascular function, independent of HbA1c.
Methods
Study population and design
We used data from The Maastricht Study, an observational prospective population-based cohort study. The rationale and methodology have been described previously [18]. In brief, The Maastricht Study focuses on the etiology, pathophysiology, complications and comorbidities of type 2 diabetes, and is characterized by an extensive phenotyping approach. All individuals aged between 40 and 75 years and living in the southern part of the Netherlands were eligible for participation. We recruited participants through mass media campaigns and from the municipal registries and the regional Diabetes Patient Registry via mailings. For reasons of efficiency, we stratified recruitment according to known type 2 diabetes status, with an oversampling of individuals with type 2 diabetes. The present report includes cross-sectional data from the first 3451 participants who completed the baseline survey between November 2010 and September 2013. All examinations were performed within a three-month time window; the OGTT and vascular measurements were performed during different research visits. The Maastricht Study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of the Netherlands (Permit 131088-105234-PG). All participants gave written informed consent.
Assessment of glucose metabolism status and incremental glucose peak
Participants underwent a standardized 2-h 75 g OGTT after fasting overnight to determine glucose metabolism status (GMS), which was defined according to the World Health Organization 2006 criteria as normal glucose metabolism (NGM), impaired fasting glucose, impaired glucose tolerance (combined as prediabetes), or type 2 diabetes [19]. For safety reasons, participants using insulin or with a fasting plasma glucose (FPG) value above 11.0 mmol/L (determined by finger prick) did not undergo the OGTT. For these individuals, we used FPG and information about their diabetes medication to determine GMS. During the OGTT, we took venous blood glucose samples at baseline and 15, 30, 45, 60, 90 and 120 min; we calculated IGP by subtracting FPG from the absolute glucose peak (AGP) value.
Assessment of arterial stiffness, intima-media thickness and circumferential wall stress
The rationale and methodology of the macrovascular measurements have been described previously [20, 21]. We determined carotid-femoral pulse wave velocity (cf-PWV) with the use of applanation tonometry (SphygmoCor, Atcor Medical, Sydney, Australia) [22], and used the median of three consecutive cf-PWV recordings in our analyses.
We measured the left common carotid artery using an ultrasound scanner equipped with a 7.5-MHz linear probe (MyLab 70, Esaote Europe B.V., Maastricht, the Netherlands) to assess local carotid distension, intima-media thickness (cIMT), and interadventitial diameter (IAD) [23]. We quantified local arterial stiffness by calculating the carotid distensibility coefficient (carDC) based on the following formula: carDC = (2*ΔD*IAD + ΔD2)/(braPP*IAD2), where IAD is interadventitial arterial diameter, ΔD distension, and braPP brachial pulse pressure [24].
We defined cIMT as the distance between the lumen-intima and media-adventitia interfaces of the far (posterior) wall [23], and IAD as the distance between the media-adventitia interfaces of the near and far wall. The median carDC, cIMT and IAD of three consecutive measurements were used. We calculated carotid lumen diameter (LD) according to the following formula [25]: LD = IAD − (2*cIMT). In parallel with the vascular measurements, we determined mean heart rate (HR) and mean arterial pressure (MAP) every 5 min with an oscillometric device (Accutorr Plus, Datascope Inc., Montvale, NJ, USA). Mean (CWSmean) and pulsatile (CWSpuls) carotid circumferential wall stress were calculated using the Lamé equation as CWSmean = (MAP*(LD/2))/cIMT and CWSpuls = (carPP*(LD/2))/cIMT, where carotid pulse pressure (carPP) was obtained from carotid pressure waveform calibration [20].
Assessment of microvascular function
The rationale and methodology of assessing the microcirculation of the retina and skin have been described previously [8]. In short, we measured the retinal microvascular dilation response to flicker light during a 50-s baseline, 40-s flicker-light provocation, and 60-s recovery phase, by use of the Dynamic Vessel Analyzer (DVA; Imedos, Jena, Germany). The integrated DVA software (version 4.51; Imedos) automatically calculated average baseline diameter size (expressed in measurement units; MUs) during the 20–50 s of baseline recording, and percentage dilation at time points 10 and 40 s during the flicker stimulation period. Two regression lines were drawn (at the 0–10-s and 10–40-s intervals) and averaged to assess average percentage dilation. We measured skin blood flow with a laser-Doppler system (Periflux 5000; Perimed, Järfalla, Sweden) equipped with a thermostatic laser-Doppler probe (PF457; Perimed) at the dorsal side of the left wrist. After a 2-min baseline recording, the probe temperature was rapidly increased to 44 °C and kept constant until the end of the registration. The heat-induced skin hyperemic response was expressed as the percentage increase in average perfusion units (PUs) during the 23-min heating phase over the average baseline PU.
Measurement of covariates
As described previously [18], we assessed history of cardiovascular disease (CVD), physical activity, and smoking status (never, former, current) by questionnaire; calculated Mediterranean diet adherence according to Trichopoulou et al. based on a food frequency questionnaire [26]; assessed lipid-modifying, antihypertensive, and glucose-lowering medication use as part of a medication interview; measured weight, height, body mass index (BMI), and waist circumference, during a physical examination; measured office and 24-h ambulatory blood pressure (BP); measured HbA1c, fasting plasma insulin and lipid profile in fasting venous blood samples; quantified insulin resistance (IR) based on the updated Homeostatic Model Assessment (HOMA2-IR); measured albumin excretion in two 24-h urine collections; calculated the estimated glomerular filtration rate (eGFR) based on both serum creatinine and cystatin C [27]; and assessed retinopathy presence in both eyes via fundus photography.
Statistical analysis
Normally distributed data are presented as mean and standard deviation (SD), non-normally distributed data as median and interquartile range (IQR), and categorical data as n (%). We used multivariable linear regression to study the associations between IGP and arterial stiffness (cf-PWV, carDC), arterial remodeling (cIMT, CWSmean, CWSpuls), and microvascular function (retinal arteriolar average dilatation, heat-induced skin hyperemia). Model 1 was the crude model, which included only IGP as a determinant; model 2 was adjusted for age and sex; model 3 was additionally adjusted for HbA1c; model 4 was additionally adjusted for MAP (or alternatively office systolic BP; or carPP) and HR in case of cf-PWV only; model 5 was additionally adjusted for cardiovascular risk and lifestyle factors (i.e. BMI, smoking status, physical activity, Mediterranean diet score, antihypertensive and lipid-modifying drug use, fasting triglycerides and total-to-high-density lipoprotein cholesterol levels). The results are presented as: regression coefficient (B) (corresponding 95% confidence interval [CI]), P value. We considered a P value of < 0.05 statistically significant. To test the robustness of our findings, we performed multiple sensitivity analyses by: (1) additionally adjusting for history of CVD, retinopathy, eGFR, and urinary albumin excretion; (2) additionally adjusting for fasting plasma insulin or HOMA2-IR; (3) replacing HbA1c with GMS or FPG; (4) replacing IGP with AGP or percentage increase from baseline (IGPpercentage = IGP/FPG*100%); (5) adjusting for alternative BP measurements (e.g. ambulatory 24-h systolic BP); and (6) replacing IGP with time to glucose peak. We incorporated interaction terms in the fully adjusted regression models to test for interactions between IGP and sex [28], as well as IGP and age, as previously advocated [29]. We considered a P value for interaction of < 0.10 statistically significant. We performed all statistical analyses with the Statistical Package for Social Sciences (Version 25.0; IBM, Chicago, IL).
Results
Study population characteristics
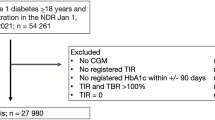
The total study population comprised 3451 individuals, from which we excluded 41 participants with diabetes types other than type 2 diabetes. Some participants had incomplete data on the seven-point OGTT, either because of missing glucose samples (n = 368) or an OGTT contraindication (n = 238; i.e. insulin use or plasma glucose levels > 11.0 mmol/L before initiation of the OGTT), resulting in a study population of 2804 individuals. Those with missing glucose samples were generally comparable to the final study population (Additional file 1: Table S1); as expected, those with an OGTT contraindication differed statistically significantly from the final study population with regard to almost all characteristics (Additional file 1: Table S1). Finally, for 46 participants all outcome data was missing. These individuals were similar to the final study population (Additional file 1: Table S1), which consisted of 2758 individuals. Since outcome and covariate data could not be obtained in all individuals (Additional file 1: Table S2), the number of individuals included in the different regression analyses varied (n = 1134–1978) (Fig. 1).
Flowchart of the IGP study population selection process. OGTT oral glucose tolerance test, IGP incremental glucose peak, cf-PWV carotid-femoral pulse wave velocity, carDC carotid distensibility coefficient, cIMT carotid intima-media thickness, CWSmean mean circumferential wall stress, CWSpuls pulsatile circumferential wall stress
Table 1 shows the general characteristics of the final study population, stratified according to IGP tertiles. Participants in the highest tertile were older, predominantly male, and had a worse cardiometabolic profile, i.e. higher BMI, waist circumference, systolic BP, and fasting glucose, 2-h post-load glucose, HbA1c, fasting plasma insulin, and triglycerides levels. They were also less physically active, more often smoker, more frequently used lipid-modifying, antihypertensive or glucose-lowering medication, and more often had a history of CVD, decreased eGFR, albuminuria, and retinopathy. Of note, IGP tertiles did not fully correspond with GMS. Several individuals with type 2 diabetes (n = 47; 17 newly diagnosed) were not in the highest IGP tertile. Individuals with prediabetes were distributed equally among the second and third tertile. Heterogeneity existed regarding the OGTT glucose peak time point (Additional file 1: Table S3); for the first, second and third IGP tertile, the most frequently occurring time points were 30, 45 and 90 min, respectively.
Incremental glucose peak and arterial stiffness
Figure 2a, b and Additional file 1: Table S4 show the associations of IGP with cf-PWV and carDC. Higher IGP was statistically significantly associated with higher cf-PWV in the crude analysis. This association persisted after adjustment for age, sex, and HbA1c (model 3), and additional adjustment for MAP and HR (model 4). After further adjustment for cardiovascular risk and lifestyle factors, the association of IGP with cf-PWV remained statistically significant (model 5, B: 0.054 m/s [0.020; 0.089], P = 0.002).
Multivariable-adjusted associations of incremental glucose peak (IGP) and arterial stiffness and arterial remodeling. Regression coefficients (B) indicate the mean difference (95% confidence interval) associated with 1 unit (mmol/L) increase of IGP. The panels depict the a associations between IGP and carotid-femoral pulse wave velocity (cf-PWV); b associations between IGP and carotid distensibility coefficient (carDC); c associations between IGP and carotid intima-media thickness (cIMT); d associations between IGP and mean circumferential wall stress (CWSmean); e associations between IGP and pulsatile circumferential wall stress (CWSpuls). Model 1: crude. Model 2: additionally adjusted for age and sex. Model 3: additionally adjusted for HbA1c. Model 4: additionally adjusted for mean arterial pressure and mean heart rate (cf-PWV), mean arterial pressure (carDC, CWSpuls), office systolic blood pressure (cIMT) or carotid pulse pressure (CWSmean). Model 5: additionally adjusted for body mass index, smoking status, physical activity, Mediterranean diet score, use of antihypertensive and lipid-modifying drugs, fasting triglycerides, and total-to-HDL cholesterol levels
Higher IGP was statistically significantly associated with lower carDC in the crude analysis. This association persisted after adjustment for age, sex, and HbA1c (model 3). The association did not remain statistically significant after adjustment for MAP, and cardiovascular risk factors and lifestyle factors (model 5, B: − 0.026 10−3/kPa [− 0.112; 0.060], P = 0.551).
Incremental glucose peak, intima-media thickness and circumferential wall stress
Figure 2c–e and Additional file 1: Table S4 show the associations of IGP with cIMT, CWSmean and CWSpuls. There was a statistically significant, positive association between IGP and cIMT in the crude analysis (crude, B: 4.157 µm [1.944; 6.370], P < 0.001). Of note, this association became negative after correction for age, sex, and HbA1c (model 3). Conversely, HbA1c was positively associated with cIMT (data not shown). Higher IGP was not statistically significantly associated with lower cIMT in the fully adjusted model (model 5, B: − 2.745 µm [− 5.736; 0.245], P = 0.072).
Higher IGP was associated with higher CWSmean in the crude model, and remained associated after adjustment for age, sex, and HbA1c (model 3). The association between IGP and CWSmean remained statistically significant after further correction for cardiovascular risk factors and lifestyle factors (model 5, B: 0.227 kPa [0.008; 0.446], P = 0.043).
IGP was positively associated with CWSpuls in the crude model, and after additional adjustment for age, sex, and HbA1c (model 3). The association between IGP and CWSpuls did not remain statistically significant after correction for cardiovascular risk and lifestyle factors (model 5, B: 0.108 kPa [− 0.054; 0.270], P = 0.192).
Incremental glucose peak and microvascular function
IGP was not associated with retinal arteriolar baseline diameter or skin baseline blood flow (Table 2). Higher IGP was statistically significantly associated with lower retinal arteriolar average dilatation and lower heat-induced skin hyperemia (crude models, Table 2). These associations did not remain statistically significant after adjustment for age, sex, and HbA1c (retinal arteriolar average dilatation), and age and sex (heat-induced skin hyperemia).
Additional analyses
Additional adjustment for history of CVD, retinopathy, eGFR, and urinary albumin excretion did not materially alter the results (Additional file 1: Tables S5 and S6), although statistical significance was not retained in the associations of IGP with CWSmean (model 6). Additional adjustment for fasting plasma insulin or HOMA2-IR did not materially affect the results (Additional file 1: Table S7). The GMS- or FPG-adjusted models yielded results that were mostly comparable with the main (i.e. HbA1c-adjusted) models (Additional file 1: Table S8). Differences from the main models with regard to statistical significance were observed for the GMS-adjusted association of IGP and cf-PWV (5a, B: 0.031 m/s [− 0.008; 0.071], P = 0.120), the GMS-adjusted association of IGP and cIMT (5a, B: − 4.282 µm [− 7.706; -0.857], P = 0.014), and the FPG-adjusted association of IGP and CWSmean (5b, B: 0.156 kPa [− 0.065; 0.377], P = 0.167). In general, the results were not materially different when AGP or IGPpercentage were used as determinant instead of IGP (Additional file 1: Tables S9 and S10). Adjustment for alternative BP measurements did not materially affect the results either (Additional file 1: Tables S11, S12 and S13). When time to glucose peak was used as determinant instead of IGP, only cf-PWV was found to be statistically significantly associated (B: 0.005 m/s [0.001; 0.008], P value = 0.007) (Additional file 1: Table S14).
The association between IGP and cf-PWV was stronger with higher age (P value for interaction < 0.001; Additional file 1: Table S15). The association between IGP and carDC was weaker with higher age (P value for interaction < 0.001; Additional file 1: Table S16). Age statistically significantly modified the association between IGP and CWSpuls (P value for interaction = 0.013; Additional file 1: Table S17). Sex did not modify the associations of IGP with arterial stiffness, arterial remodeling, or microvascular function.
Discussion
In the present study, we investigated cross-sectional associations of IGP with arterial stiffness, arterial remodeling and microvascular function. Our study has two main findings. First, higher IGP was independently associated with higher aortic stiffness (cf-PWV) and higher CWSmean, but not with carotid stiffness (carDC), cIMT and CWSpuls. Second, IGP was not independently associated with measures of microvascular function.
Our study shows that IGP measured during an OGTT provides additional information on top of established glycemic indices (i.e. HbA1c, GMS, and FPG). We found that IGP not fully corresponds with GMS, as individuals with prediabetes were equally distributed among the second and third IGP tertile (Table 1). Furthermore, we showed that the associations of IGP with cf-PWV and CWSmean were independent of HbA1c.
Current observations in the perspective of prior research
This is the first study to report on the association of IGP with arterial stiffness measures. Our findings are in concordance with studies using comparable determinants or outcomes. Hulman et al. for example, showed that individuals with the highest glucose peak during an OGTT were characterized by a worse cardiometabolic risk factor profile (i.e. age, sex, smoking status, BP, plasma lipids) [30]. Moreover, the 1-h OGTT value has previously been found to be independently associated with cf-PWV [31] and brachial-ankle PWV [29]. Still, in our study IGP was observed at this time point in only 20.8% of the participants (Additional file 1: Table S3). Our independent association between time to glucose peak and arterial stiffness (Additional file 1: Table S14) is also in line with Hulman et al.’s findings on glucose peak time point and cardiometabolic risk [30]. By contrast, in a study by the same research group no independent association between OGTT glucose peak and incident CVD was found [32]. However, their use of just a three-point OGTT entails a major limitation.
We observed a negative, albeit not statistically significant, association of IGP with cIMT, which is in contrast with two studies that have found a positive association between IGP and cIMT [33, 34]. These associations, however, were not adjusted for HbA1c or other glycaemic indices. Adjustment for HbA1c, GMS or FPG consistently resulted in a negative association between IGP and cIMT.
The absence of a HbA1c-independent association between IGP and measures of microvascular function is in line with current literature to the extent that the association of glucose variability, as assessed by continuous glucose monitoring, with macroalbuminuria disappeared after adjustment for mean sensor glucose [11]. This could imply that mean glucose values, rather than glucose peaks, are an important determinant of microvascular function.
Mechanistic explanations
The biological mechanism underlying the relationship between IGP and aortic stiffness remains to be elucidated. Previous research has shown that the glucose peak during an OGTT correlates well with glucose variability based on a self-determined ten-point home glucose profile [17]. Greater daily glucose variability may lead to greater oxidative stress [14, 15], which in turn could lead to advanced glycation end product (AGE) formation [2]. AGEs are thought to induce arterial stiffening by accumulating in the arterial wall and forming cross-links between elastin and collagen [3, 35]. Of interest, a previous study by our group showed that AGE precursor levels peaked in parallel with glucose values during an OGTT [36]. This supports the mechanistic concept that the mean glucose (reflected by HbA1c) and glucose variability (reflected by IGP) both contribute to arterial stiffness, mediated by AGEs. Alternatively, elevated IGP could be a hallmark of higher IR, which may, just as hyperinsulinemia, cause arterial stiffening [3, 37]. Indeed, glucose peak height and time point were associated with higher indices of IR in our study, as recently reported by Wang et al. [38]. However, additional adjustment for fasting plasma insulin or HOMA-IR did not substantially alter the results (Additional file 1: Table S7).
Reflections on unexpected findings
Our analyses yielded several interesting findings. First, IGP was independently associated with cf-PWV, but not with carDC. This difference could be due to structural differences between the aorta (mixed elastic and muscular) and carotid artery (predominately elastic) [22]. Indeed, while an association of tissue and circulating AGEs with cf-PWV has been reported [39], no link has been established for carDC [40]. Second, the inverse association between IGP and cIMT was unexpected, in particular because we observed a positive association of cIMT with HbA1c, and found a statistically significant positive association between IGP and CWSmean, which normally should stimulate arterial remodeling to increase arterial wall thickness [25]. Our findings could therefore imply that individuals with high IGP values experience maladaptive arterial remodeling of the carotid artery, as has previously been demonstrated in patients with type 2 diabetes [25]. Alternatively, although the assumptions of linear regression were met and the sensitivity analyses showed comparable results, our findings could still be spurious (type 1 error). Third, IGP was statistically significantly associated with CWSmean, but not with CWSpuls. Based on this finding and on the notion that the regression coefficients decreased more from model 3 to 4 for CWSpuls compared to CWSmean, we conclude that IGP is more strongly associated with MAP than with carPP, and thus that in our study population IGP corresponds more with mean than pulsatile vascular stress. Fourth, while we observed an independent association of IGP with the macrovasculature, no such association was found with the microvasculature, which might be attributable to sample size differences. Future research should focus on further elucidating this discrepancy. Fifth, the regression coefficients of the HbA1c-adjusted models differed in magnitude from the GMS- and FPG-adjusted models, which were performed as sensitivity analyses. This could be a result of using a categorical (i.e. GMS) instead of a continuous (i.e. HbA1c and FPG) confounder [41]. Still, these associations were not statistically significantly dissimilar, as the 95% CIs strongly overlap and include each other’s regression coefficients [42]. The loss of statistical significance after adjustment for additional variables (e.g. history of CVD, eGFR) could be due to smaller sample size or overadjustment bias [43].
Clinical relevance
Aortic stiffness, as measured by cf-PWV, is an independent determinant of CVD, cardiovascular mortality, and all-cause mortality [22]. We found that after adjustment for HbA1c and all other relevant confounders cf-PWV was 0.054 m/s higher per IGP unit (mmol/L). This corresponds with six months of vascular aging per 1 mmol/L higher IGP [44]. Accordingly, the 5.9 mmol/L difference in median IGP between the first and third IGP tertile reflects a three year vascular aging difference. Our results may imply that, even in case of well-controlled HbA1c, the harmful effects of glucose peaks on aortic stiffness are still present. Future studies should investigate whether these findings translate to daily glucose fluctuations. If they are replicated using continuous glucose monitoring data, it would further justify therapeutic interventions that specifically target glucose variability.
Strengths and limitations
This study has several strengths and limitations. Strengths are (1) the use of multiple, state-of-the-art measurements to study arterial stiffness, arterial remodeling, and microvascular function; (2) the study sample size and the extensive participant characterization, allowing adjustment for a broad array of possible confounders; and (3) the robustness of the results, reflected by the consistency of several sensitivity analyses. Our study had certain limitations. First, we could only calculate the main determinant using one OGTT, which is known for its moderate reproducibility [45]. The consequent random measurement error in IGP may have resulted in underestimated associations (i.e. attenuation bias) [46]. Second, a relatively large number of individuals were excluded due to missing determinant, outcome and/or confounder data (Fig. 1). Still, individuals with missing data were generally comparable to the final study population (Additional file 1: Table S1), except for the participants with an OGTT contraindication, who were characterized by a more adverse cardiometabolic profile. The inability to calculate IGP in this relatively unhealthy subgroup might have affected the precision of the associations. Third, the cross-sectional design renders us unable to rule out reverse causality. Arterial stiffness, which has been associated with incident diabetes, could theoretically influence glucose values [47]. Fourth, our study population is mostly Caucasian, which limits the generalizability of our results. Fifth, although the models were adjusted for a large number of cardiovascular risk and lifestyle factors, residual confounding may still be present.
Conclusions
We show that higher IGP is independently associated with greater aortic stiffness and maladaptive carotid remodeling, but not with carotid stiffness, cIMT, or microvascular function. Taken together, these findings support the concept that glucose peaks have harmful macrovascular effects, regardless of mean glucose levels. Further research is needed to elucidate how these findings translate to daily glucose fluctuations and to what extent CVD could be prevented by reducing glucose variability.
Data availability
Data are available from The Maastricht Study for any researcher who meets the criteria for access to confidential data; the corresponding author may be contacted to request data.
Abbreviations
- AGE:
-
advanced glycation end product
- AGP:
-
absolute glucose peak (during an OGTT)
- B:
-
regression coefficient
- BMI:
-
body mass index
- BP:
-
blood pressure
- braPP:
-
brachial pulse pressure
- carDC:
-
carotid distensibility coefficient
- carPP:
-
carotid pulse pressure
- cf-PWV:
-
carotid-femoral pulse wave velocity
- CI:
-
confidence interval
- cIMT:
-
carotid intima-media thickness
- CVD:
-
cardiovascular disease
- CWSmean :
-
mean circumferential wall stress
- CWSpuls :
-
pulsatile circumferential wall stress
- DVA:
-
Dynamic Vessel Analyzer
- eGFR:
-
estimated glomerular filtration rate
- FPG:
-
fasting plasma glucose
- GMS:
-
glucose metabolism status
- HOMA2-IR:
-
updated Homeostatic Model Assessment
- HR:
-
heart rate
- IAD:
-
interadventitial diameter
- IGP:
-
incremental glucose peak (during an OGTT)
- IGPpercentage :
-
percentage increase from baseline (during an OGTT)
- IQR:
-
interquartile range
- IR:
-
insulin resistance
- LD:
-
lumen diameter
- MAP:
-
mean arterial pressure
- MU:
-
measurement unit
- NGM:
-
normal glucose metabolism
- OGTT:
-
oral glucose tolerance test
- PU:
-
perfusion unit
- SD:
-
standard deviation
References
Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–88.
Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–25.
Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51(4):527–39.
Swoboda PP, Erhayiem B, Kan R, McDiarmid AK, Garg P, Musa TA, et al. Cardiovascular magnetic resonance measures of aortic stiffness in asymptomatic patients with type 2 diabetes: association with glycaemic control and clinical outcomes. Cardiovasc Diabetol. 2018;17(1):35.
van Sloten TT, Henry RM, Dekker JM, Nijpels G, Unger T, Schram MT, et al. Endothelial dysfunction plays a key role in increasing cardiovascular risk in type 2 diabetes: the Hoorn study. Hypertension. 2014;64(6):1299–305.
Stehouwer CDA. Microvascular dysfunction and hyperglycemia: a vicious cycle with widespread consequences. Diabetes. 2018;67(9):1729–41.
Henry RM, Kostense PJ, Spijkerman AM, Dekker JM, Nijpels G, Heine RJ, et al. Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation. 2003;107(16):2089–95.
Sorensen BM, Houben AJ, Berendschot TT, Schouten JS, Kroon AA, van der Kallen CJ, et al. Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: The Maastricht Study. Circulation. 2016;134(18):1339–52.
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86.
Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295(14):1707–8.
Jin SM, Kim TH, Oh S, Baek J, Joung JY, Park SM, et al. Association between the extent of urinary albumin excretion and glycaemic variability indices measured by continuous glucose monitoring. Diabet Med. 2015;32(2):274–9.
Xu F, Zhao LH, Su JB, Chen T, Wang XQ, Chen JF, et al. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr. 2014;6(1):139.
Sartore G, Chilelli NC, Burlina S, Lapolla A. Association between glucose variability as assessed by continuous glucose monitoring (CGM) and diabetic retinopathy in type 1 and type 2 diabetes. Acta Diabetol. 2013;50(3):437–42.
Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–7.
Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–54.
Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–40.
Meier JJ, Baller B, Menge BA, Gallwitz B, Schmidt WE, Nauck MA. Excess glycaemic excursions after an oral glucose tolerance test compared with a mixed meal challenge and self-measured home glucose profiles: is the OGTT a valid predictor of postprandial hyperglycaemia and vice versa? Diabetes Obes Metab. 2009;11(3):213–22.
Schram MT, Sep SJ, van der Kallen CJ, Dagnelie PC, Koster A, Schaper N, et al. The Maastricht Study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol. 2014;29(6):439–51.
WHO. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. Geneva: WHO; 2006.
Geijselaers SL, Sep SJ, Schram MT, van Boxtel MP, van Sloten TT, Op Het Roodt J, et al. Carotid circumferential wall stress is not associated with cognitive performance among individuals in late middle age: The Maastricht Study. Atherosclerosis. 2018;276:15–22.
van Sloten TT, Czernichow S, Houben AJ, Protogerou AD, Henry RM, Muris DM, et al. Association between arterial stiffness and skin microvascular function: The SUVIMAX2 Study and The Maastricht Study. Am J Hypertens. 2015;28(7):868–76.
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–605.
Willekes C, Hoeks AP, Bots ML, Brands PJ, Willigers JM, Reneman RS. Evaluation of off-line automated intima-media thickness detection of the common carotid artery based on M-line signal processing. Ultrasound Med Biol. 1999;25(1):57–64.
Reneman RS, Meinders JM, Hoeks AP. Non-invasive ultrasound in arterial wall dynamics in humans: what have we learned and what remains to be solved. Eur Heart J. 2005;26(10):960–6.
Henry RM, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Kamp O, et al. Carotid arterial remodeling: a maladaptive phenomenon in type 2 diabetes but not in impaired glucose metabolism: the Hoorn study. Stroke. 2004;35(3):671–6.
Trichopoulou A, Orfanos P, Norat T, Bueno-de-Mesquita B, Ocke MC, Peeters PH, et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ. 2005;330(7498):991.
Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–9.
Schiebinger L, Leopold SS, Miller VM. Editorial policies for sex and gender analysis. Lancet. 2016;388(10062):2841–2.
Nakagomi A, Sunami Y, Okada S, Ohno Y, Shoji T, Fujisawa T, et al. Association between 1-h post-load plasma glucose levels and arterial stiffness in normotensive subjects with normal glucose tolerance. Diab Vasc Dis Res. 2018;15(1):39–45.
Hulman A, Simmons RK, Vistisen D, Tabak AG, Dekker JM, Alssema M, et al. Heterogeneity in glucose response curves during an oral glucose tolerance test and associated cardiometabolic risk. Endocrine. 2017;55(2):427–34.
Sciacqua A, Maio R, Miceli S, Pascale A, Carullo G, Grillo N, et al. Association between one-hour post-load plasma glucose levels and vascular stiffness in essential hypertension. PLoS ONE. 2012;7(9):e44470.
Hulman A, Vistisen D, Glumer C, Bergman M, Witte DR, Faerch K. Glucose patterns during an oral glucose tolerance test and associations with future diabetes, cardiovascular disease and all-cause mortality rate. Diabetologia. 2018;61(1):101–7.
Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23(12):1830–4.
Hu Y, Liu W, Huang R, Zhang X. Postchallenge plasma glucose excursions, carotid intima-media thickness, and risk factors for atherosclerosis in Chinese population with type 2 diabetes. Atherosclerosis. 2010;210(1):302–6.
Prenner SB, Chirinos JA. Arterial stiffness in diabetes mellitus. Atherosclerosis. 2015;238(2):370–9.
Maessen DE, Hanssen NM, Scheijen JL, van der Kallen CJ, van Greevenbroek MM, Stehouwer CD, et al. Post-glucose load plasma alpha-dicarbonyl concentrations are increased in individuals with impaired glucose metabolism and type 2 diabetes: The CODAM Study. Diabetes Care. 2015;38(5):913–20.
Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17(1):41.
Wang X, Zhao X, Zhou R, Gu Y, Zhu X, Tang Z, et al. Delay in glucose peak time during the oral glucose tolerance test as an indicator of insulin resistance and insulin secretion in type 2 diabetes patients. J Diabetes Investig. 2018;9(6):1288–95.
van Eupen MG, Schram MT, van Sloten TT, Scheijen J, Sep SJ, van der Kallen CJ, et al. Skin autofluorescence and pentosidine are associated with aortic stiffening: The Maastricht Study. Hypertension. 2016;68(4):956–63.
Baumann M, Richart T, Sollinger D, Pelisek J, Roos M, Kouznetsova T, et al. Association between carotid diameter and the advanced glycation end product N-epsilon-carboxymethyllysine (CML). Cardiovasc Diabetol. 2009;8:45.
Groenwold RH, Klungel OH, Altman DG, van der Graaf Y, Hoes AW, Moons KG, et al. Adjustment for continuous confounders: an example of how to prevent residual confounding. CMAJ. 2013;185(5):401–6.
Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31(4):337–50.
Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–95.
McEniery CM, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46(9):1753–60.
Mooy JM, Grootenhuis PA, de Vries H, Kostense PJ, Popp-Snijders C, Bouter LM, et al. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia. 1996;39(3):298–305.
Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289.
Muhammad IF, Borne Y, Ostling G, Kennback C, Gottsater M, Persson M, et al. Arterial stiffness and incidence of diabetes: a population-based cohort study. Diabetes Care. 2017;40(12):1739–45.
Acknowledgements
Not applicable.
Funding
The Maastricht Study was supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs (grant 31O.041), Stichting De Weijerhorst (Maastricht, the Netherlands), the Pearl String Initiative Diabetes (Amsterdam, the Netherlands), School for Cardiovascular Diseases (CARIM, Maastricht, the Netherlands), Care and Public Health Research Institute (CAPHRI, Maastricht, the Netherlands), School for Nutrition and Translational Research in Metabolism (NUTRIM, Maastricht, the Netherlands), Stichting Annadal (Maastricht, the Netherlands), Health Foundation Limburg (Maastricht, the Netherlands), and by unrestricted grants from Janssen-Cilag B.V. (Tilburg, the Netherlands), Novo Nordisk Farma B.V. (Alphen aan den Rijn, the Netherlands), Sanofi-Aventis Netherlands B.V. (Gouda, the Netherlands), and Medtronic (Tolochenaz, Switzerland).
Author information
Authors and Affiliations
Contributions
YDF and MCGJB contributed to conception and design, participated in acquisition of data, analyzed and interpreted data, drafted the manuscript, revised the manuscript critically for important intellectual content, and provided final approval of the version to be published. KDR, NCS, and CDAS contributed to conception and design, contributed to analyses and interpretation of data, revised the manuscript critically for important intellectual content, and provided final approval of the version to be published. TTJMB, MCJMvD, SJPME, MMJvG, RMAH, AJHMH, CJHvdK, AAK, and MTS contributed to conception and design, revised the manuscript critically for important intellectual content, and provided final approval of the version to be published. YDF and MCGJB are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Maastricht Study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of the Netherlands (Permit 131088-105234-PG). All participants gave written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Additional tables.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Foreman, Y.D., Brouwers, M.C.G.J., Berendschot, T.T.J.M. et al. The oral glucose tolerance test-derived incremental glucose peak is associated with greater arterial stiffness and maladaptive arterial remodeling: The Maastricht Study. Cardiovasc Diabetol 18, 152 (2019). https://doi.org/10.1186/s12933-019-0950-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-019-0950-x