Abstract
Both obesity and type 2 diabetes are important risk factors for atrial fibrillation (AF), possibly because they both cause an expansion of epicardial adipose tissue, which is the source of proinflammatory adipocytokines that can lead to microvascular dysfunction and fibrosis of the underlying myocardium. If the derangement of epicardial fat adjoins the left atrium, the result is an atrial myopathy, which is clinically manifest as AF. In patients with AF, there is a close relationship between epicardial fat volume and the severity of electrophysiological abnormalities in the adjacent myocardial tissues, and epicardial fat mass predicts AF in the general population. The expansion of epicardial adipose tissue in obesity and type 2 diabetes may also affect the left ventricle, impairing its distensibility and leading to heart failure with a preserved ejection fraction (HFpEF). Patients with obesity or type 2 diabetes with AF often have HFpEF, but the diagnosis may be missed, if dyspnea is attributed to increased body mass or to the arrhythmia. The expected response to the treatment for obesity, diabetes or AF may be influenced by their effects on epicardial inflammation and the underlying atrial and ventricular myopathy. Bariatric surgery and metformin reduce epicardial fat mass and ameliorate AF, whereas insulin promotes adipogenesis and cardiac fibrosis, and its use is accompanied by an increased risk of AF. Rate control strategies for AF may impair exercise tolerance, because they allow for greater time for ventricular filling in patients who cannot tolerate volume loading because of cardiac fibrosis and HFpEF. At the same time, both obesity and diabetes decrease the expected success rate of rhythm control strategies for AF (e.g., electrical cardioversion or catheter ablation), because increased epicardial adipose tissue volumes and cardiac fibrosis are important determinants of AF recurrence following these procedures.
Similar content being viewed by others
Both obesity and type 2 diabetes are important risk factors for the development of atrial fibrillation (AF). Although hypertension has long been the primary determinant of AF in the general community, obesity represents the second highest population-attributable risk for AF, and its importance is growing [1]. An increase in body mass contributes to the development of AF in 20% of patients with AF, and short-term weight gain elevates the risk of AF by 40% over a follow-up of 5 years [1, 2]. At the same time, diabetes also contributes significantly to the development of AF; the severity of hyperglycemia predicts the incidence of AF [3].
Role of obesity- and diabetes-driven epicardial adipose tissue expansion in mediating the development of atrial fibrillation
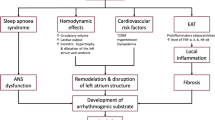
What mechanisms drive the development of AF in these two common metabolic disorders? Both obesity and type 2 diabetes are accompanied by an expansion and biological transformation of epicardial adipose tissue [4,5,6,7], which can be the source of proinflammatory mediators that are capable of causing microvascular dysfunction and fibrosis of the underlying myocardium [8,9,10]. If the derangement of epicardial fat adjoins the left atrium, the result is electroanatomical remodeling leading to an atrial myopathy (Fig. 1) [11]. In patients with AF and other cardiovascular disorders, there is a close relationship between the volume and inflammatory state of epicardial fat, the presence of atrial fibrosis, and the severity of electrophysiological abnormalities in the adjacent myocardial tissues [5, 12,13,14]. Epicardial fat mass predicts the incidence of AF in the general population [15]; it increases as AF progresses from a paroxysmal to a persistent arrhythmia [16]; and it identifies patients at risk of major adverse cardiovascular events [17]. The powerful link between obesity and the risk of AF in epidemiological studies is entirely explained by the underlying atrial myopathy [18].
Importantly, the expansion and inflammation of epicardial adipose tissue in obesity and type 2 diabetes affects not only the atria, but also the ventricles [11]. The derangements of epicardial fat can lead to inflammation, microcirculatory dysfunction and fibrosis in the adjoining myocardium, impairing the distensibility of the left ventricle (LV) and restraining its ability to tolerate volume (Fig. 1) [11, 19, 20]. LV filling pressure rises, causing exertional dyspnea and leading to heart failure with a preserved ejection fraction (HFpEF) [19]. Incident AF increases the risk of a subsequent diagnosis of heart failure, particularly HFpEF [21, 22]. Even when heart failure has not been formally diagnosed, many patients with AF (particularly with exercise intolerance) have increased LV filling pressure at rest or during exercise on echocardiography or by cardiac catheterization [23,24,25]. Therefore, patients with obesity or type 2 diabetes with AF often have underlying latent HFpEF, but the diagnosis is frequently not made, because dyspnea is often attributed to increased body mass or to the arrhythmia.
Disease–treatment interactions in patients with obesity or type 2 diabetes who have atrial fibrillation
How should patients with obesity or diabetes who have AF be managed? Physicians could (1) treat the causal metabolic disorder or (2) directly address the arrhythmia, either with rate- or rhythm-control strategies. However, the expected response to these interventions may be influenced by epicardial adipose tissue inflammation and by the underlying atrial and ventricular myopathy.
Influence of weight loss and antihyperglycemic drugs on AF
Epicardial fat is relatively resistant to weight loss regimens [26]; thus, the modest weight loss that is typically seen with caloric restriction has minimal effect on epicardial adipose tissue [27] and exerts little benefit on AF [28]. In contrast, marked weight loss (e.g., with bariatric surgery) can decrease both the mass and inflammation of epicardial fat [29, 30]. In both observational studies and randomized controlled trials, striking degrees of weight loss can reduce the burden of AF or restore sinus rhythm in patients with established AF [31, 32]. Interestingly, this degree of weight loss is also paralleled by an amelioration of the diastolic filling abnormalities typically seen in HFpEF [33].
Additionally, in patients with type 2 diabetes, the effects of antihyperglycemic drugs on AF may parallel their actions on epicardial adipose tissue. Insulin promotes adipogenesis and cardiac fibrosis [34, 35] (exacerbating the atrial myopathy) and exerts antinatriuretic effects (increasing atrial wall stress) [36]. Also, by promoting episodic hypoglycemia, insulin may activate the sympathetic nervous system and enhance arrhythmogenesis. Accordingly, insulin use is accompanied by an increased risk of AF [37, 38]. In contrast, metformin exerts anti-inflammatory effects on adipose tissue and decreases the release of proinflammatory adipokines from the epicardium [39, 40], and its use has been accompanied by a decreased risk of AF [41]. In addition, by promoting PPAR-γ signaling, pioglitazone can reverse the dysfunctional state of epicardial fat [42, 43], thereby attenuating atrial inflammation and fibrosis. Pioglitazone ameliorates AF in experimental models [44], and use of the drug has been associated with a lower risk of new-onset or recurrent AF in observational studies in the clinical setting [45, 46]. However, thiazolidinediones did not reduce AF events in two randomized controlled trials of patients with insulin resistance or type 2 diabetes [47], possibly because PPAR-γ agonism promotes sodium retention and increases cardiac volumes [48]. The resulting atrial distension could negate any benefits on AF that might be expected from the action of these drugs to reduce epicardial adipose inflammation.
Rate- and rhythm-control strategies for AF in obesity and diabetes
Even though patients with obesity and type 2 diabetes are at high risk of undiagnosed HFpEF, physicians will often ascribe complaints of dyspnea to the presence of AF, and thus, treatments are likely to be directed towards control of AF. However, efforts at both rate and rhythm control are often unsuccessful and carry important risks, if patients have a metabolic disorder or underlying HFpEF.
Rate control strategies
The intent of rate control in AF is to prevent tachyarrhythmia-related cardiac injury. However, in most patients with heart failure and AF, a rapid ventricular rate does not have adverse functional or prognostic significance. When compared with patients with faster heart rates, patients with greater rate control do not have improved long-term outcomes [49]. Furthermore, if there is underlying HFpEF, heart rate slowing can impair exercise tolerance, presumably because it allows greater time for ventricular filling in patients who cannot tolerate volume loading because of cardiac fibrosis [50]. The use of atrioventricular nodal blocking drugs (e.g., digoxin, amiodarone and dronedarone) has been associated with an increased risk of death in patients with AF [51, 52]. Furthermore, although beta-blockers reduce morbidity and mortality in patients with a reduced ejection fraction in sinus rhythm, they do not exert these benefits in those with AF, particularly if the ejection fraction is preserved [53]. Fibrosis-related conduction system disease may increase the risk of serious bradyarrhythmias if patients are prescribed rate-control agents [54]. These experiences raises important doubts about the value of intensive rate control of AF in patients with obesity or diabetes, who are likely to have an underlying inflammatory myopathy.
Rhythm control strategies
Given these challenges, physicians frequently turn to rhythm control strategies for AF, i.e., electrical or chemical cardioversion or catheter ablation. However, both obesity and type 2 diabetes decrease the success rate (i.e., maintenance of sinus rhythm) following electrical cardioversion [55, 56], presumably because increased epicardial adipose tissue volume is a major determinant of AF recurrence following the procedure [57]. Furthermore, the post-cardioversion administration of anti-arrhythmic drugs carries an important risk of proarrhythmia and worsening heart failure, particularly in patients who have an underlying atrial or ventricular myopathy [52].
Catheter ablation may be used to abolish AF in patients with obesity or type 2 diabetes. However, the left atrium and LV in these individuals is typically affected with extensive fibrosis [58], especially if they have long-standing AF [59]. Unfortunately, patients with AF who have myocardial fibrosis are unlikely to maintain sinus rhythm following ablation [60, 61]—especially if epicardial adipose tissue volume is increased [62, 63]—thus explaining the high rate of AF recurrence in patients with obesity or type 2 diabetes [64,65,66]. Marked weight loss produced by bariatric surgery (which reduces epicardial fat volume) can improve the success of ablation procedures [67]. However, if epicardial adiposity persists, the presence of fibrosis may attenuate any benefit that abolition of AF might otherwise have on LV structure and function. In the only trial that has reported favorable effects of catheter ablation on LV ejection fraction using magnetic resonance imaging, the observed benefit was confined to those without preprocedural myocardial fibrosis [68]. More worrisome, if a patient with AF also has an underlying atrial myopathy as a result of epicardial inflammation caused by obesity or diabetes, ablation may add to the pre-existing fibrotic burden of the left atrium, further compromising chamber capacitance and leading to post-procedural increases in pulmonary venous pressures and worsening heart failure, particularly in those with underlying HFpEF [69, 70].
Conclusions
Patients with obesity or type 2 diabetes are at markedly increased risk of AF. The management of the metabolic disorder can influence the course of AF, and conversely, efforts to treat AF (with rate or rhythm control) may have limited efficacy in patients with these coexistent conditions. It is hypothesized that these complex interactions are mediated by an expansion of epicardial adipose tissue, which not only drives the development of AF, but whose biology may also be influenced by the management of the underlying metabolic diseases. Longitudinal studies using magnetic resonance imaging to quantify epicardial fat and cardiac fibrosis are poised to confirm or refute this hypothesis.
Availability of data and materials
There are no new data presented; this is a commentary
Abbreviations
- AF:
-
atrial fibrillation
- HFpEF:
-
heart failure with a preserved ejection fraction
- PPAR-γ:
-
peroxisome proliferator-activated receptor-gamma
References
Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–8.
Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol. 2010;55:2319–27.
Huxley RR, Alonso A, Lopez FL, Filion KB, Agarwal SK, Loehr LR, Soliman EZ, Pankow JS, Selvin E. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart. 2012;98:133–8.
Groves EM, Erande AS, Le C, Salcedo J, Hoang KC, Kumar S, Mohar DS, Saremi F, Im J, Agrawal Y, Nadeswaran P, Naderi N, Malik S. Comparison of epicardial adipose tissue volume and coronary artery disease severity in asymptomatic adults with versus without diabetes mellitus. Am J Cardiol. 2014;114:686–91.
Mahajan R, Nelson A, Pathak RK, Middeldorp ME, Wong CX, Twomey DJ, Carbone A, Teo K, Agbaedeng T, Linz D, de Groot JR, Kalman JM, Lau DH, Sanders P. Electroanatomical remodeling of the atria in obesity: impact of adjacent epicardial fat. JACC Clin Electrophysiol. 2018;4:1529–40.
Colom C, Viladés D, Pérez-Cuellar M, Leta R, Rivas-Urbina A, Carreras G, Ordóñez-Llanos J, Pérez A, Sánchez-Quesada JL. Associations between epicardial adipose tissue, subclinical atherosclerosis and high-density lipoprotein composition in type 1 diabetes. Cardiovasc Diabetol. 2018;17(1):156. https://doi.org/10.1186/s12933-018-0794-9.
Kang J, Kim YC, Park JJ, Kim S, Kang SH, Cho YJ, Yoon YE, Oh IY, Yoon CH, Suh JW, Cho YS, Youn TJ, Chae IH, Choi DJ. Increased epicardial adipose tissue thickness is a predictor of new-onset diabetes mellitus in patients with coronary artery disease treated with high-intensity statins. Cardiovasc Diabetol. 2018;17(1):10. https://doi.org/10.1186/s12933-017-0650-3.
Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360–72.
Greulich S, Maxhera B, Vandenplas G, de Wiza DH, Smiris K, Mueller H, Heinrichs J, Blumensatt M, Cuvelier C, Akhyari P, Ruige JB, Ouwens DM, Eckel J. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation. 2012;126:2324–34.
Gruzdeva O, Uchasova E, Dyleva Y, Borodkina D, Akbasheva O, Belik E, Karetnikova V, Brel N, Kokov A, Kashtalap V, Barbarash O. Relationships between epicardial adipose tissue thickness and adipo-fibrokine indicator profiles post-myocardial infarction. Cardiovasc Diabetol. 2018;17(1):40. https://doi.org/10.1186/s12933-018-0679-y.
Packer M. The epicardial adipose inflammatory triad: coronary atherosclerosis, atrial fibrillation, and heart failure with a preserved ejection fraction. Eur J Heart Fail. 2018;20:1567–9.
Mazurek T, Kiliszek M, Kobylecka M, Skubisz-Głuchowska J, Kochman J, Filipiak K, Królicki L, Opolski G. Relation of proinflammatory activity of epicardial adipose tissue to the occurrence of atrial fibrillation. Am J Cardiol. 2014;113:1505–8.
Wang Q, Xi W, Yin L, Wang J, Shen H, Gao Y, Min J, Zhang Y, Wang Z. Human epicardial adipose tissue cTGF expression is an independent risk factor for atrial fibrillation and highly associated with atrial fibrosis. Sci Rep. 2018;8(1):3585. https://doi.org/10.1038/s41598-018-21911-y.
Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clément K, Hatem SN. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J. 2015;36:795–805a.
Bos D, Vernooij MW, Shahzad R, Kavousi M, Hofman A, van Walsum T, Deckers JW, Ikram MA, Heeringa J, Franco OH, van der Lugt A, Leening MJG. Epicardial fat volume and the risk of atrial fibrillation in the general population free of cardiovascular disease. JACC Cardiovasc Imaging. 2017;10:1405–7.
Oba K, Maeda M, Maimaituxun G, Yamaguchi S, Arasaki O, Fukuda D, Yagi S, Hirata Y, Nishio S, Iwase T, Takao S, Kusunose K, Yamada H, Soeki T, Wakatsuki T, Harada M, Masuzaki H, Sata M, Shimabukuro M. Effect of the epicardial adipose tissue volume on the prevalence of paroxysmal and persistent atrial fibrillation. Circ J. 2018;82:1778–87.
Chu CY, Lee WH, Hsu PC, Lee MK, Lee HH, Chiu CA, Lin TH, Lee CS, Yen HW, Voon WC, Lai WT, Sheu SH, Su HM. Association of increased epicardial adipose tissue thickness with adverse cardiovascular outcomes in patients with atrial fibrillation. Medicine. 2016;95(11):e2874. https://doi.org/10.1097/MD.0000000000002874.
Wang TJ, Parise H, Levy D, D’Agostino RB Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–7.
Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19.
Cho DH, Joo HJ, Kim MN, Lim DS, Shim WJ, Park SM. Association between epicardial adipose tissue, high-sensitivity C-reactive protein and myocardial dysfunction in middle-aged men with suspected metabolic syndrome. Cardiovasc Diabetol. 2018;17(1):95. https://doi.org/10.1186/s12933-018-0735-7.
Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL, Van Gilst WH, Van Gelder IC, Rienstra M. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol. 2015;66:1000–7.
Pandey A, Kim S, Moore C, Thomas L, Gersh B, Allen LA, Kowey PR, Mahaffey KW, Hylek E, Peterson ED, Piccini JP, Fonarow GC, ORBIT-AF Investigators and Patients. Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail. 2017;5:44–52.
Chen SM, He R, Li WH, Li ZP, Chen BX, Feng XH. Relationship between exercise induced elevation of left ventricular filling pressure and exercise intolerance in patients with atrial fibrillation. J Geriatr Cardiol. 2016;13:546–51.
Meluzin J, Starek Z, Kulik T, Jez J, Lehar F, Wolf J, Dusek L, Leinveber P, Novak M. Prevalence and predictors of early heart failure with preserved ejection fraction in patients with paroxysmal atrial fibrillation. J Card Fail. 2017;23:558–62.
Reddy YNV, Obokata M, Gersh BJ, Borlaug BA. High prevalence of occult heart failure with preserved ejection fraction among patients with atrial fibrillation and dyspnea. Circulation. 2018;137:534–5.
Wu FZ, Huang YL, Wu CC, Wang YC, Pan HJ, Huang CK, Yeh LR, Wu MT. Differential effects of bariatric surgery versus exercise on excessive visceral fat deposits. Medicine. 2016;95(5):e2616. https://doi.org/10.1097/MD.0000000000002616.
Gaborit B, Jacquier A, Kober F, Abdesselam I, Cuisset T, Boullu-Ciocca S, Emungania O, Alessi MC, Clément K, Bernard M, Dutour A. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol. 2012;60:1381–9.
Alonso A, Bahnson JL, Gaussoin SA, Bertoni AG, Johnson KC, Lewis CE, Vetter M, Mantzoros CS, Jeffery RW, Soliman EZ. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the Look AHEAD randomized trial. Am Heart J. 2015;170:770–777.e5.
Rabkin SW, Campbell H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta-analysis. Obes Rev. 2015;16:406–15.
Fu CP, Sheu WH, Lee IT, Tsai IC, Lee WJ, Liang KW, Lee WL, Lin SY. Effects of weight loss on epicardial adipose tissue thickness and its relationship between serum soluble CD40 ligand levels in obese men. Clin Chim Acta. 2013;421:98–103.
Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, Abhayaratna WP, Kalman JM, Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–60.
Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–69.
Kurnicka K, Domienik-Karłowicz J, Lichodziejewska B, Bielecki M, Kozłowska M, Goliszek S, Dzikowska-Diduch O, Lisik W, Kosieradzki M, Pruszczyk P. Improvement of left ventricular diastolic function and left heart morphology in young women with morbid obesity six months after bariatric surgery. Cardiol J. 2018;25:97–105.
Klemm DJ, Leitner JW, Watson P, Nesterova A, Reusch JE, Goalstone ML, Draznin B. Insulin-induced adipocyte differentiation. Activation of CREB rescues adipogenesis from the arrest caused by inhibition of prenylation. J Biol Chem. 2001;276:28430–5.
Cieslik KA, Trial J, Carlson S, Taffet GE, Entman ML. Aberrant differentiation of fibroblast progenitors contributes to fibrosis in the aged murine heart: role of elevated circulating insulin levels. FASEB J. 2013;27:1761–71.
Trevisan R, Fioretto P, Semplicini A, Opocher G, Mantero F, Rocco S, Remuzzi G, Morocutti A, Zanette G, Donadon V, Perico N, Giorato C, Nosadini R. Role of insulin and atrial natriuretic peptide in sodium retention in insulin-treated IDDM patients during isotonic volume expansion. Diabetes. 1990;39:289–98.
Liou YS, Yang FY, Chen HY, Jong GP. Antihyperglycemic drugs use and new-onset atrial fibrillation: a population-based nested case control study. PLoS ONE. 2018;13(8):e0197245. https://doi.org/10.1371/journal.pone.0197245.
Chen HY, Yang FY, Jong GP, Liou YS. Antihyperglycemic drugs use and new-onset atrial fibrillation in elderly patients. Eur J Clin Investig. 2017;47:388–93.
Qi T, Chen Y, Li H, Pei Y, Woo SL, Guo X, Zhao J, Qian X, Awika J, Huo Y, Wu C. A role for PFKFB3/iPFK2 in metformin suppression of adipocyte inflammatory responses. J Mol Endocrinol. 2017;59:49–59.
Zulian A, Cancello R, Girola A, Gilardini L, Alberti L, Croci M, Micheletto G, Danelli P, Invitti C. In vitro and in vivo effects of metformin on human adipose tissue adiponectin. Obes Facts. 2011;4:27–33.
Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kuo CF, Wen MS, Chen WJ, Yeh YH, See LC. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;10(13):123. https://doi.org/10.1186/s12933-014-0123-x.
Yang W, Yang C, Luo J, Wei Y, Wang W, Zhong Y. Adiponectin promotes preadipocyte differentiation via the PPARγ pathway. Mol Med Rep. 2018;17:428–35.
Antonopoulos AS, Margaritis M, Verheule S, Recalde A, Sanna F, Herdman L, Psarros C, Nasrallah H, Coutinho P, Akoumianakis I, Brewer AC, Sayeed R, Krasopoulos G, Petrou M, Tarun A, Tousoulis D, Shah AM, Casadei B, Channon KM, Antoniades C. Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-γ/adiponectin signalling. Circ Res. 2016;118:842–55.
Liu C, Liu R, Fu H, Li J, Wang X, Cheng L, Korantzopoulos P, Tse G, Li G, Liu T. Pioglitazone attenuates atrial remodeling and vulnerability to atrial fibrillation in alloxan-induced diabetic rabbits. Cardiovasc Ther. 2017. https://doi.org/10.1111/1755-5922.12284.
Pallisgaard JL, Lindhardt TB, Staerk L, Olesen JB, Torp-Pedersen C, Hansen ML, Gislason GH. Thiazolidinediones are associated with a decreased risk of atrial fibrillation compared with other antidiabetic treatment: a nationwide cohort study. Eur Heart J Cardiovasc Pharmacother. 2017;3:140–6.
Gu J, Liu X, Wang X, Shi H, Tan H, Zhou L, Gu J, Jiang W, Wang Y. Beneficial effect of pioglitazone on the outcome of catheter ablation in patients with paroxysmal atrial fibrillation and type 2 diabetes mellitus. Europace. 2011;13:1256–61.
Zhang Z, Zhang X, Korantzopoulos P, Letsas KP, Tse G, Gong M, Meng L, Li G, Liu T. Thiazolidinedione use and atrial fibrillation in diabetic patients: a meta-analysis. BMC Cardiovasc Disord. 2017;17(1):96. https://doi.org/10.1186/s12872-017-0531-4.
Dorkhan M, Dencker M, Stagmo M, Groop L. Effect of pioglitazone versus insulin glargine on cardiac size, function, and measures of fluid retention in patients with type 2 diabetes. Cardiovasc Diabetol. 2009;20(8):15. https://doi.org/10.1186/1475-2840-8-15.
Sartipy U, Savarese G, Dahlström U, Fu M, Lund LH. Association of heart rate with mortality in sinus rhythm and atrial fibrillation in heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21:471–9.
Kosmala W, Holland DJ, Rojek A, Wright L, Przewlocka-Kosmala M, Marwick TH. Effect of If-channel inhibition on hemodynamic status and exercise tolerance in heart failure with preserved ejection fraction: a randomized trial. J Am Coll Cardiol. 2013;62:1330–8.
Qureshi W, O’Neal WT, Soliman EZ, Al-Mallah MH. Systematic review and meta-analysis of mortality and digoxin use in atrial fibrillation. Cardiol J. 2016;23:333–43.
Lafuente-Lafuente C, Valembois L, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;3:CD005049. https://doi.org/10.1002/14651858.cd005049.pub4.
Mulder BA, van Veldhuisen DJ, Crijns HJ, Böhm M, Cohen-Solal A, Babalis D, Roughton M, Flather MD, Coats AJ, Van Gelder IC. Effect of nebivolol on outcome in elderly patients with heart failure and atrial fibrillation: insights from SENIORS. Eur J Heart Fail. 2012;14:1171–8.
Mareev Y, Cleland JG. Should β-blockers be used in patients with heart failure and atrial fibrillation? Clin Ther. 2015;37:2215–24.
Voskoboinik A, Moskovitch J, Plunkett G, Bloom J, Wong G, Nalliah C, Prabhu S, Sugumar H, Paramasweran R, McLellan A, Ling LH, Goh CY, Noaman S, Fernando H, Wong M, Taylor AJ, Kalman JM, Kistler PM. Cardioversion of atrial fibrillation in obese patients: results from the cardioversion-BMI randomized controlled trial. J Cardiovasc Electrophysiol. 2019;30:155–61.
Potpara T, Marinković-Erić J, Grujić M, Radojković-Cirović B, Vujisić-Tesić B, Petrović M. Effect of diabetes mellitus in recovery and maintenance of sinus rhythm in patients with persistent atrial fibrillation. Srp Arh Celok Lek. 2002;130:189–92.
Dereli S, Bayramoğlu A, Yontar OC, Cerşit S, Gürsoy MO. Epicardial fat thickness: a new predictor of successful electrical cardioversion and atrial fibrillation recurrence. Echocardiography. 2018;35:1926–31.
Storz C, Hetterich H, Lorbeer R, Heber SD, Schafnitzel A, Patscheider H, Auweter S, Zitzelsberger T, Rathmann W, Nikolaou K, Reiser M, Schlett CL, von Knobelsdorff-Brenkenhoff F, Peters A, Schulz-Menger J, Bamberg F. Myocardial tissue characterization by contrast-enhanced cardiac magnetic resonance imaging in subjects with prediabetes, diabetes, and normal controls with preserved ejection fraction from the general population. Eur Heart J Cardiovasc Imaging. 2018;19:701–8.
Siebermair J, Suksaranjit P, McGann CJ, Peterson KA, Kheirkhahan M, Baher AA, Damal K, Wakili R, Marrouche NF, Wilson BD. Atrial fibrosis in non-atrial fibrillation individuals and prediction of atrial fibrillation by use of late gadolinium enhancement magnetic resonance imaging. J Cardiovasc Electrophysiol. 2019;30:550–6.
Chelu MG, King JB, Kholmovski EG, Ma J, Gal P, Marashly Q, AlJuaid MA, Kaur G, Silver MA, Johnson KA, Suksaranjit P, Wilson BD, Han FT, Elvan A, Marrouche NF. Atrial fibrosis by late gadolinium enhancement magnetic resonance imaging and catheter ablation of atrial fibrillation: 5-year follow-up data. J Am Heart Assoc. 2018;7(23):e006313. https://doi.org/10.1161/JAHA.117.006313.
Luetkens JA, Wolpers AC, Beiert T, Kuetting D, Dabir D, Homsi R, Meendermann H, Dayé NA, Knappe V, Karsdal M, Nielsen SH, Genovese F, Stöckigt F, Linhart M, Thomas D, Nickenig G, Schild HH, Schrickel JW, Andrié RP. Cardiac magnetic resonance using late gadolinium enhancement and atrial T1 mapping predicts poor outcome in patients with atrial fibrillation after catheter ablation therapy. Sci Rep. 2018;8(1):13618. https://doi.org/10.1038/s41598-018-31916-2.
Sanghai SR, Sardana M, Hansra B, Lessard DM, Dahlberg ST, Aurigemma GP, Fitzgibbons TP, McManus DD. Indexed left atrial adipose tissue area is associated with severity of atrial fibrillation and atrial fibrillation recurrence among patients undergoing catheter ablation. Front Cardiovasc Med. 2018;19(5):76. https://doi.org/10.3389/fcvm.2018.00076.
Sepehri Shamloo A, Dagres N, Dinov B, Sommer P, Husser-Bollmann D, Bollmann A, Hindricks G, Arya A. Is epicardial fat tissue associated with atrial fibrillation recurrence after ablation? A systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2019;22:132–8.
Lu ZH, Liu N, Bai R, Yao Y, Li SN, Yu RH, Sang CH, Tang RB, Long DY, Du X, Dong JZ, Ma CS. HbA1c levels as predictors of ablation outcome in type 2 diabetes mellitus and paroxysmal atrial fibrillation. Herz. 2015;40(Suppl 2):130–6.
Glover BM, Hong KL, Dagres N, Arbelo E, Laroche C, Riahi S, Bertini M, Mikhaylov EN, Galvin J, Kiliszek M, Pokushalov E, Kautzner J, Calvo N, Blomström-Lundqvist C, Brugada J, ESC-EHRA Atrial Fibrillation Ablation Long-Term Registry Investigators. Impact of body mass index on the outcome of catheter ablation of atrial fibrillation. Heart. 2019;105:244–50.
Lakkireddy DR, Blake GE, Patel D, Rotter M, Verma A, Ryschon K, Khan M, Schweikert R, Haissaguerre M, Natale A. Success of radiofrequency catheter ablation of atrial fibrillation: does obesity influence the outcomes? J Atr Fibrillation. 2008;1(1):36. https://doi.org/10.4022/jafib.36.
Donnellan E, Wazni OM, Kanj M, Baranowski B, Cremer P, Harb S, McCarthy CP, McEvoy JW, Elshazly MB, Aagaard P, Tarakji KG, Jaber WA, Schauer PR, Saliba WI. Association between pre-ablation bariatric surgery and atrial fibrillation recurrence in morbidly obese patients undergoing atrial fibrillation ablation. Europace. 2019. https://doi.org/10.1093/europace/euz183 (Epub ahead of print).
Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB, Pathik B, Nalliah CJ, Wong GR, Azzopardi SM, Gutman SJ, Lee G, Layland J, Mariani JA, Ling LH, Kalman JM, Kistler PM. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol. 2017;70:1949–61.
Packer M. Effect of catheter ablation on pre-existing abnormalities of left atrial systolic, diastolic, and neurohormonal functions in patients with chronic heart failure and atrial fibrillation. Eur Heart J. 2019;40:1873–9.
Witt CM, Fenstad ER, Cha YM, Kane GC, Kushwaha SS, Hodge DO, Asirvatham SJ, Oh JK, Packer DL, Powell BD. Increase in pulmonary arterial pressure after atrial fibrillation ablation: incidence and associated findings. J Interv Card Electrophysiol. 2014;40:47–52.
Acknowledgements
Not applicable.
Funding
There was no funding.
Author information
Authors and Affiliations
Contributions
There is only one author who takes full responsibility for the work. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
There is only one author who takes full responsibility for the work and consents to its submission.
Competing interests
Dr. Packer has recently consulted for Abbvie, Actavis, Akcea, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Gilead, Johnson & Johnson, NovoNordisk, Pfizer, Relypsa, Sanofi, Synthetic Biologics and Theravance. None of these relationships are relevant to the topic of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Packer, M. Disease–treatment interactions in the management of patients with obesity and diabetes who have atrial fibrillation: the potential mediating influence of epicardial adipose tissue. Cardiovasc Diabetol 18, 121 (2019). https://doi.org/10.1186/s12933-019-0927-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-019-0927-9