Abstract
Background
Carotid artery intima-media thickness (cIMT) progression is a surrogate marker of atherosclerosis with a high predictive value for future CVD risk. This study evaluates the comparative efficacies of lipid lowering, hypoglycemic, antihypertensive and antiplatelet medications on cIMT progression.
Methods
We conducted a network meta-analysis (NMA) to evaluate the relative efficacies of several drug classes in modifying cIMT progression. After a literature search in several electronic databases, studies were selected by following predetermined eligibility criteria. An inverse variance-heterogeneity model was used for NMA. Sensitivity analyses were performed to check the reliability of the overall NMA, and transitivity analyses were performed to examine the effects of modifiers on the NMA outcomes.
Results
Data were taken from 47 studies (15,721 patients; age: 60.2 years [95% confidence interval (CI) 58.8, 61.6]; BMI: 27.2 kg/m2 [95% CI 26.4, 28.0]; and gender: 58.3% males [95% CI 48.3, 68.3]). Treatment duration was 25.8 months [95% CI 22.9, 28.7]. Of the 13 drug classes in the network, treatment with phosphodiesterase III inhibitors was the most effective in retarding annual mean cIMT against network placebo (weighted mean difference (WMD) − 0.059 mm [95% CI − 0.099, − 0.020) followed by the calcium channel blockers (WMD − 0.055 mm [95% CI − 0.099, 0.001]) and platelet adenosine diphosphate inhibitors (WMD − 0.033 mm [95% CI − 0.058, 0.008]). These 3 drug classes also attained the same positions when the NMA was conducted by using first-year changes in mean cIMT. In transitivity analyses, longer treatment duration, higher body mass index (BMI), and a higher baseline cIMT were found to be independently associated with a lesser reduction in annual mean cIMT. However, in a multivariate analysis with these 3 modifiers, none of these factors was significantly associated with annual change in mean cIMT. In the placebo group, age was inversely associated with annual change in mean cIMT independently.
Conclusion
Phosphodiesterase III inhibitors and calcium channel blockers are found more effective than other drug classes in retarding cIMT progression. Age, BMI, and baseline cIMT may have some impact on these outcomes.
Similar content being viewed by others
Introduction
Metabolic diseases constitute a major global public health challenge. An aggregation of conditions like visceral obesity, dyslipidemia, hyperglycemia, hypertension, and insulin resistance also makes an individual vulnerable to type 2 diabetes and cardiovascular disease (CVD) [1, 2]. One of the most important associations of metabolic diseases is atherosclerosis, which is difficult to detect in younger healthy populations because of its gradual progression perhaps continuing throughout life depending on the presence of one or more risk factors [3]. Atherosclerosis risk factors include male gender, old age, smoking, hypertension, dyslipidemia (hypertriglyceridemia, higher low-density lipoproteins (LDL)-cholesterol and lower high-density lipoprotein (HDL)-cholesterol), hyperinsulinemia, family history, obesity, and diabetes mellitus [4].
A surrogate marker of atherosclerosis is intima-media thickness (IMT), which is the combined thickness of the tunica intima and media of a circulatory vessel detectable non-invasively with ultrasonographic techniques [5]. It is a valuable indicator of CVD risk, significantly correlated with current and future CVD when its absolute value and progression rates are determined [6]. Based on data from many studies, carotid artery IMT (cIMT) progression is considered an indicator of atherosclerosis with a high predictive value for future CVD and related mortality [7].
Several drugs are used to treat diabetes and other metabolic diseases, and evaluation of their efficacies is an active area of clinical research. Several trials have also reported the effect of various drugs on cIMT dynamics. We have undertaken a systematic review of relevant trials which evaluated the efficacy of one or more drug/s and reported on cIMT changes. Data acquired from these studies were used to conduct network meta-analysis (NMA) for evaluating the relative efficacies of different drug classes in changing mean cIMT.
Method
Eligibility criteria
A study was included if it (a) evaluated the efficacy of a drug/s used for the management of metabolic diseases (antihypertensive, antiplatelet, hypoglycemic, lipid lowering) in changing mean cIMT; (b) recruited patients with diabetes mellitus, impaired glucose tolerance, CVD, or metabolic syndrome and treated patients for at least 6 months; (c) reported annual changes in mean cIMT or baseline, yearly and end of study mean cIMT values; (d) had a placebo or comparative drug control group to compare the outcomes; and (e) investigated one or more of the following classes of drugs—alpha-glucosidase inhibitors, angiotensin I converting enzyme (ACE) inhibitor, angiotensin II receptor blockers, beta blockers, cyclooxygenase inhibitors, calcium channel blockers, dipeptidyl peptidase-4 (DPP4) inhibitors, glucagon-like peptide 1 (GLP-1) analogues, hydroxy-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors, incretin-based therapies, insulin secretagogues, peroxisome proliferator-activated receptor (PPAR) alpha/gamma agonists, phosphodiesterase III inhibitors, sodium-chloride cotransporter inhibitors, or sodium-glucose cotransporter-2 (SGLT2) inhibitors.
A study was excluded if it (a) compared a nutrient rather than a drug against a placebo or a comparative drug, (b) used a combination of drugs in one or both arms, (c) was a single arm trial, or (d) reported an endpoint other than mean cIMT (e.g., maximum IMT, etc.).
Literature search
The literature search was conducted out in electronic databases (Embase, Google Scholar, Ovid SP, and PubMed). Important Medical Subject Headings and key terms were used as combination phrases. Primary combination phrases included: carotid intima-media thickness—atherosclerosis—drug therapy. In a secondary search, the primary combination was used with several other keywords. The full search strategy is given in Additional file 1: Appendix S1. Bibliographies of important research and review articles, as well as database corroborations were also screened.
Data and analyses
Required cIMT measurement data along with important demographic and clinical information about the patients and study characteristics were extracted from the research articles and synthesized in datasheets for use in the NMA. The endpoints for NMAs were (a) annual change in mean cIMT and (b) first-year change in mean cIMT. NMAs were performed with MetaXL software (version 5.3; EpiGear International Pty Ltd) using continuous data under the inverse variance-heterogeneity (IVhet) model. Direct and repeated adjusted indirect comparisons were based on Generalized Pairwise Modeling (GPM) framework under the assumption that common control nodes are sufficiently similar [8, 9]. We used weighted mean differences as an effect estimator. IVhet is a distributional assumption-free model that tends to yield model-based estimator variance closer to the observed variance by re-scaling overdispersion. For this, IVhet adapts a quasi-likelihood approach in which individual comparisons are performed under a fixed effect assumption (inverse variance weighting) but the variance of the overall effect estimate is inflated to account for the heterogeneity [9].
As sensitivity analyses, NMAs were performed by using placebo-controlled studies (no comparator-controlled arms) under the IVhet model with MetaXL software and a random-effects conventional meta-analysis of mean differences between active drugs and their placebos in changing annual mean cIMT was performed with RevMan software (version 5.3; Cochrane Collaboration).
To assess the quality of the included studies, the Cochrane Collaboration’s Tool for the Quality Assessment of Randomized Controlled Trials was used. Assessment of publication bias was performed with Begg’s test. Transitivity analyses were performed to evaluate the impact of modifiers on NMA outcomes (annual change in mean cIMT) [10]. For this purpose, the effect sizes of all treatment and placebo arms were pooled separately and were subjected to metaregression with Stata software (Stata Corporation; Texas, USA) to evaluate the strength of associations between outcomes and modifiers.
Results
Data characteristics
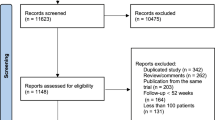
Forty-seven studies [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] were selected for inclusion in the NMA (Fig. 1). Available datasets (trial arms) were 53. Important characteristics of the included studies are presented in Additional file 1: Table S1. In these studies, 10,254 patients were treated with a therapeutic drug and 5467 patients were treated with placebo. The age and body mass index (BMI) of these patients were 60.2 years [95% confidence interval (CI): 58.8, 61.6] and 27.2 kg/m2 [95% CI 26.4, 28.0], respectively. In this population, the percentage of males was 58.3% [95% CI 48.3, 68.3] and the percentage of current smokers was 29.2% [95% CI 22.4, 35.9]. This population consisted of patients with type 2 diabetes mellitus (48.6%), dyslipidemia (18.7%), coronary syndromes (12.8%), hypertension (12.05%), and impaired glucose tolerance (7.8%).
NMA outcomes
In the overall network (Fig. 2), 13 drug classes (Table S2) were subjected to direct and indirect comparisons. Treatment duration was 25.8 months [22.9, 28.7]. Phosphodiesterase III inhibitors were found to be the most effective in retarding annual mean cIMT against network placebo (weighted mean difference (WMD) − 0.059 mm [95% CI − 0.099, − 0.020]) followed by the calcium channel blockers (WMD − 0.055 mm [95% CI − 0.099, 0.001]), platelet ADP inhibitors (WMD − 0.033 mm [95% CI − 0.058, 0.008] and cyclooxygenase inhibitors (WMD − 0.033 mm [95% CI − 0.054, 0.011]; Fig. 3). The outcomes of direct and indirect comparisons are given in Table S3 and the outcomes of all systematic NMAs are presented in Additional file 1: Figure S1a–m.
In a parallel NMA, instead of annual changes, only first year changes in mean cIMT reported by the included studies were used; the outcomes were similar. In this NMA, Phosphodiesterase III inhibitors (WMD − 0.059 mm [95% CI − 0.099, − 0.0196]), calcium channel blockers (WMD − 0.055 mm [95% CI − 0.099, 0.0099]), platelet ADP inhibitors (WMD − 0.033 mm [95% CI − 0.058, 0.008], and cyclooxygenase inhibitors (WMD − 0.033 mm [95% CI − 0.058, 0.0084]) were found to be the most effective in retarding annual mean cIMT progression (Fig. 4).
Sensitivity analyses
As a sensitivity analysis, a placebo-controlled network (no comparator in network) consisting of 10 classes studied in 36 trial arms was performed. In this network, treatment with phosphodiesterase III inhibitors (WMD − 0.051 mm [95% CI − 0.091, − 0.00]) was associated with the maximum reduction in annual mean cIMT, followed by the cyclooxygenase inhibitors (WMD − 0.03 mm [95% CI − 0.060, − 0.008]), and the platelet ADP inhibitors (WMD − 0.033 mm [95% CI − 0.064, − 0.002]; Additional file 1: Figure S2).
The outcomes of the placebo-controlled IV-het NMA were also compared with a placebo-controlled random-effects frequentist meta-analysis. In this meta-analysis, the phosphodiesterase III inhibitors (WMD − 0.05 mm [95% CI − 0.10, 0.00]; p = 0.05), platelet ADP inhibitors (WMD − 0.03 mm [95% CI − 0.06, − 0.00]; p = 0.04), PPAR-alpha agonists (WMD − 0.01 mm [95% CI − 0.03, 0.00]; p = 0.05), and PPAR-gamma agonists (WMD − 0.01 mm [95% CI − 0.01, − 0.00]; p = 0.008) showed significant reductions in annual change in mean cIMT (Additional file 1: Figure S3).
Quality of evidence
With some caveats discussed in the limitation section, the quality of the evidence generated herein is moderate based on the following observations. Except for 2 prospective non-randomized trials, all the included studies were randomized and controlled and were of moderate (mainly due to blinding constraints) to high quality (Additional file 1: Table S4). Although, the Begg’s test showed no significant publication (adjusted Kendall’s Score − 157 ± 105.62 (SD); p = 0.137; Additional file 1: Figure S4), weightage could be affected by the imbalance in the number of included studies for different drug classes.
Transitivity analyses
To determine the possible impact of modifiers on the NMA outcome, associations of the outcome (annual change in mean cIMT in treated and placebo groups) with important explanatory variables were estimated using metaregression (Additional file 1: Table S5). In the treatment group (any drug class), longer treatment duration, higher BMI, and a higher baseline cIMT were found to be independently associated with a lesser reduction in annual mean cIMT. However, in multivariate analysis with these 3 modifiers, none of these variables was significantly associated with the change in mean cIMT per annum. In placebo group, only increasing age was independently associated with a lesser reduction in mean cIMT per year. In correlation analyses, age was positively associated with baseline mean cIMT in both treatment and placebo groups (Additional file 1: Table S5).
Discussion
Metabolic diseases can increase the risk of heart disease, stroke and diabetes. Therefore, medication is required not only to control active disease but also to reduce risk of possible future cardiovascular events. In this NMA, we compared the effects of 13 drug classes on the progression of cIMT and found that phosphodiesterase III inhibitors and calcium channel blockers were more effective than other therapies, although most drug classes were associated with retardation of cIMT progression. Age, baseline cIMT and BMI were identified as potential modifiers affecting the change.
A previous meta-analysis of 5 RCTs found that cilostazol, a selective phosphodiesterase III inhibitor, significantly reduced the progression of cIMT (WMD: − 0.08 mm [95% CI − 0.13, − 0.04; P = 0.00003) in a patient population of which 85% had type 2 diabetes mellitus [58], which is comparable to the annual change observed for this drug (WMD: − 0.059 mm [95% CI − 0.099, − 0.020]) in the present NMA. Phosphodiesterase III inhibitors may attenuate atherosclerosis by increasing cyclic adenosine monophosphate (cAMP) levels in platelets, vascular smooth cells and endothelial cells which then inhibit smooth muscle cell migration and proliferation [12]. Cilostazol is reported to inhibit high glucose or angiotensin II-induced proliferation of human vascular smooth muscle cells by inhibiting cell cycle transcription factor, E2F, or type 1 plasminogen activator inhibitor expression, in vitro [59, 60]. Cilostazol also decreases serum triglyceride and LDL-cholesterol levels, increases HDL-cholesterol levels, improves insulin sensitivity, increases nitric oxide production, prevents production of adhesion molecules, and attenuates endothelial dysfunction [28].
Calcium channel blockers may also reduce cIMT progression by several mechanisms. For example, amlodipine has been reported to inhibit the growth and migration of vascular smooth muscles [61] and to manifest antioxidant effects to retard atherosclerosis [62]. Lacidipine, in addition to exerting antioxidant effects, also reduces adhesion molecule expression in endothelial cells and restores endothelium dependent vasodilatation in essential hypertensive patients [63, 64]. In a review of RCTs, calcium-channel blockers were found to be more effective than other antihypertensive drugs including diuretics, beta-blockers, and ACE inhibitors in blunting cIMT progression [65].
Previous reviews have also found that statins [66], and alpha-glucosidase inhibitors [67] are capable of attenuating cIMT progression. Statins may reverse cIMT by reducing lipids, inflammation, and oxidative stress, and they may change the histological characteristics of plaque [68]. Improved insulin sensitivity may play a role in atheroprotection [69] and an increase in HDL-cholesterol levels [69] and reduction in LDL-cholesterol [70] are associated with reduction of cIMT progression.
To date, less data are available for GLP-1 agonists, sodium-chloride transporter inhibitors, and SGLT2 inhibitors in association with cIMT progression. A meta-analysis of 5 studies found that 3–12 months of GLP-1 based therapies decreased cIMT, although statistically non-significantly [71]. A prospective cohort study of 35 type 2 diabetes outpatients found significantly reduced IMT in the empagliflozin and liraglutide-treated patients after 3 months of treatment [72]. Sitagliptin and liraglutide treatment also improved arterial stiffness, diastolic function and myocardial strain by reducing oxidative stress in subjects with newly diagnosed type 2 diabetes [73, 74].
In diabetes patients, atherosclerosis is a leading cause of mortality due to cardiovascular or cerebrovascular disease or sudden death [75]. CVD is 2–4 times more prevalent in diabetes patients than in the normal population. The mean difference in IMT observed between diabetes patients and non-diabetes individuals is 0.05–0.08 mm [76]. Glycosylated hemoglobin and the duration of diabetes have also been found to be positively associated with cIMT [77, 78]. For these reasons, prevention or retardation of atherosclerosis is more important in diabetes patients.
In the transitivity analyses, we also observed that a higher BMI was independently associated with a lesser reduction in annual mean cIMT. In a community-based study of 676 individuals, BMI was found to be a significant predictor of progression of cIMT in black but not white individuals after controlling for age, race, sex, and traditional CVD risk factors [79]. Moreover, BMI has been found to have a significantly positive correlation with cIMT [80, 81].
Some limitations of the present study should to be considered. An important limitation was the unavailability of an adequate number of studies for some drug classes such as sodium-chloride transporter inhibitors, SGLT2 inhibitors and GLP-1 receptor agonists. Another factor possibly affecting the NMA outcomes could be the varying number of studies for a drug class in the network as we had more data available for some classes such as PPAR-gamma agonists and statins but less for others such as platelet ADP inhibitors and cyclooxygenase inhibitors. Another shortcoming could arise from the grouping of PPAR-gamma agonists because pioglitazone and rosiglitazone are reported to have contrasting effects on cardiovascular outcomes. However, a sub-analysis of the present study showed were no significant differences between these 2 drugs in reducing cIMT.
Results of this study indicate that cardiovascular risk factors play a role in the progression of cIMT, and the drug environment may modify this process, reiterating the importance of timely therapeutic interventions in the control of cardiovascular risk factors. Because atherosclerosis is a progressive process and is affected by several risk factors, reduction in cIMT or slowing its progression can lead to the prevention or delaying of CVD events. Our finding (which is mainly based on primary prevention studies) that drugs, especially the phosphodiesterase III inhibitors and calcium channel blockers, significantly retard cIMT progression indicates that medication can significantly influence the relationship between IMT progression and CVD risk. We have also observed that a greater reduction in cIMT occurred in patients with high cIMT values at baseline. Thus, earlier treatment can improve the chances of a better prognosis in the long run. As Rundek et al. pointed out that the presence of plaque may be an important confounder in the predictive models of IMT, therefore a separate characterization of plaque and IMT is necessary for vascular disease risk assessment [82]. Our results identify medication use as another confounder in the predictive models of IMT progression and vascular disease risk assessment.
Conclusion
In this NMA, 13 drug classes used for the management of metabolic disorders (antihypertensive, antiplatelet, hypoglycemic, and lipid lowering) studied in 47 trials involving 15,721 subjects treated for approximately 2 years (25.8 months [95% CI 22.9, 28.7]), phosphodiesterase III inhibitors and calcium channel blockers were found more effective than others in retarding cIMT per annum. Age, BMI, and baseline cIMT may have some impact on these outcomes. Currently, less data are available for platelet ADP inhibitors, GLP-1 receptor agonists, sodium-chloride transporter inhibitors, and SGLT2 inhibitors.
Abbreviations
- ADDIS:
-
Aggregate Data Drug Information System
- ADP:
-
adenosine diphosphate
- BMI:
-
body mass index
- cIMT:
-
carotid artery intima-media thickness
- CVD:
-
cardiovascular disease
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- GLP-1:
-
glucagon like peptide-1
- GPM:
-
Generalized Pairwise Modeling
- HDL:
-
high-density lipoprotein
- HMG CoA:
-
hydroxy-methylglutaryl coenzyme A
- IMT:
-
intima-media thickness
- IVhet:
-
inverse variance-heterogeneity
- LDL:
-
low-density lipoproteins
- MCMC:
-
Markov Chain Monte Carlo
- NMA:
-
network meta-analysis
- PPAR:
-
peroxisome proliferator-activated receptor
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- RCTs:
-
randomized controlled trials
- SGLT2:
-
sodium-glucose cotransporter-2
References
Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–62.
Aguilar-Salinas CA, Rojas R, Gómez-Pérez FJ, Mehta R, Franco A, Olaiz G, et al. The metabolic syndrome: a concept hard to define. Arch Med Res. 2005;36(3):223–31.
Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Völzke H, Tuomainen TP, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379(9831):2053–62.
Chambless LE, Folsom AR, Davis V, Sharrett R, Heiss G, Sorlie P, et al. Risk factors for progression of common carotid atherosclerosis: the Atherosclerosis Risk in Communities Study, 1987–1998. Am J Epidemiol. 2002;155(1):38–47.
Lundby-Christensen L, Almdal TP, Carstensen B, Tarnow L, Wiinberg N. Carotid intima-media thickness in individuals with and without type 2 diabetes: a reproducibility study. Cardiovasc Diabetol. 2010;9:40. https://doi.org/10.1186/1475-2840-9-40.
Mita T, Katakami N, Shiraiwa T, Yoshii H, Gosho M, Shimomura I, et al. Changes in carotid intima-media thickening in patients with type 2 diabetes mellitus: subanalysis of the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation. J Diabetes Investig. 2017;8(2):254–5.
Peters SA, Grobbee DE, Bots ML. Carotid intima–media thickness: a suitable alternative for cardiovascular risk as outcome? Eur J Cardiovasc Prev Rehabil. 2011;18(2):167–74.
Barendregt JJ, Doi SA. MetaXL Version 5.3. User Guide. EpiGear International Pty Ltd. http://www.epigear.com.
Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trials. 2015;45(Pt A):130–8.
Mavridis D, Giannatsi M, Cipriani A, Salanti G. A primer on network meta-analysis with emphasis on mental health. Evid Based Ment Health. 2015;18(2):40–6.
Ahn CM, Hong SJ, Park JH, Kim JS, Lim DS. Cilostazol reduces the progression of carotid intima-media thickness without increasing the risk of bleeding in patients with acute coronary syndrome during a 2-year follow-up. Heart Vessels. 2011;26(5):502–10.
Ahn CW, Lee HC, Park SW, Song YD, Huh KB, Oh SJ, et al. Decrease in carotid intima media thickness after 1 year of cilostazol treatment in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2001;52(1):45–53.
Ariff B, Zambanini A, Vamadeva S, Barratt D, Xu Y, Sever P, et al. Candesartan- and atenolol-based treatments induce different patterns of carotid artery and left ventricular remodeling in hypertension. Stroke. 2006;37(9):2381–4.
Asselbergs FW, van Roon AM, Hillege HL, de Jong PE, Gans RO, Smit AJ, et al. Effects of fosinopril and pravastatin on carotid intima-media thickness in subjects with increased albuminuria. Stroke. 2005;36(3):649–53.
Baguet JP, Asmar R, Valensi P, Nissedurgeat S, Mallion JM. Effects of candesartan cilexetil on carotid remodeling in hypertensive diabetic patients: the MITEC study. Vascular Health Risk Manag. 2009;5(1):175–83.
Beishuizen ED, van de Ree MA, Jukema JW, Tamsma JT, van der Vijver JCM, Meinders AE, et al. Two-year statin therapy does not alter the progression of intima-media thickness in patients with type 2 diabetes without manifest cardiovascular disease. Diabetes Care. 2004;27:2887–92.
Bots ML, Palmer MK, Dogan S, Plantinga Y, Raichlen JS, Evans GW, et al. Intensive lipid lowering may reduce progression of carotid atherosclerosis within 12 months of treatment: the METEOR study. J Intern Med. 2010;265(6):698–707.
de Groot E, Jukema JW, van Boven AJ, Reiber JH, Zwinderman AH, Lie KI, et al. Effect of pravastatin on progression and regression of coronary atherosclerosis and vessel wall changes in carotid and femoral arteries: a report from the Regression Growth Evaluation Statin Study. Am J Cardiol. 1995;76:40C–6C.
Elkeles RS, Diamond JR, Poulter C, Dhanjil S, Nicolaides AN, Mahmood S, et al. Cardiovascular outcomes in type 2 diabetes: a double-blind placebo-controlled study of bezafibrate: the St. Mary’s, Ealing, Northwick Park Diabetes Cardiovascular Disease Prevention (SENDCAP) study. Diabetes Care. 1998;21:641–8.
Hanefeld M, Chiasson JL, Koehler C, Henkel E, Schaper F, Temelkova-Kurktschiev T. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke. 2004;35(5):1073–8.
Hedblad B, Wikstrand J, Janzon L, Wedel H, Berglund G. Low-dose metoprolol CR/XL and fluvastatin slow progression of carotid intima-media thickness: main results from the Beta-Blocker Cholesterol-Lowering Asymptomatic Plaque Study (BCAPS). Circulation. 2001;103(13):1721–6.
Hedblad B, Zambanini A, Nilsson P, Janzon L, Berglund G. Rosiglitazone and carotid IMT progression rate in a mixed cohort of patients with type 2 diabetes and the insulin resistance syndrome: main results from the Rosiglitazone Atherosclerosis Study. J Intern Med. 2007;261(3):293–305.
Hiukka A, Westerbacka J, Leinonen ES, Hulten LM, Taskinen MR, Hiukka A, et al. Long-term effects of fenofibrate on carotid intima-media thickness and augmentation index in subjects with type 2 diabetes mellitus. J Am College Cardiol. 2008;52(25):2190–7.
Hodis HN, Mack WJ, Zheng L, Li Y, Torres M, Sevilla D, et al. Effect of peroxisome proliferator-activated receptor gamma agonist treatment on subclinical atherosclerosis in patients with insulin-requiring type 2 diabetes. Diabetes Care. 2006;29(7):1545–53.
Hosomi N, Mizushige K, Ohyama H, Takahashi T, Kitadai M, Hatanaka Y, et al. Angiotensin-converting enzyme inhibition with enalapril slows progressive intima-media thickening of the common carotid artery in patients with non-insulin-dependent diabetes mellitus. Stroke. 2001;32(7):1539–45.
Igase M, Kohara K, Tabara Y, Nagai T, Ochi N, Kido T, et al. Low-dose rosuvastatin improves the functional and morphological markers of atherosclerosis in asymptomatic postmenopausal women with dyslipidemia. Menopause. 2012;19(12):1294–9.
Ishikawa S, Shimano M, Watarai M, Koyasu M, Uchikawa T, Ishii H, et al. Impact of sitagliptin on carotid intima-media thickness in patients with coronary artery disease and impaired glucose tolerance or mild Diabetes Mellitus. Am J Cardiol. 2014;114(3):384–8.
Katakami N, Kim YS, Kawamori R, Yamasaki Y. The phosphodiesterase inhibitor cilostazol induces regression of carotid atherosclerosis in subjects with type 2 diabetes mellitus: principal results of the Diabetic Atherosclerosis Prevention by Cilostazol (DAPC) study: a randomized trial. Circulation. 2010;52(5):1421–9.
Kodama M, Yamasaki Y, Sakamoto K, Yoshioka R, Matsuhisa M, Kajimoto Y, et al. Antiplatelet drugs attenuate progression of carotid intima-media thickness in subjects with type 2 diabetes. Thromb Res. 2000;97(4):239–45.
Koshiyama H, Tanaka S, Minamikawa J. Effect of calcium channel blocker amlodipine on the intima-media thickness of carotid arterial wall in type 2 diabetes. J Cardiovasc Pharmacol. 1999;33:894–6.
Koshiyama H, Shimono D, Kuwamura N, Minamikawa J, Nakamura Y. Inhibitory effect of pioglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab. 2001;86:3452–6.
Koyasu M, Ishii H, Watarai MK, Inden Y, Takeshita K, Amano T, et al. Impact of acarbose on carotid intima-media thickness in patients with newly diagnosed impaired glucose tolerance or mild type 2 diabetes mellitus: a one-year, prospective, randomized, open-label, parallel-group study in Japanese adults with established cor. Clin Ther. 2010;32(9):1610–7.
Langenfeld MR, Forst T, Hohberg C. Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus: results from a controlled randomized study. Circulation. 2005;14(12):22–9.
Lonn EM, Gerstein HC, Sheridan P, Smith S, Diaz R, Mohan V, et al. Effect of ramipril and of rosiglitazone on carotid intima-media thickness in people with impaired glucose tolerance or impaired fasting glucose: STARR (STudy of Atherosclerosis with Ramipril and Rosiglitazone). J Am College Cardiol. 2009;53(22):2036–8.
Ludwig M, Stapff M, Ribeiro A, Fritschka E, Tholl U, Smith RD, et al. Comparison of the effects of losartan and atenolol on common carotid artery intima-media thickness in patients with hypertension: results of a 2-year, double-blind, randomized, controlled study. Clin Ther. 2002;24(7):1175–93.
Mazzone T, Meyer PM, Feinstein SB, Davidson MH, Kondos GT, D’Agostino RB, et al. Effect of pioglitazone compared with glimepiride on carotid intima-media thickness in type 2 diabetes: a randomized trial. JAMA. 2006;296(21):2572–81.
Minamikawa J, Tanaka S, Yamauchi M, Inoue D, Koshiyama H. Potent inhibitory effect of troglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab. 1998;83:1818–20.
Mita T, Watada H, Shimizu T, Tamura Y, Sato F, Watanabe T, et al. Nateglinide reduces carotid intima-media thickening in type 2 diabetic patients under good glycemic control. Arterioscler Thromb Vasc Biol. 2007;27(11):2456–62.
Mita T, Katakami N, Yoshii H, Onuma T, Kaneto H, Osonoi T, et al. Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: the Study of Preventive Effects of Alogliptin on Diabetic Atherosclerosis (SPEAD-A). Diabetes Care. 2016;39(1):139–48.
Mita T, Katakami N, Shiraiwa T, Yoshii H, Onuma T, Kuribayashi N, et al. Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the Sitagliptin Preventive Study of Intima-Media Thickness Evaluation (SPIKE): A randomized controlled trial. Diabetes Care. 2016;39(3):455–64.
Mitsuhashi N, Tanaka Y, Kubo S, Ogawa S, Hayashi C, Uchino H, et al. Effect of cilostazol, a phosphodiesterase inhibitor, on carotid IMT in Japanese type 2 diabetic patients. Endocr J. 2004;51(6):545–50.
Mörtsell D, Malmqvist K, Held C, Kahan T. Irbesartan reduces common carotid artery intima-media thickness in hypertensive patients when compared with atenolol: the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA) study. J Intern Med. 2010;261(5):472–9.
Nakamura T, Matsuda T, Kawagoe Y, Ogawa H, Takahashi Y, Sekizuka K, et al. Effect of pioglitazone on carotid intima-media thickness and arterial stiffness in type 2 diabetic nephropathy patients. Metabolism. 2004;53(10):1382–6.
Ohta Y, Kawano Y, Iwashima Y, Hayashi S, Yoshihara F, Matayoshi T, et al. Control of home blood pressure with an amlodipine- or losartan-based regimen and progression of carotid artery intima-media thickness in hypertensive patients: the HOSP substudy. Clin Exp Hypertens. 2013;35(4):279–84.
Olsen MH, Wachtell K, Neland K, Bella JN, Rokkedal J, Dige-Petersen H, et al. Losartan but not atenolol reduces carotid artery hypertrophy in essential hypertension. A LIFE substudy. Blood Press. 2005;14:177–83.
Oyama T, Saiki A, Endoh K, Ban N, Nagayama D, Ohhira M, et al. Effect of acarbose, an alpha-glucosidase inhibitor, on serum lipoprotein lipase mass levels and common carotid artery intima-media thickness in type 2 diabetes mellitus treated by sulfonylurea. J Atheroscler Thromb. 2008;15(3):154–9.
Oyama J, Murohara T, Kitakaze M, Ishizu T, Sato Y, Kitagawa K, et al. The Effect of sitagliptin on carotid artery atherosclerosis in type 2 diabetes: the PROLOGUE Randomized Controlled Trial. PLoS Med. 2016;13(6):e1002051. https://doi.org/10.1371/journal.pmed.1002051.
Patel YR, Kirkman MS, Considine RV, Hannon TS, Mather KJ. Effect of acarbose to delay progression of carotid intima–media thickness in early diabetes. Diabetes Metab Res Rev. 2013;29(7):582–91.
Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, et al. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST). J Am Coll Cardiol. 2002;39(4):610–6.
Shinoda-Tagawa T, Yamasaki Y, Yoshida S, Kajimoto Y, Tsujino T, Hakui N, et al. A phosphodiesterase inhibitor, cilostazol, prevents the onset of silent brain infarction in Japanese subjects with Type II diabetes. Diabetologia. 2002;45(2):188–94.
Sidhu JS, Kaposzta Z, Markus HS, Kaski JC. Effect of rosiglitazone on common carotid intima-media thickness progression in coronary artery disease patients without diabetes mellitus. Arterioscler Thromb Vasc Biol. 2004;24(5):930.
Stanton AV, Chapman JN, Mayet J, Sever PS, Poulter NR, Hughes AD, et al. Effects of blood pressure lowering with amlodipine or lisinopril on vascular structure of the common carotid artery. Clin Sci (Lond). 2001;101:455–64.
Stumpe KO, Agabitirosei E, Zielinski T, Schremmer D, Scholze J, Laeis P, et al. Carotid intima-media thickness and plaque volume changes following 2-year angiotensin II-receptor blockade. The Multicentre Olmesartan atherosclerosis Regression Evaluation (MORE) study. Ther Adv Cardiovasc Dis. 2007;1(2):97–106.
Wiklund O, Hulthe J, Wikstrand J, Schmidt C, Olofsson SO, Bondjers G. Effect of controlled release/extended release metoprolol on carotid intima-media thickness in patients with hypercholesterolemia: a 3-year randomized study. Stroke. 2002;33(2):572–7.
Xiang AH, Peters RK, Kjos SL, Ochoa C, Marroqin A, Goico J, et al. Effect of thiazolidinedione treatment on progression of subclinical atherosclerosis in postmenopausal women at high risk for type 2 diabetes. J Clin Endocrinol Metabol. 2005;90:1986–91.
Yamamoto K, Ozaki H, Takayasu K, Akehi N, Fukui S, Sakai A, et al. The Effect of losartan and amlodipine on left ventricular diastolic function and atherosclerosis in Japanese patients with mild-to-moderate hypertension (J-ELAN) study. Hypertens Res. 2010;34(3):325–30.
Yasunari E, Takeno K, Funayama H, Tomioka S, Tamaki M, Fujitani Y, et al. Efficacy of pioglitazone on glycemic control and carotid intima-media thickness in type 2 diabetes patients with inadequate insulin therapy. J Diabetes Investig. 2011. https://doi.org/10.1111/j.2040-1124.2010.00064.x.
Geng DF, Deng J, Jin DM, Wu W, Wang JF. Effect of cilostazol on the progression of carotid intima-media thickness: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;220(1):177–83.
Kim MJ, Park KG, Lee KM, Kim HS, Kim SY, Kim CS, et al. Cilostazol inhibits vascular smooth muscle cell growth by downregulation of the transcription factor E2F. Hypertension. 2005;45(4):552–6.
Lee KM, Lee HJ, Kim MK, Kim HS, Jung GS, Hur SH, et al. Cilostazol inhibits high glucose- and angiotensin II-induced type 1 plasminogen activator inhibitor expression in artery wall and neointimal region after vascular injury. Atherosclerosis. 2009;207(2):391–8.
Stepien O, Gogusev J, Zhu DL, Iouzalen L, Herembert T, Drueke TB, et al. Amlodipine inhibition of serum-, thrombin-, or fibroblast growth factor-induced vascular smooth-muscle cell proliferation. J Cardiovasc Pharmacol. 1998;31:786–93.
Zhang X, Hintze TH. Amlodipine releases nitric oxide from canine coronary microvessels: an unexpected mechanism of action of a calcium channel-blocking agent. Circulation. 1998;95:576–80.
Cristofori P, Lanzoni A, Quartaroli M, Pastorino AM, Zancanaro C, Cominacini L, et al. The calcium-channel blocker lacidipine reduces the development of atherosclerotic lesions in the apoE deficient mouse. J Hypertens. 2000;18:1429–36.
Taddei S, Virdis A, Ghiadoni L, Magagna A, Pasini AF, Garbin U, Cominacini L, Salvetti A. Effect of calcium antagonist or beta blockade treatment on nitric oxide-dependent vasodilation and oxidative stress in essential hypertensive patients. J Hypertens. 2001;19(8):1379–86.
Cuspidi C, Negri F, Giudici V, Capra A, Sala C. Effects of antihypertensive drugs on carotid intima-media thickness: Focus on angiotensin II receptor blockers. A review of randomized, controlled trials. Integr Blood Press Control. 2009;2:1–8.
Huang Y, Li W, Dong L, Li R, Wu Y. Effect of statin therapy on the progression of common carotid artery intima-media thickness: an updated systematic review and meta-analysis of randomized controlled trials. J Atheroscler Thromb. 2013;20(1):108–21.
Geng DF, Jin DM, Wu W, Fang C, Wang JF. Effect of alpha-glucosidase inhibitors on the progression of carotid intima-media thickness: a meta-analysis of randomized controlled trials. Atherosclerosis. 2011;218(1):214–9.
Mita T, Katakami N, Shiraiwa T, Yoshii H, Gosho M, Shimomura I, et al. The effect of sitagliptin on the regression of carotid intima-media thickening in patients with type 2 diabetes mellitus: a post hoc analysis of the sitagliptin preventive study of intima-media thickness evaluation. Int J Endocrinol. 2017. https://doi.org/10.1155/2017/1925305.
Davidson M, Meyer PM, Haffner S, Feinstein S, D’Agostino R Sr, Kondos GT, et al. Increased high-density lipoprotein cholesterol predicts the pioglitazone-mediated reduction of carotid intima-media thickness progression in patients with type 2 diabetes mellitus. Circulation. 2008;117(16):2123–30.
Labreuche J, Deplanque D, Touboul PJ, Bruckert E, Amarenco P. Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis: systematic review and meta-regression analysis. Atherosclerosis. 2010;212(1):9–15.
Song X, Jia H, Jiang Y, Wang L, Zhang Y, Mu Y, Liu Y. Anti-atherosclerotic effects of the glucagon-like peptide-1 (GLP-1) based therapies in patients with type 2 Diabetes Mellitus: a meta-analysis. Sci Rep. 2015;5:10202. https://doi.org/10.1038/srep10202.
Irace C, Casciaro F, Scavelli FB, Oliverio R, Cutruzzolà A, Cortese C, Gnasso A. Empagliflozin influences blood viscosity and wall shear stress in subjects with type 2 diabetes mellitus compared with incretin-based therapy. Cardiovasc Diabetol. 2018;17(1):52. https://doi.org/10.1186/s12933-018-0695-y.
Katakami N, Mita T, Irie Y, Takahara M, Matsuoka TA, Gosho M, et al. Effect of sitagliptin on tissue characteristics of the carotid wall in patients with type 2 diabetes: a post hoc sub-analysis of the sitagliptin preventive study of intima-media thickness evaluation (SPIKE). Cardiovasc Diabetol. 2018;17(1):24. https://doi.org/10.1186/s12933-018-0666-3.
Lambadiari V, Pavlidis G, Kousathana F, Varoudi M, Vlastos D, Maratou E, et al. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):8. https://doi.org/10.1186/s12933-017-0646-z.
Burchfiel CM, Reed DM, Marcus EB, Strong JP, Hayashi T. Associations of diabetes mellitus with coronary atherosclerosis and myocardial lesions: an autopsy study from the Honolulu Heart Program. Am J Epidemiol. 1993;137:1328–40.
Folsom AR, Eckfeldt HJ, Weitzman S, Ma J, Chambless LE, Barnes RW, et al. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Stroke. 1994;25:66–73.
Kawamori R, Yamasaki Y, Matsushima H, Nishizawa H, Nao K, Hougaku H, et al. Prevalence of carotid atherosclerosis in diabetic patients. Ultrasound high-resolution B-mode imaging on carotid arteries. Diabetes Care. 1992;15(10):1290–4.
Kanters S, Alga A, Banga JD. Carotid intima-media thickness in hyperlipidemic type I and type II diabetic patients. Diabetes Care. 1997;3:276–80.
Alonso CF, Barshop R, Galazka P, Li S, Chen W, Berenson GS. Body mass index is associated with progression of carotid intima-media thickness in black, but not in white young adults: The Bogalusa Heart Study. Arterioscler Thromb Vasc Biol. 2018;35:Ab495.
Asaleye AA, Braimoh KT, Oyinloye OI, Asaleye CM, Omisore AD. Variation of carotid intima media thickness with body mass index in healthy adults of black African descent. J Ultrasound Med. 2018. https://doi.org/10.1002/jum.14673.
Eric OU, Atinuke MA, Abiodun OA, Ademola JA. Body mass index as a determinant of carotid intima-media thickness in Nigerian adults with primary hypertension. Ann Afr Med. 2014;13(4):151–6.
Rundek T, Brook RD, Spence JD. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007. https://doi.org/10.1161/circulationaha.107.696385.
Authors’ contributions
Designed research: LJ and RH. Performed literature search: RH, YX, YC, and ZH. Synthesized and analyzed data: KM, JR, HH and YL. Wrote Manuscript: RH and LJ. All authors read and approved the final manuscript.
Acknowledgements
None.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Please contact authors for data requests.
Consent for publication
All authors consent to submission and publication of this research.
Ethics approval and consent to participate
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China under Grant No. 31300137. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1.
Additional figures and tables.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Huang, R., Mills, K., Romero, J. et al. Comparative effects of lipid lowering, hypoglycemic, antihypertensive and antiplatelet medications on carotid artery intima-media thickness progression: a network meta-analysis. Cardiovasc Diabetol 18, 14 (2019). https://doi.org/10.1186/s12933-019-0817-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-019-0817-1