Abstract
Background
Hyperinsulinemia and insulin resistance have been recently recognized as an important cause of atherosclerosis. Clinical studies have also found that expression of the estrogen receptor is closely related to the incidence of atherosclerosis. This study investigate the effects of insulin and estrogen receptor α (ER-α) in atherosclerosis.
Methods
Double knockout ApoE/Lepr mice were given intraperitoneal injections of insulin, and their aortae were harvested for hematoxylin-eosin staining and immunohistochemical analysis. In addition, vascular smooth muscle cells (VSMCs) were treated with insulin or infected with a lentivirus encoding exogenous ER-α, and changes in gene expression were detected by real-time polymerase chain reaction and western blotting. The methylation levels of the ER-α gene were tested using bisulfite sequencing PCR, and flow cytometry and EdU assay were used to measure VSMCs proliferation.
Results
Our results showed that insulin can induce the formation of atherosclerosis. Gene expression analysis revealed that insulin promotes the expression of DNA methyltransferases and inhibits ER-α expression, while 5-aza-2′-deoxycytidine can inhibit this effect of insulin. Bisulfite sequencing PCR analysis showed that methylation of the ER-α second exon region increased in VSMCs treated with insulin. The results also showed that ER-α can inhibit VSMCs proliferation.
Conclusions
Our data suggest that insulin promotes the expression of DNA methyltransferases, induces methylation of ER-α second exon region and decreases the expression of ER-α, thereby interfering with estrogen regulation of VSMCs proliferation, resulting in atherosclerosis.
Similar content being viewed by others
Background
Atherosclerosis is a major cardiovascular disease affecting human health that is associated with a variety of factors such as obesity, abnormal lipid metabolism, hypertension, hyperinsulinemia and insulin resistance [1]. Insulin resistance leads to increased blood insulin levels, termed hyperinsulinemia, which has been confirmed by several clinical studies to be a predictor of cardiovascular disease [2, 3]. Abundant evidence also indicates that high concentrations of insulin can stimulate vascular smooth muscle cells (VSMCs) proliferation and migration [4, 5], which are currently thought to be the direct causes of atherosclerosis [6, 7].
An epidemiological study found that the incidence of cardiovascular diseases and insulin resistance are low in premenopausal women, with no significant differences between postmenopausal women and men, suggesting that endogenous estrogen in premenopausal women tends to reduce the risk of these diseases [8, 9]. Population-based observational studies have found that the application of estrogen in the early stage of postmenopausal women was associated with a reduced incidence of cardiovascular diseases and an improvement of insulin resistance. Estrogens exert their atheroprotective effects by binding to the estrogen receptor (ER), which regulates the transcription of many genes [10]. ER-α, ER-β and G protein-coupled estrogen receptor 1 (GPER1) are the three known receptors that mediate the effects of estrogen. The protective effect on vessels is retained in ER-β knockout mice but lost in ER-α knockout mice, suggesting that ER-α is essential for the protective effect of estrogen on the vasculature [11–13]. Lundholm et al. [14] found that ER-α activation with propyl pyrazole triol (PPT) reverses high fat diet-induced insulin resistance. Other researcher found that some male patients with ER-α deficiency exhibited insulin resistance, impaired glucose metabolism and hyperinsulinemia [15]. Estrogen can combine with ER-α to generate nitrogen monoxide (NO) in endothelial cells (ECs), which has a protective function on the vessels [1, 12, 16, 17] and suppresses smooth muscle cell proliferation by inhibiting the expression of extracellular signal-regulated kinase, c-Jun N-terminal kinase, P38, and other genes [18]. The expression of ER-α is decreased in the late stage of postmenopausal women, meaning that taking an estrogen supplement cannot reverse the risk of atherosclerosis, which is consistent with the findings of other studies [19–21]. Together, these data suggest that ER-α expression is one of the most important factors protecting premenopausal women from atherosclerosis.
Estrogen signaling is tightly intertwined with epigenetic regulation [22, 23], especially DNA methylation associated with gene repression. Methylation of ER genes is an important mechanism of reducing the expression of ER, and ER-α promoter methylation is increased in atherosclerotic patients and/or postmenopausal women [24]. At the same time, a growing body of evidence indicates the involvement of epigenetic alterations in atherosclerotic plaque formation and progression. The mechanism through which insulin promotes atherosclerosis also involves epigenetic modifications; high insulin levels reduce methylation of the leptin and adiponectin promoters, which contributes to atherosclerosis [25]. However, whether insulin induces ER-α methylation has not been investigated. To date, the nature of the relationship between hyperinsulinemia, ER-α expression and atherosclerosis is unclear.
In this study, we propose and test the hypothesis that high insulin levels can induce ER-α methylation and reduce ER-α expression, ultimately resulting in atherosclerosis. Double knockout ApoE/Lepr mice were given intraperitoneal injections of insulin for 12 weeks, and their aortae were harvested for hematoxylin–eosin (HE) staining and immunohistochemical analysis to investigate the formation of atherosclerotic plaques and differences in ER-α expression. We also tested ER-α and DNA methyltransferases (DNMTs) expression both in mRNA and protein levels in VSMCs treated with insulin. We also tested the methylation levels of the ER-α exon regions in VSMCs with or without insulin treatment. In addition, we measured the VSMCs proliferation after infection with a lentivirus encoding exogenous ER-α.
Methods
Materials
Specific pathogen free-grade ApoE/Lepr double knockout mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The lentivirus LV5-Esr1 (Rat) NM_012689.1 carrying an ER-α expression vector and the zero-load LV5NC (Suzhou Genepharma Co.,Ltd, Suzhou, China), a rat VSMC cell line, 5-Aza (Sigma-Aldrich, St. Louis, MO, USA), insulin for injection and insulin for cell culture (Roche, Basel, Switzerland), RIPA lysis buffer (Beyotime, Jiangsu, China), TriPure Isolation Reagent (Roche), anti-GAPDH antibody (PTSolution Biotechnology Co., Ltd, Guangzhou, China), anti-ER-α antibody (Merck Millipore, Bedford, MA, USA), anti-DNMT1 antibody (Santa Cruz Biotechnology, Dallas, TX, USA), anti-DNMT3a antibody (Abnova, Taibei, Taiwan), iTaq™ Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA), and reverse transcription PrimeScript™ RT reagent kit with gDNA Eraser (Perfect Real Time, TaKaRa, Dalian, Japan) were used in this study.
Animals and treatment
All animal work was approved by the Animal Care and Use Committee of the Third Military Medical University (Chongqing, China), and the methods were carried out in accordance with the approved guidelines. Mice were housed in a room with controlled temperature and 12 h light–dark cycle and had free access to water and standard chow. Eight-week-old ApoE/Lepr double knockout female mice (n = 8) were randomly divided in two groups: control group (N; n = 4) and insulin group (INS; n = 4), which received intraperitoneal injections of saline or insulin (4 IU) every other day for 12 weeks, respectively. Body weights and blood glucose were recorded once a week. At the end of the protocol, mice were euthanized by anesthesia for tissue harvesting.
Cell culture and treatment
Rat VSMCs were cultured in Dulbecco’s modified Eagle’s medium (HyClone, Logan City, UT, USA) with 10% fetal bovine serum. Cells were treated with various combination of insulin (100 nM) and/or 5-Aza (10 μM), or infected with lentivirus carrying an ER-α expression vector and then were harvested for the indicated assays.
Immunohistochemical staining
Fresh tissues were collected and fixed in 4% paraformaldehyde and embedded in paraffin. Tissue slides were obtained through serial sectioning and processing with standard procedures. The slides were stained with hematoxylin-eosin or ER-α antibody diluted at 1:100. After being washed, the slides were incubated with a biotinylated secondary antibody, and the color reaction was performed using ABC reagent (Zhongshan Co., Beijing, China) then counterstained with hematoxylin.
Real-time PCR
Following the instructions of the PrimeScript™ RT reagent kit, 1 μg total RNA was reverse transcribed to cDNA. Quantitative PCR amplification was performed with a 10 µl final reaction mixture consisting of 0.5 µl of reverse transcription reaction, 0.1 µM of both the sense and antisense primers (Table 1) and the SYBR Green Master mix. The PCR conditions were an initial denaturation at 95 °C for 5 min followed by 40 cycles of PCR reaction: denaturation at 95 °C for 10 s; annealing at 58 °C for 20 s and elongation at 72 °C for 30 s. Gene expression was normalized to the corresponding Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels and presented as the fold difference relative to GAPDH.
Western blotting
Cell pellets were harvested at the indicated times and were homogenized in ice-cold RIPA buffer (Beyotime) containing complete protease inhibitor cocktail. Total protein was resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. After blocking in 3% nonfat milk for 1 h, membranes were incubated with antibodies against total ER-α, DNMT1, DNMT3a, and GAPDH, followed by incubation with the corresponding secondary antibodies (Zhongshan Co.). The bands were visualized using the enhanced chemiluminescence system. The expression of individual proteins were normalized to GAPDH.
Bisulfite sequencing PCR
The CpG island of ER-α situates in its second exon, the sequence was obtained by searching the University of California Santa Cruz (UCSC) Genome Bioinformatics website (http://genome.ucsc.edu/) and localizes in chr1:42669631–42670064. The BSP primers were designed by Methyl Primer Express v1.0 (Table 1). DNA was extracted from the rat VSMCs using Apoptosis, DNA Ladder Extracion Kit with Spin Column (Beyotime, Shanghai, China). Bisulfite modification of genomic DNA was performed by EZ DNA Methylation-Gold™ Kit (Zymo Research Corp, Orange, CA) (DNA sample was then treated with sodium bisulfate, converting unmethylated cytosine (C) to uracil and keeping methylated C intact). Then the bisulfate-modified DNA were amplified by PCR. The reaction conditions were as follows: pre-degeneration at 98 °C for 4 min, 94 °C for 45 s, 66 °C for 45 s, where the annealing temperature was reduced by 0.5 °C in each cycle and 72 °C for 1 min for a total of 20 cycles; 94 °C for 45 s, 56 °C for 45 s, and 72 °C for 1 min for a total of 20 cycles, with a final elongation step of 72 °C for 8 min. The PCR products were separated by electrophoresis in a 2% agarose gel and recycled by the gel extraction kit (Sigma-Aldrich), followed by linking with T-vectors (Suzhou Genepharma Co., Ltd, Suzhou, China) and white-blue plaque selection, 6–10 of positive clones were sequenced in each sample at BGI. The sequencing results were then imported into BIQ Analyzer software (a software tool for easy visualization and quality control of DNA methylation data from bisulfate sequencing, http://biq-analyzer.bioinf.mpi-inf.mpg.de/) for comparing and calculating the methylation ratio.
Lentiviral infection
Approximately 1 × 104 VSMCs were inoculated in culture flasks with 50 µl of LV5-Esr1 or LV5NC to make up the final volume of 2 ml complete medium. The medium was changed after 24 h, and fluorescence was observed after 72 h.
EdU assay and flow cytometry
VSMCs were inoculated in 96-well plates, approximately 5000/well. Then following the manufacturer’s instructions of the EdU (5-ethynyl-29-deoxyuridine) assay kit (Ribobio, Guangzhou, China), cells proliferation was calculated. The cells were exposed to 50 µM of EdU for 2 h at 37 °C. The cells were then fixed with 4% formaldehyde for 30 min at room temperature and treated with 0.5% Triton X-100 for 10 min at room temperature for permeabilization. After wash with PBS, the cells were treated with 100 µl of 1 × ApolloR reaction cocktail for 30 min. Subsequently, the DNA contents of each well of cells were stained with 100 µl of 1 × Hoechst 33,342 for 10 min and visualized under a fluorescent microscope (Olympus, Japan).
VSMCs were collected, resuspended in 70% ethanol, and stored at −20 °C overnight. Prior to mounting, the cells were thrice rinsed with phosphate buffer saline (PBS), stained with propidium iodide, and incubated at room temperature in the dark, then analyzed using flow cytometry.
Statistical analysis
The data are presented as mean ± SD. For the comparison between multiple groups, quantitative data were analyzed using one-way ANOVA and Spearman rank correlation analysis, and qualitative data were analyzed using Chi square test. Values less than P < 0.05 were considered significant.
Results
Insulin decreased ER-α expression in VSMCs in the atherosclerosis plaque of mice
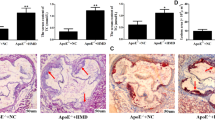
Mice in the experimental group showed significant atherosclerotic plaques (mean plaque area: 104,733 ± 11.3 × 103 µm2), thickened walls, and a disordered arrangement of nuclei after the 12-week treatment of intraperitoneal insulin injections compared with the control group (Fig. 1a; P < 0.01). Immunohistochemistry revealed that the expression of ER-α protein in the vascular smooth muscle nuclei was significantly decreased in the experimental group compared with the control group (Fig. 1b; P < 0.05). Moreover, the insulin level and HOMA-IR of the experimental group were higher significantly compare with the control group. But there were no differences in mean body weight or blood glucose between the two groups (Additional file 1: Table S1, Additional file 2: Figure S1). Therefore, we hypothesized that insulin induced atherosclerosis and was responsible for the decreased expression of ER-α.
Insulin inhibited ER-α expression and promoted DNMTs expression in VSMCs
To directly test whether insulin inhibited ER-α expression, we treated VSMCs with insulin for 24, 48 and 72 h. Quantitative reverse transcriptase polymerase chain reaction (RT-PCR) analysis confirmed that insulin treatment significantly decreased the mRNA level of ER-α compared with the control group (Fig. 2a; P < 0.01). To further confirm this effect of insulin, ER-α protein was measured by western blot. The results showed that insulin treatment reduced the expression of ER-α compared with the control group (Fig. 2b; P < 0.01). These data clearly indicate that insulin inhibits ER-α expression in VSMCs.
It has been reported that the majority of cellular DNMT activity is contributed by DNMT1, DNMT3a and DNMT3b. DNMT3a and DNMT3b have similar functions, so we tested the expression of DNMT1 and DNMT3a to determine whether their expression correlated with the inhibition of ER-α expression by insulin. The effect of insulin on DNA methyltransferase expression was tested using RT-PCR and western blot. The results showed that DNMT1 and DNMT3a were significantly increased both in mRNA (Fig. 2c) and protein (Fig. 2d) levels in the insulin treatment group compared with the control group (P < 0.01), further demonstrating that insulin epigenetically inhibited ER-α expression.
5-aza-2′-deoxycytidine (5-Aza) inhibited the insulin-dependent down-regulation of ER-α
To determine if the regulation of ER-α expression by insulin involved epigenetics we treated VSMCs with 5-Aza and insulin and tested the expression of ER-a by RT-PCR and western-blot. The mRNA level of ER-α was increased when VSMCs were treated with 5-Aza alone or in combination with insulin compared with the control group (Fig. 3a; P < 0.01). And the protein level of ER-α was not decreased when VSMCs were treated with 5-Aza and insulin compare with control (Fig. 3b; P > 0.05). The data suggest that 5-Aza can inhibit the insulin-induced suppression of ER-α.
Effect of insulin on the methylation status of the ER-α second exon region
DNMTs inhibits gene expression by promoting DNA methylation, and the results of bisulfite sequencing PCR showed that the extent of methylation in the ER-α second exon region was significantly increased in the insulin treatment group compared with the control group (Fig. 4; P < 0.05). Moreover, the extent of methylation in the ER-α second exon region was significantly decreased after VSMCs were treated with 5-Aza compared with the insulin group (P < 0.01). These data suggest that insulin signaling promotes the expression of DNMTs, which promotes methylation of the ER-α second exon, and ultimately inhibits ER-α expression.
ER-α inhibited VSMCs proliferation
To study the effect of ER-α in VSMC proliferation we over-expressed ER-α by transducing a lentiviral expression vector containing ER-α. ER-α expression in VSMCs was then tested by RT-PCR and western blot, and the results showed that ER-α expression was significantly higher in VSMCs infected with LV5-Esrl, a lentivirus carrying a V5-tagged ER-α expression vector, than in the control and zero-load lentiviral infection groups (Fig. 5; P < 0.01). The ER-α mRNA and protein levels did not differ significantly in VSMCs in the control and zero-load viral infection groups. The results of EdU assay showed that ER-α inhibited VSMCs proliferation (Fig. 6a; P < 0.01), and cell cycle analysis by flow cytometry revealed that the proportion of S-phase cells was significantly decreased in the LV5-Esrl-infected group compared with the control and zero-load virus-infected groups, and the differences were statistically significant (Fig. 6b; P < 0.01). These results suggest that ER-α inhibits VSMCs proliferation.
Discussion
In this study, we found significant atherosclerotic plaques and decreased ER-α expression in aortae of double knockout ApoE/Lepr mice treated with insulin, and we demonstrated that insulin signaling leads to epigenetic modifications that promote atherosclerosis, including inducing hypermethylation of the ER-α second exon region, which subsequently decreased ER-α mRNA and protein expression. We also demonstrated that VSMCs proliferation is inhibited by exogenous ER-α expression.
Hyperinsulinemia is now considered a risk factor of cardiovascular diseases such as hypertension, atherosclerosis, and intimal injury. Although there are abundant studies about the mechanism of insulin signaling pathway to atherosclerosis [26], the specific pathogenic mechanism is still unclear, it is generally believed to be associated with hyperinsulinemia-induced phenotypic modulation of VSMCs. Lineage-tracing studies have illustrated that significant plasticity is maintained in the VSMCs of adult animals [25, 26], which might perform reversible phenotypic modulation when subjected to vascular injury, cytokines, growth factors or diseases. These shifts in phenotype tend to be from contractile to synthetic types, resulting in enhanced VSMCs proliferation and migration, which can lead to atherosclerosis [26–29]. Previously, our study had found that insulin promotes VSMCs proliferation and migration via microRNA-208-mediated downregulation of p21 and inducing dopamine D1 receptor dysfunction, and activation of the dopamine D4 receptor suppresses the effect of insulin on VSMCs [30–32]. Our present in vivo experiments confirmed that insulin can promote atherosclerotic plaque formation and VSMCs proliferation. The results of HE staining showed thickened aorta walls, disordered nuclei arrangements and significant atherosclerotic plaques that are consistent with previous studies.
Human ER-α is considered an atheroprotective gene that regulates the beneficial effects of estrogen on endothelial cells and VSMCs. ER-α expression was found to be significantly decreased in aortic VSMCs from patients with atherosclerosis compared with normal control subjects, and accompanied by increased methylation of the ER-α promoter [33, 34]. However, the mechanisms underlying these changes in ER-α promoter methylation are unknown, but may be due to the impact of the environment and diet according to the epigenetic regulation theory.
Insulin is an atherogenic molecule, and meanwhile, is part of the complex external environment that influences cell behaviors and phenotypes, and a change in insulin concentration has been confirmed to alter DNA methylation patterns. It can lead to different methylation patterns of the same genes in different cell types as well as cause different methylation patterns of various genes within the same cell type [35–37]. However, insulin-induced methylation of ER-α in VSMCs had not been previously reported, so we tested ER-α expression in the aorta wall of the double knockout ApoE/Lepr mice primarily. The results showed that ER-α expression was lower in the insulin-treated group than the control group, suggesting that insulin-induced atherosclerosis was mediated by decreased ER-α expression. We subsequently validated these results in vitro using a rat VSMC line. We found that ER-α expression was also decreased in the VSMCs treated with insulin.
ER-α expression is related to DNA methylation (cytosine methylation in CpG dinucleotides), which is typically associated with reduced gene transcription through a chemically stable epigenetic modification carried out by DNMTs [38]. Hence, we tested DNMT1 and DNMT3a expression in VSMCs treated with insulin. The results showed that DNMT1 and DNMT3a mRNA and protein expression were both increased after insulin treatment. We also found that the effect on ER-α expression could be inhibited by the DNMT inhibitor, 5-Aza; ER-α expression was increased in VSMCs treated with insulin and 5-Aza compared to cells treated with insulin alone. These data further indicate that the insulin-induced ER-α repression was through DNA methylation. We also measured the extent of ER-α second exon region methylation using bisulfite sequencing PCR. These results confirmed previous observations and directly showed that DNA methylation levels in the ER-α second exon region were increased in the insulin treatment group, but were significantly decreased after co-treatment with 5-Aza. These results also highlighted that the baseline methylation level of ER-α in the control group was high. The VSMCs we used were senescing, and perhaps this could explain the high baseline level of ER-α methylation, which was consistent with the previous study concluded that methylation of the ER gene is associated with aging [24]. To determine whether ER-α inhibits VSMCs proliferation, we tested VSMCs proliferation after over-expressing ER-α. We found that VSMCs proliferation was lower after infection with LV5-Esrl.
Atherosclerosis is a chronic inflammatory condition of the vessel wall, and a large number of studies have investigated the molecular mechanisms underlying plaque formation and progression [39]. Recently, a growing body of evidence has indicated the involvement of epigenetic alterations in plaque formation and progression and in other atherosclerosis-related diseases. To date, epigenetic modifications have been reported to regulate some of the genes implicated in extracellular matrix formation, inflammation, and cell proliferation, which are involved in atherosclerosis [40–42]. For example, the NOS3 gene (encoding the endothelial eNOS) promoter in endothelial cells is hypomethylated, but hypermethylation in smooth muscle cells [43, 44]. The main epigenetic mechanisms include DNA methylation, histone modifications, and RNA-based mechanisms regulating gene silencing and activation. This study revealed, for the first time, that insulin promotes atherosclerosis through epigenetic modifications, specifically, by inducing hypermethylation of the ER-α second exon region, which reduces ER-α expression and promotes atherosclerosis. Our data reveal a new mechanism and provide new evidence for insulin promoting atherosclerosis. This work will help us find new therapeutic targets for atherosclerosis and choose better therapeutic regimens for patients with diabetes and menopause problems.
Conclusion
High blood insulin concentration is an important factor in the incidence of atherosclerosis, especially for women in the latter stages of menopause. Our manuscript is the first report that high levels of insulin lower ER-α expression via inducing hypermethylation of the ER-α second exon region. This decreases estrogen signaling and allows for upregulated VSMCs proliferation. Therefore, in addition to controlling blood sugar levels, controlling insulin levels is essential for patients with type 2 diabetes mellitus, because of this link with atherosclerosis-associated diseases. Nevertheless, there are some unresolved questions for us to elucidate. First, we are not sure if there will be the same significant change in ER-α hypermethylation in VSMCs of atherosclerotic patients treated with insulin; second, the effect of insulin on ER-α transcriptional activity in VSMCs has not been studied. All these questions and more will be the focus of our further studies.
Abbreviations
- ER-α:
-
estrogen receptor α
- VSMCs:
-
vascular smooth muscle cells
- PCR:
-
polymerase chain reaction
- GPER1:
-
G protein-coupled estrogen receptor 1
- NO:
-
nitrogen monoxide
- ECs:
-
endothelial cells
- HE:
-
hematoxylin–eosin
- DNMT:
-
DNA methyltransferase
- PBS:
-
phosphate buffer saline
References
Monnier L, Hanefeld M, Schnell O, et al. Insulin and atherosclerosis: how are they related? Diabetes Metab. 2013;39(2):111–7.
Pyöräla K. Relationship of glucose tolerance and plasma insulin to the incidence of coronary heart disease: results from two population studies in Finland. Diabetes Care. 1979;2(2):131–41.
Després JP, Lamarche B, Mauriège P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334(15):952–7.
Isenović ER, Kedees MH, Tepavcević S, et al. Role of PI3K/AKT, cPLA2 and ERK1/2 signaling pathways in insulin regulation of vascular smooth muscle cells proliferation. Cardiovasc Hematol Disord Drug Targets. 2009;9(3):172–80.
Yang M, Kahn AM. Insulin-inhibited and stimulated cultured vascular smooth muscle cell migration are related to divergent effects on protein phosphatase-2A and autonomous calcium/calmodulin-dependent protein kinase II. Atherosclerosis. 2008;196(1):227–33.
Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95(2):156–64.
Mudau M, Genis A, Lochner A, et al. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr. 2012;23(4):222–31.
Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–7.
Heianza Y, Arase Y, Kodama S, et al. Effect of postmenopausal status and age at menopause on type 2 diabetes and prediabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 17 (TOPICS 17). Diabetes Care. 2013;36(12):4007–14.
O’Malley BW, Tsai MJ. Molecular pathways of steroid receptor action. Biol Reprod. 1992;46(2):163–7.
Brouchet L, Krust A, Dupont S, et al. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation. 2001;103(3):423–8.
Bansal S, Chopra K. Distinct role of estrogen receptor-alpha and beta on postmenopausal diabetes-induced vascular dysfunction. Gen Comp Endocrinol. 2014;206:51–9.
Xiang J, Wang Y, Su K, et al. Ritonavir binds to and downregulates estrogen receptors: molecular mechanism of promoting early atherosclerosis. Exp Cell Res. 2014;327(2):318–30.
Lundholm L, Bryzgalova G, Gao H, et al. The estrogen receptor a-selective agonist propyl pyrazole triol improves glucose tolerance in ob/ob mice; potential molecular mechanisms. J Endocrinol. 2008;199(2):275–86.
Zirilli Lucia, Rochira Vincenzo, Diazzi Chiara, et al. Human models of aromatase deficiency. J Steroid Biochem Mol Biol. 2008;109(3–5):212–8.
Nevzati E, Shafighi M, Bakhtian KD, et al. Estrogen induces nitric oxide production via nitric oxide synthase activation in endothelial cells. Acta Neurochir Suppl. 2015;120:141–5.
Li QY, Chen L, Zhu YH, et al. Involvement of estrogen receptor-β in farrerol inhibition of rat thoracic aorta vascular smooth muscle cell proliferation. Acta Pharmacol Sin. 2011;32(4):433–40.
Holm A, Nilsson BO. Identification and characterization of new mechanisms in vascular oestrogen signalling. Basic Clin Pharmacol Toxicol. 2013;113(5):287–93.
Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33.
Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–13.
Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95(7 Suppl 1):s1–66.
Gallou-Kabani C, Vigé A, Gross MS, et al. Nutri-epigenomics: lifelong remodelling of our epigenomes by nutritional and metabolic factors and beyond. Clin Chem Lab Med. 2007;45(3):321–7.
Kuhlmann JD, Rasch J, Wimberger P, et al. microRNA and the pathogenesis of ovarian cancer–a new horizon for molecular diagnostics and treatment? Clin Chem Lab Med. 2012;50(4):601–15.
Post WS, Goldschmidt-Clermont PJ, Wilhide CC, et al. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res. 1999;43(4):985–91.
García-Cardona MC, Huang F, García-Vivas JM, et al. DNA methylation of leptin and adiponectin promoters in children is reduced by the combined presence of obesity and insulin resistance. Int J Obes (Lond). 2014;38(11):1457–65.
Hopkins PN. Molecular biology of atherosclerosis. Physiol Rev. 2013;93(3):1317–542.
Lacolley P, Regnault V, Nicoletti A, et al. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovasc Res. 2012;95(2):194–204.
Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31(7):1495–505.
Cao W, Ning J, Yang X, et al. Excess exposure to insulin is the primary cause of insulin resistance and its associated atherosclerosis. Curr Mol Pharmacol. 2011;4(3):154–66.
Zhang Y, Wang Y, Wang X, et al. Insulin promotes vascular smooth muscle cell proliferation via microRNA-208-mediated downregulation of p21. J Hypertens. 2011;29(8):1560–8.
Fu J, Han Y, Wang H, et al. Impaired dopamin D1 receptor-mediated vasorelaxation of mesenteric arteries in obese Zuker rats. Cardiovasc Diabetol. 2014;13:50.
Yu C, Wang Z, Han Y, et al. Dopamine D4 receptors inhibit proliferation and migration of vascular smooth muscle cells induced by insulin via down-regulation of insulin receptor expression. Cardiovasc Diabetol. 2014;13:97.
Kim JW, Oh MM, Yoon CY, et al. The effect of diet-induced insulin resistance on DNA methylation of the androgen receptor promoter in the penile cavernosal smooth muscle of mice. Asian J Androl. 2013;15(4):487–91.
Huang YS, Zhi YF, Wang SR. Hypermethylation of estrogen receptor-alpha gene in atheromatosis patients and its correlation with homocysteine. Pathophysiology. 2009;16(4):259–65.
Ting W, Yanyan Q, Jian H, et al. The relationship between insulin resistance and CpG island methylation of LMNA gene in polycystic ovary syndrome. Cell Biochem Biophys. 2013;67(3):1041–7.
Morvan D, Steyaert JM, Schwartz L, et al. Normal human melanocytes exposed to chronic insulin and glucose supplementation undergo oncogenic changes and methyl group metabolism cellular redistribution. Am J Physiol Endocrinol Metab. 2012;302(11):1407–18.
Sosa-Larios TC, Cerbón MA, Morimoto S. Epigenetic alterations caused by nutritional stress during fetal programming of the endocrine pancreas. Arch Med Res. 2015;46(2):93–100.
Barrès R, Osler ME, Yan J, et al. Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab. 2009;10(3):189–98.
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–25.
Schleithoff C, Voelter-Mahlknecht S, Dahmke IN, et al. On the epigenetics of vascular regulation and disease. Clin Epigenetics. 2012;4(1):7.
Udali S, Guarini P, Moruzzi S, et al. Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Aspects Med. 2013;34(4):883–901.
Krishna SM, Dear A, Craig JM, et al. The potential role of homocysteine mediated DNA methylation and associated epigenetic changes in abdominal aortic aneurysm formation. Atherosclerosis. 2013;228(2):295–305.
Yan MS, Matouk CC, Marsden PA. Epigenetics of the vascular endothelium. J Appl Physiol. 2010;109(3):916–26.
Chan Y, Fish JE, D’Abreo C, et al. The cell-specific expression of endothelial nitric-oxide synthase: a role for DNA methylation. J Biol Chem. 2004;279(33):35087–100.
Authors’ contributions
JM, ZWT, WY, BY, WXK raised the hypothesis and designed the study protocol; ZB, WY gave us technical guidance in the experiments; JM, ZWT, CP, PY carried out all of the experiments, and collated the data; JM and ZWT analyzed the data; and JM was the major contributor in writing the manuscript. All authors had carefully read this manuscript, and they all agreed with this signature. All authors read and approved the final manuscript.
Acknowledgements
We appreciate all colleagues of the Department of Medical Genetics, College of Basic Medicine, Third Military Medical University, they gave us selfless help during the period of our study.
Competing interests
We declare that all authors have not any financial and personal relationships with other people or organizations that could inappropriately influence our work.
Availability of data and materials
Not applicable. The conclusions of the manuscript are base on relevant datasets available in the manuscript.
Source of funding
This study was supported by the National Natural Science Foundation of China (Grant Number 81370367).
Author information
Authors and Affiliations
Corresponding authors
Additional files
12933_2016_471_MOESM1_ESM.doc
Additional file 1: Table S1. The mean body weight, blood glucose, insulin level and HOMA-IR of the ApoE/Lepr double knockout mice after 12 weeks.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Min, J., Weitian, Z., Peng, C. et al. Correlation between insulin-induced estrogen receptor methylation and atherosclerosis. Cardiovasc Diabetol 15, 156 (2016). https://doi.org/10.1186/s12933-016-0471-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-016-0471-9