Abstract
Background
Several antidiabetic drugs (i.e., sulfonylureas; SU, rosiglitazone) have been reported to be associated with increased risks of cardiovascular diseases (CVD) in patients with type 2 diabetes mellitus (T2DM). Dipeptidyl peptidase-4 inhibitors (DPP4i) are newly available antidiabetic drugs. Most studies only compared DPP4i with a placebo or SU, or targeted a specific CVD event of interest (i.e., heart failure; HF). Comparative research of CVD risks of DPP4i with other antidiabetic drugs (i.e., metformin, thiazolidinediones, meglitinides, acarbose, and insulin) remains scarce. This study was aimed to assess comparative risks of CVD, including ischemic stroke, myocardial infarction (MI) and HF, and hypoglycemia of DPP4i with other antidiabetic drugs.
Methods
We utilized Taiwan’s National Health Insurance Research Database. A total of 123,050 T2DM patients newly prescribed oral antidiabetic treatments were identified in 2009–2010 and followed until 2013. Outcome endpoints included a composite of CVD events: hospitalizations for ischemic stroke, MI and HF, and hypoglycemia. Time-varying Cox proportional hazards regression was applied to assess the time to event hazards of various antidiabetic drugs, adjusted for patients’ demographics, comorbidity, diabetic complications, and co-medications. Additional analyses were performed for the patients with and without CVD history, respectively.
Results
DPP4i users had significantly lower CVD risks as compared to that of non-DPP4i users (adjusted hazard ratio [aHR]: 0.83, 95 % confidence interval [CI]: 0.76–0.91). Compared to DPP4i users, meglitinides (aHR 1.3, 95 % CI 1.20–1.43) and insulin users (aHR 3.73, 95 % CI 3.35, 4.14) had significantly higher risks for composite CVD, as well as those for stroke, MI, HF, and hypoglycemia. Additionally, metformin users had significantly lower risks for composite CVD risk (aHR 0.87, 95 % CI 0.79–0.94), as well as those for MI, HF, and hypoglycemia, as compared to those of DPP4i users. Although there was a trend toward low CVD risks in pioglitazone users, the role of potential confounding by indication cannot be excluded.
Conclusions
DPP4i-treated T2DM patients had lower risks for CVD as compared to those for non-DPP4i users, except metformin users.
Similar content being viewed by others
Background
Cardiovascular diseases (CVD) are highly prevalent complications in patients with type 2 diabetes mellitus (T2DM), which are the leading of deaths in such individuals [1]. However, with emerging trials evaluating cardiovascular effects of antidiabetic drugs, not all the drugs appear to reduce CVD risks in T2DM patients. Sulfonylureas (SU) have been reported to be associated with increased CVD risks [2, 3]. The meta-analyses of clinical trials [4] and observational studies [5] showed that rosiglitazone was associated with excess myocardial infarction (MI) and heart failure (HF) risks, although a recent large prospective trial, the RECORD, did not have sufficient data to determine if it yields a higher risk for MI as compared to metformin or SU [6]. Conversely, the cardiovascular benefits have been seen with several antidiabetic drugs: metformin for T2DM patients with overweight [7], acarbose for the patients with impaired glucose tolerance (IGT) [8, 9], and empagliflozin in those at high risk of CVD events [10].
Dipeptidyl peptidase-4 inhibitors (DPP4i) are newly available oral hypoglycemic agents (OHAs). DPP4i suppresses the breakdown of incretin hormones glucagon-like peptide-1 and glucose-dependent insulinotropic peptide, achieving glycemic control. The CVD risks associated with DPP4i treatment have been investigated. DPP4i was shown to not have increased risks for ischemic stroke and MI [11–19]. Recent trials noticed that saxagliptin (SAVOR-TIMI 53 trial [12]) and alogliptin (EXAMINE [13]) had a higher risk for heart failure (HF) as compared to placebo, while a large observational study of 127,555 T2DM patients in Italy showed a significantly lower HF risk of DPP4i as compared with SU [20]. However, most studies only compared DPP4i with a placebo [12, 13] or SU [11, 15, 17, 18, 21], or targeted a specific CVD event of interest (i.e., HF [20]). Research that assesses comparative CVD risks of DPP4i with other antidiabetic drugs (i.e., metformin, thiazolidinediones (TZDs), meglitinides, acarbose, and insulin) remains scarce.
The present study utilizes a comprehensive national cohort of diabetic patients in Taiwan to evaluate the risks of CVD, including stroke, MI and HF, and hypoglycemia associated with DPP4i as compared with those of other antidiabetic drugs.
Methods
The Institutional Review Board of National Cheng Kung University Hospital approved the study before commencement (A-ER-103-298).
Data source
We used the Longitudinal Cohort of Diabetes Patients database (LHDB) 1996–2013, retrieved from the National Health Insurance Research Database (NHIRD), provided by Taiwan’s National Health Research Institutes. Taiwan’s NHIRD is population-based and derived from the claims data from the National Health Insurance program, a mandatory-enrollment, single-payment system that covers over 99 % of Taiwan’s population. The LHDB consists of a random sample of 120,000 de-identified diabetes incident cases from 1996 to 2013, who were tracked back to 1996 and followed up to 2013 to establish a longitudinal cohort. The LHDB is most representative of Taiwan’s diabetic population and provides the opportunity to conduct longitudinal studies to evaluate long-term outcomes of diabetes treatments.
Study cohort
We identified patients aged ≥20 years with T2DM diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification, ICD-9-CM = 250.0X–250.9X, X = 0 or 2) from 1999–2013 LHDB. We further selected cases with any antidiabetic drug exposure during 2009–2010. The first claim date of an antidiabetic drug prescribed in 2009–2010 was defined as the index date. Patients who had any antidiabetic drugs before the index date were excluded, in order to include new users of antidiabetic drugs. The observation for each case started from the index date to the date of death or the end of 2013. The maximum follow-up time was 5 years (2009–2013) and the minimum was 3 years (2011–2013). The primary outcome of interest was major adverse cardiovascular events (MACEs) (a composite of CVD events including hospitalizations for ischemic stroke [ICD-9 codes 430–438], MI [ICD-9 codes 410, 412], and HF [ICD-9 codes 428]) and individual components of CVD. The secondary outcome was a hospitalization for hypoglycemia (ICD-9 codes: 250.8, 251.0, 251.1, and 251.2).
Exposure to antidiabetic drugs
Medication utilization was identified using drug_no in the NHIRD and linked to the Anatomical Therapeutic Chemical (ATC) Classification System used to classify active ingredients of antidiabetic drugs: metformin, SU, DPP4i, pioglitazone, rosiglitazone, meglitinides, acarbose, and insulin. Patients were considered as unexposed to antidiabetic drugs (no antidiabetic drug exposure) if there was a gap of 30 days or more between two consecutive antidiabetic drug refills (“grace period”).
Statistics
Population characteristics were analyzed using descriptive statistics, including means, standard deviation, frequency, and proportion. The crude incidence rate of CVD was calculated as the total number of CVD events during the follow-up period divided by person-years at risk. The person-years at risk was defined as the sum of patients from the index date (the first antidiabetic drug claim) to the diagnosis of the first CVD event, death, or the end of 2013, whichever came first. The time-varying Cox proportional hazards model was applied to evaluate the time to event for the effect of exposure to antidiabetic agents, adjusted for patients’ baseline characteristics: age, gender, comorbidity from 1 year prior to the index date (via Charlson comorbidity index; CCI [22]), diabetic complications (via adapted Diabetes Complication Severity Index; aDCSI [23–25]), CVD history, and co-medications for CVD, including α-blockers, β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, anti-platelet agents, anti-coagulants, diuretics, digoxin, and nitroglycerin.
The aforementioned baseline characteristics were treated as fixed effects in the Cox model, while antidiabetic drug exposure in the follow-up period was the random effect. Time-varying exposure to antidiabetic drugs was based on the expected duration of each prescription by using the “days supplied” field in the NHIRD. We first analyzed the outcomes of various antidiabetic drugs (i.e., DPP4i vs. non-DPP4i exposure, which included metformin, SU, pioglitazone, rosiglitazone, meglitinides, acarbose, and insulin, and non-antidiabetic drug exposure). Second, we assessed the comparative outcomes of antidiabetic drugs, where DPP4i served as the reference for comparison with other antidiabetic drugs. We attributed outcome events to the drugs the patient was expected to be receiving at the time of the event. We assumed no legacy or carryover effects of remote exposure to any of the antidiabetic drugs. Subgroup analyses were stratified by patients’ CVD history. Hazard ratios (HRs) and associated 95 % confidence intervals (CIs) adjusted for cluster variance were computed. The significance level of this study was set at 0.05. SAS software (version 9.4) was utilized for the aforementioned analyses.
Results
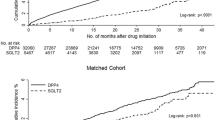
A total of 123,050 new users of oral antidiabetic drugs were included (Fig. 1), where metformin was the most commonly prescribed antidiabetic drug, and followed for a total of 362,656 person-years.
Table 1 shows the patients’ characteristics according to antidiabetic drug exposure at any point during the study. We identified 28,508 (8.1 %) patients who received DPP4i. Meglitinides users were relatively older and had more comorbidities and diabetic complications as compared to those exposed to other antidiabetic drugs.
Table 2 shows CVD risks for each antidiabetic drug as compared with non-exposure to a given antidiabetic drug (e.g., DPP4i users vs. non-DPP4i users). DPP4i, SU, acarbose, metformin, and pioglitazone users had significantly lower CVD risks than those of their counterparties (non-exposure to these drugs), while meglitinides and insulin users had significantly higher CVD risks as compared with those of patients without exposure to these drugs. There was no statistical difference in CVD risks between rosiglitazone users and non-rosiglitazone users.
Table 3 shows the comparative CVD and hypoglycemic risks of antidiabetic drugs, where DPP4i served as the reference. DPP4i users had a significantly lower risk for MACEs than that of meglitinides and insulin users, but higher than that of metformin and pioglitazone users. Also, DPP4i users had a significantly lower stroke risk than that of meglitinides and insulin users, but higher than that of pioglitazone users. DPP4i users had a significantly lower MI risk than that of meglitinides and insulin users, but higher than that of metformin users. DPP4i users had a significantly lower HF risk than that of meglitinides and insulin users, but higher than that of SU and metformin users. DPP4i users had a significantly lower hypoglycemic risk than that of meglitinides and insulin users, but higher than that of metformin users. Subgroup analyses for the patients with and without CVD history (Tables 4, 5) show trends similar to those from the primary analysis (Table 3).
Discussion
This was a large cohort study with a long-term follow-up to assess CVD and hypoglycemic risks of DPP4i as compare with other antidiabetic drugs. DPP4i users had significantly lower CVD risks as compared to non-DPP4i users. DPP4i users had significantly lower CVD risks than that for those treated with meglitinides and insulin, but not than that for those treated with metformin and pioglitazone.
Comparison with previous studies
Several studies have evaluated CVD risks in DPP4i users as compared to those of non-DPP4i users, which are similar to the approach in the first part of our analysis (the results presented in Table 2). Eurich et al.’s study, based on commercially insured US claims, showed that sitagliptin users did not have increased risks for CVD-related hospitalizations as compared with those of non-sitagliptin users (0.90, 95 % CI 0.77–1.07) [26]. Kim et al. utilized US commercial insurance claims data and found that DPP4i users had significantly lower risks for composite CVD events (including stroke, MI, and HF) (0.87, 95 % CI 0.79–0.96), with similar trends observed in patients with CVD history [16]. Chen et al.’s study utilized Taiwan’s NHIRD 2009–2011 and found that DPP4i use in T2DM patients with stroke [27] or MI [28] history was not associated with increased CVD risks as compared with non-DPP4i users. Consistent with the aforementioned studies, the present research based on Taiwan’s NHIRD 1999–2013 found that DPP4i users did not have increased risks for CVD as compared with non-DPP4i users (Table 2).
Comparative CVD risks of DPP4i with other antidiabetic drugs
We further performed comparative analysis of CVD risks of antidiabetic drugs. DPP4i users appeared to have lower CVD risks as compared with those of insulin and meglitinides users, but higher CVD risks than those of metformin and pioglitazone users. Eurich et al.’s study found that insulin users had significantly higher risks for CVD-related hospitalizations than those of non-insulin users (HR 2.15, 95 CI %: 1.85–2.51) [26]. Several possible reasons may explain the high CVD risks in insulin users. First, a harmful effect of insulin on the vascular endothelium has been suggested and increased insulin dosage appeared to be associated with increased CVD risks [29]. Second, hypoglycemia is a common side effect observed in insulin users. Hypoglycemia has been associated with increased CVD risks. The present study found that insulin-treated T2DM patients had 4.55 times higher hypoglycemic risk as compared to that of those treated with DPP4i (Table 3), which might in part explain higher CVD risks in insulin users.
Meglitinides have been related to variable degrees of undesirable CVD risks [30]. Repaglinide, a meglitinide that acts by closing ATP-dependent potassium channels, appears to be associated with a risk of adverse cardiovascular sequelae similar to that for SU [30]. Additionally, we found that meglitinides users appeared to be older and advanced diabetic patients, in terms of comorbidity and diabetic complications, as compared to those treated with other OHAs (i.e., DPP4i) (Table 1). And, meglitinides users had a significantly higher CVD risks as compared with non-meglitinides users (Table 2). Meglitinides users might thus have poor prognosis and higher CVD risks as compared to those on other OHAs such as DPP4i (Table 3).
Previous evidence showed that metformin users had significantly lower CVD risks as compared with non-metformin users, with a possible mechanism being the attenuation of atrial cell tachycardia-induced myolysis oxidative stress [31]. Limited research has compared the CVD risks of DPP4i users with those of metformin users. A recent clinical trial showed that, in terms of glycemic control, metformin monotherapy was superior to DPP4i monotherapy; however, no difference in CVD risks between the two treatment groups were noticed, in part due to the limited study period (i.e., 12 months) [32]. This observational study with a relatively long follow-up time showed that metformin appeared to be associated with significantly lower CVD risks as compared to DPP4i.
TZDs (i.e., rosiglitazone,pioglitazone) have been associated with increased risks for stroke, HF, and all-cause mortality [33–36], which appeared to be largest in the elderly (i.e., >60 years) and in patients treated with rosiglitazone [36]. Practice guidelines state that rosiglitazone and pioglitazone are not recommended for diabetic patients with pre-existing heart diseases or at risk for CVD (i.e., decreased ventricular function) [37]. Accordingly, the patients who receive TZDs are likely to be underlying at low CVD risks. Based on our cohort, we found that TZDs users had relatively lower hypertension, dyslipidemia, coronary artery diseases, HF, and stroke at baseline as compared to those of patients exposed to DPP4i (Table 1), which might explain lower CVD risks in TZDs-treated patients (e.g., pioglitazone) as compared with those of DPP4i users. In other words, there appears to be potential confounding by indication regarding TZDs use, which might influence our comparative analysis of CVD risks between TZDs and DPP4i. Although we adjusted for patients’ baseline comorbidities (i.e., CVD history) in the analysis and stratified the analysis by patients’ CVD history, other unmeasured biases (e.g., weight gain, diet, exercise, physicians’ behaviors) for CVD outcomes may still exist. We were thus unable to determine whether DPP4i use is associated with lower or higher CVD risks as compared to TZDs because potential confounding by indication could not be excluded.
A recent review of vitro studies and preliminary trials concluded potential cardiovascular benefits of alpha-glucosidase inhibitors (i.e., acarbose) in diabetic patients [38], particularly for those with IGT [8, 9]. Intervening on postprandial hyperglycemia, a key component of mechanisms linked to increased CVD incidences [39, 40], acarbose was associated with a favorable impact on CVD surrogate markers [41, 42]. Our analysis showed that acarbose use was associated with lower CVD risks as compared to non-acarbose use (Table 2). However, lack of previous research evaluated comparative CVD risks between DPP4i and alpha-glucosidase inhibitors. Although we found no significant difference in CVD risks between DPP4i and acarbose (Table 3), further study is needed to confirm our findings, especially for diabetic patients with IGT.
Today, most studies compared the CVD risks of DPP4i with SU as a group [17, 43, 44], not individual SU. However, individual SU appear to have different degrees of desirable or undesirable cardiovascular effects. Glimepiride, a third-generation SU, might provide potential cardiovascular benefits because of its favorable glycemic control, especially postprandial glucose lowering effects [45], anti-oxidative properties [46], and maintaining myocardial preconditioning [47]. In terms of reducing CVD risks, glimepiride or gliclazide with a specific influence on pancreatic ATP-sensitive K+ channels might be superior to glibenclamide [45], which blocks mitochondrial ATP-sensitive K+ channels in cardiac myocytes, resulting in the inhibition of ischemic preconditioning [48]. Also, a previous population-based cohort study showed that glipizide was associated with increased CVD risks as compared to other SU. Hence, further study is anticipated to assess whether pharmacological differences between individual drugs translate into differences in their associated CVD risks. Our additional analyses showed that, as compared to glibenclamide, gliclazide had a significantly lower risk for MACEs, and gliclazide and glimepiride had a significantly lower risk for stroke (Additional file 1: Table S1). In this regard, we further compared the CVD risks of DPP4i with individual SU. The results showed that, as compared to DPP4i, glipizide and glibenclamide had significantly higher risks for MACEs and stroke (Additional file 1: Table S2).
Study limitations
First, laboratory data (e.g., HbA1c) were not available in the NHIRD claims data. However, we used surrogat indicators to adjust for patients’ baseline diabetes severity, including aDCSI and diabetes duration. Second, because of the nature of an observational study, potential confounding by indication could not be eliminated. Also, potential residual confounding by incomplete adjustment for unmeasured biases (i.e., lifestyle risk factors, physicians’ preferences and behaviors) for study outcomes may exist. Third, another class of incretin drug, namely GLP-1 receptor agonists (GLP-1 RA), was not analyzed. GLP-1 RA was introduced to Taiwan’s national formulary in 2013. Since our data were only available up to the end of 2013, GLP-1 RA users only accounted for a small proportion of our study population. Fourth, potential misclassification may exist when defining CVD events based on Taiwan’s NHIRD. However, previous validation studies for the identification of CVD events (i.e., MI [49], stroke [50]) from the NHIRD showed high sensitivity and positive predictive values. Fifth, individual DPP4i might be associated with variable degrees of desirable or undesirable cardiovascular outcomes [51]. The SAVOR-TIMI 53 trial reported an increased HF risk in saxagliptin-treated patients [12], which was not seen with other DPP4i. The present study analyzed individual DPP4i as a group because our preliminary analyses showed no significant difference in comparative risks for MACEs and HF of individual DPP4i (sitagliptin, vildagliptin, saxagliptin, linagliptin) (Additional file 1: Table S3). Further study is anticipated to clarify the mechanisms underlying the difference in CVD risks among individual DPP4i. Lastly, our results might only be generalizable to a Chinese population under universal healthcare insurance coverage.
Conclusions
Understanding comparative effects of antidiabetic drugs provides a basis for guiding clinical care for T2DM patients. The present study shows that the use of DPP4i was not associated with increased CVD risks and that DPP4i-treated patients appeared to have lower CVD risks as compared with non-DPP4i users, except metformin users.
Abbreviations
- aDCSI:
-
adapted Diabetes Complication Severity Index
- ATC:
-
Anatomical Therapeutic Chemical
- CCI:
-
Charlson comorbidity index
- CIs:
-
confidence intervals
- CVD:
-
cardiovascular diseases
- DPP4i:
-
dipeptidyl peptidase-4 inhibitors
- GLP-1 RA:
-
GLP-1 receptor agonists
- HF:
-
heart failure
- HRs:
-
hazard ratios
- ICD-9-CM:
-
International Classification of Diseases, Ninth Revision, Clinical Modification
- IGT:
-
impaired glucose tolerance
- LHDB:
-
Longitudinal Cohort of Diabetes Patients database
- MACEs:
-
major adverse cardiovascular events
- MI:
-
myocardial infarction
- NHIRD:
-
National Health Insurance Research Database
- OHAs:
-
oral hypoglycemic agents
- SU:
-
sulfonylureas
- TZDs:
-
thiazolidinediones
References
Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the US population, 1971–1993. Diabetes Care. 1998;21(7):1138–45.
Jorgensen CH, Gislason GH, Andersson C, Ahlehoff O, Charlot M, Schramm TK, Vaag A, Abildstrom SZ, Torp-Pedersen C, Hansen PR. Effects of oral glucose-lowering drugs on long term outcomes in patients with diabetes mellitus following myocardial infarction not treated with emergent percutaneous coronary intervention–a retrospective nationwide cohort study. Cardiovasc Diabetol. 2010;9:54.
Margolis DJ, Hoffstad O, Strom BL. Association between serious ischemic cardiac outcomes and medications used to treat diabetes. Pharmacoepidemiol Drug Saf. 2008;17(8):753–9.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–71.
Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ. 2011;342:d1309.
Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ, Team RS. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125–35.
UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–65.
Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Group S-NTR: acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–94.
Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Group S-NTR: acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072–7.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Gallwitz B, Rosenstock J, Rauch T, Bhattacharya S, Patel S, von Eynatten M, Dugi KA, Woerle H-J. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380(9840):475–83.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26.
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–35.
Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–42.
Mogensen U, Andersson C, Fosbøl E, Schramm T, Vaag A, Scheller NM, Torp-Pedersen C, Gislason G, Køber L. Cardiovascular safety of combination therapies with incretin-based drugs and metformin compared with a combination of metformin and sulphonylurea in type 2 diabetes mellitus–a retrospective nationwide study. Diabetes Obes Metab. 2014;16(10):1001–8.
Kim SC, Glynn RJ, Liu J, Everett BM, Goldfine AB. Dipeptidyl peptidase-4 inhibitors do not increase the risk of cardiovascular events in type 2 diabetes: a cohort study. Acta Diabetol. 2014;51(6):1015–23.
Morgan CL, Mukherjee J, Jenkins-Jones S, Holden S, Currie C. Combination therapy with metformin plus sulphonylureas versus metformin plus DPP-4 inhibitors: association with major adverse cardiovascular events and all-cause mortality. Diabetes Obes Metab. 2014;16(10):977–83.
Chang YC, Chuang LM, Lin JW, Chen ST, Lai MS, Chang CH. Cardiovascular risks associated with second-line oral antidiabetic agents added to metformin in patients with Type 2 diabetes: a nationwide cohort study. Diabet Med. 2015;32(11):1460–9.
Ou S-M, Shih C-J, Chao P-W, Chu H, Kuo S-C, Lee Y-J, Wang S-J, Yang C-Y, Lin C-C, Chen T-J. Effects on clinical outcomes of adding dipeptidyl peptidase-4 inhibitors versus sulfonylureas to metformin therapy in patients with type 2 diabetes mellitus. Ann Intern Med. 2015.
Fadini GP, Avogaro A, Degli Esposti L, Russo P, Saragoni S, Buda S, Rosano G, Pecorelli S, Pani L. Risk of hospitalization for heart failure in patients with type 2 diabetes newly treated with DPP-4 inhibitors or other oral glucose-lowering medications: a retrospective registry study on 127,555 patients from the Nationwide OsMed Health-DB Database. Eur Heart J. 2015;36(36):2454–62.
Kannan S, Pantalone KM, Matsuda S, Wells BJ, Karafa M, Zimmerman RS. The risk of overall mortality and cardiovascular events in patients with type 2 diabetes on dual drug therapy including metformin: a large database study from Cleveland Clinic. J Diabetes. 2015.
Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51.
Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, Everson-Stewart S, Kinder L, Oliver M, Boyko EJ, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14(1):15–23.
Chang H-Y, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care. 2012;18(11):721–6.
Chen H-L, Hsiao F-Y. Risk of hospitalization and healthcare cost associated with Diabetes Complication Severity Index in Taiwan’s National Health Insurance Research Database. J Diabetes Complications. 2014;28(5):612–6.
Eurich D, Simpson S, Senthilselvan A, Asche C, Sandhu-Minhas J, McAlister F. Comparative safety and effectiveness of sitagliptin in patients with type 2 diabetes: retrospective population based cohort study. BMJ. 2013;346:f2267.
Chen D-Y, Wang S-H, Mao C-T, Tsai M-L, Lin Y-S, Su F-C, Chou C-C, Wen M-S, Wang C-C, Hsieh I-C. Sitagliptin After ischemic stroke in type 2 diabetic patients: a nationwide cohort study. Medicine 2015;94(28).
Wang S-H, Chen D-Y, Lin Y-S, Mao C-T, Tsai M-L, Hsieh M-J, Chou C-C, Wen M-S, Wang C-C, Hsieh I-C. Cardiovascular outcomes of sitagliptin in type 2 diabetic patients with acute myocardial infarction, a population-based cohort study in Taiwan. PLoS ONE. 2015;10(6):e0131122.
Muis M, Bots M, Grobbee D, Stolk R. Insulin treatment and cardiovascular disease; friend or foe? A point of view. Diabetic Med. 2005;22(2):118–26.
Huang Y, Abdelmoneim AS, Light P, Qiu W, Simpson SH. Comparative cardiovascular safety of insulin secretagogues following hospitalization for ischemic heart disease among type 2 diabetes patients: a cohort study. J Diabetes Complicat. 2015;29(2):196–202.
Chang S-H, Wu L-S, Chiou M-J, Liu J-R, Yu K-H, Kuo C-F, Wen M-S, Chen W-J, Yeh Y-H, See L-C. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13:123.
Bosi E, Dotta F, Jia Y, Goodman M. Vildagliptin plus metformin combination therapy provides superior glycaemic control to individual monotherapy in treatment-naive patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2009;11(5):506–15.
Lu C-J, Sun Y, Muo C-H, Chen R-C, Chen P-C, Hsu CY. Risk of stroke with thiazolidinediones: a ten-year nationwide population-based cohort study. Cerebrovasc Diseases. 2013;36(2):145–51.
Filion KB, Joseph L, Boivin JF, Suissa S, Brophy JM. Thiazolidinediones and the risk of incident congestive heart failure among patients with type 2 diabetes mellitus. Pharmacoepidemiol Drug Saf. 2011;20(8):785–96.
Berthet S, Olivier P, Montastruc J-L, Lapeyre-Mestre M. Drug safety of rosiglitazone and pioglitazone in France: a study using the French PharmacoVigilance database. BMC Pharmacol Toxicol. 2011;11(1):5.
Gallagher AM, Smeeth L, Seabroke S, Leufkens H, Van Staa TP. Risk of death and cardiovascular outcomes with thiazolidinediones: a study with the general practice research database and secondary care data. PLoS ONE. 2011;6(12):e28157.
Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S. Thiazolidinedione use, fluid retention, and congestive heart failure a consensus statement from the American Heart Association and American Diabetes Association. Circulation. 2003;108(23):2941–8.
Standl E, Theodorakis MJ, Erbach M, Schnell O, Tuomilehto J. On the potential of acarbose to reduce cardiovascular disease. Cardiovasc Diabetol. 2014;13:81.
Heine R, Balkau B, Ceriello A, Del Prato S, Horton E, Taskinen MR. What does postprandial hyperglycaemia mean? Diabet Med. 2004;21(3):208–13.
Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab. 2010;12(4):288–98.
Shimabukuro M, Higa N, Chinen I, Yamakawa K, Takasu N. Effects of a single administration of acarbose on postprandial glucose excursion and endothelial dysfunction in type 2 diabetic patients: a randomized crossover study. J Clin Endocrinol Metab. 2006;91(3):837–42.
Kato T, Inoue T, Node K. Postprandial endothelial dysfunction in subjects with new-onset type 2 diabetes: an acarbose and nateglinide comparative study. Cardiovasc Diabetol. 2010;9:12.
Yu OHY, Yin H, Azoulay L. The combination of DPP-4 inhibitors versus sulfonylureas with metformin after failure of first-line treatment in the risk for major cardiovascular events and death. Can J Diabetes. 2015;39(5):383–9.
Seong J-M, Choi N-K, Shin J-Y, Chang Y, Kim Y-J, Lee J, Kim J-Y, Park B-J. Differential cardiovascular outcomes after dipeptidyl peptidase-4 inhibitor, sulfonylurea, and pioglitazone therapy, all in combination with metformin, for type 2 diabetes: a population-based cohort study. PLoS ONE. 2015;10(5):e0124287.
Onuma H, Inukai K, Watanabe M, Sumitani Y, Hosaka T, Ishida H. Effects of long-term monotherapy with glimepiride vs glibenclamide on glycemic control and macrovascular events in Japanese Type 2 diabetic patients. J Diabetes Mellitus. 2014;4:33–7.
Nakamura I, Oyama J-I, Komoda H, Shiraki A, Sakamoto Y, Taguchi I, Hiwatashi A, Komatsu A, Takeuchi M, Yamagishi S-I. Possible effects of glimepiride beyond glycemic control in patients with type 2 diabetes: a preliminary report. Cardiovasc Diabetol. 2014;13:15.
Klepzig H, Kober G, Matter C, Luus H, Schneider H, Boedeker K, Kiowski W, Amann F, Gruber D, Harris S. Sulfonylureas and ischaemic preconditioning. Eur Heart J. 1999;20:439–46.
Sato T, Nishida H, Miyazaki M, Nakaya H. Effects of sulfonylureas on mitochondrial ATP-sensitive K + channels in cardiac myocytes: implications for sulfonylurea controversy. Diabetes Metab Res Rev. 2006;22(5):341–7.
Cheng C-L, Lee C-H, Chen P-S, Li Y-H, Lin S-J, Yang Y-HK. Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J Epidemiol. 2014;24(6):500.
Hsieh C-Y, Chen C-H, Li C-Y, Lai M-L. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc. 2015;114(3):254–9.
Fisman EZ, Tenenbaum A. Antidiabetic treatment with gliptins: focus on cardiovascular effects and outcomes. Cardiovasc Diabetol. 2015;14(1):129.
Authors’ contributions
HTO and KCC contributed substantially to the study concept and design, acquisition of data, analysis, and interpretation of data. CYL contributed to the analysis and interpretation of data. HTO wrote the first draft of the manuscript, and CYL and JSW critically revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We gratefully thank National Cheng Kung University and its affiliated hospital for all their support.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional file
12933_2016_350_MOESM1_ESM.docx
Additional file 1: Table S1. Hazards ratios of cardiovascular diseases as compared individual sulfonylureas with glibenclamide as reference. Table S2. Hazards ratios of cardiovascular diseases as compared individual sulfonyureas with DPP4i as reference. Table S3. Hazards ratios of cardiovascular diseases as compared individual DPP4i with sitagliptin as reference.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ou, HT., Chang, KC., Li, CY. et al. Risks of cardiovascular diseases associated with dipeptidyl peptidase-4 inhibitors and other antidiabetic drugs in patients with type 2 diabetes: a nation-wide longitudinal study. Cardiovasc Diabetol 15, 41 (2016). https://doi.org/10.1186/s12933-016-0350-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-016-0350-4