Abstract
Background
The associations between urine uric acid excretion (UUAE) and chronic kidney disease (CKD)/atherosclerosis have not been investigated. Our aims were to investigate the relationships between UUAE and CKD and carotid atherosclerotic lesions in hospitalized Chinese patients with type 2 diabetes.
Methods
This was a cross-sectional study that was conducted with 2627 Chinese inpatients with type 2 diabetes. UUAE was determined enzymatically using a single 24-h urine collection. The subjects were stratified into quartiles according to their UUAE levels. Carotid atherosclerotic lesions, including carotid intima-media thickness (CIMT), plaque and stenosis, were assessed by Doppler ultrasound. Both CKD and carotid atherosclerotic lesions were compared between the UUAE quartile groups.
Results
After adjustment for confounding factors, there was a significant decrease in the prevalence of CKD in the patients with type 2 diabetes across the UUAE quartiles (16.9%, 8.5%, 5.9%, and 4.9%; p < 0.001). Multiple logistic regression analyses revealed that the UUAE quartiles were significantly and inversely associated with the presence of CKD (p < 0.001). Compared with the diabetics in the highest UUAE quartile, those in the lowest quartile exhibited a nearly 4.2-fold increase in the risk of CKD (95% CI: 2.272-7.568; p < 0.001). The CIMT value (0.91 ± 0.22 mm for the diabetics with CKD and 0.82 ± 0.20 mm for the diabetics without CKD, p = 0.001) and the prevalence of carotid plaques (62.1% for the diabetics with CKD and 41.8% for the diabetics without CKD, p = 0.025) were significantly higher in the diabetics with CKD than in those without CKD. However, there was no obvious difference in carotid atherosclerotic lesions across the UUAE quartiles after controlling for the confounding factors.
Conclusions
Decreased UUAE was closely associated with the presence of CKD but not with carotid atherosclerotic lesions in hospitalized Chinese patients with type 2 diabetes. Our results suggest that UUAE is an independent risk factor for CKD in type 2 diabetes. In selected populations, such as patient with type 2 diabetes, the role of uric acid in atherosclerosis might be the result of other concomitant atherosclerotic risk factors, such as CKD.
Similar content being viewed by others
Introduction
In recent decades, accumulated clinical and epidemiological studies have confirmed the positive correlation between serum uric acid (SUA) and chronic kidney disease (CKD). For example, a large follow-up clinical trial that included 177,570 patients reported that the subjects in the highest SUA quartile exhibited a 2.14-fold increase in the risk for CKD compared with those in the lowest SUA quartile over 25 years [1].
In contrast, many studies have also investigated the relationship between SUA and atherosclerosis. However, unlike the association between SUA and CKD, which has been repeatedly demonstrated, the correlation between SUA and atherosclerosis remains controversial. For example, a previous study indicated that SUA is an independent risk factor for carotid atherosclerosis in type 2 diabetes [2]. However, another study by Iribarren et al. reported that the correlation between SUV levels and carotid intimal-medial thickness (CIMT) did not remain significant after controlling for other risk factors for atherosclerosis [3]. Similarly, in our previous study, we also found no significant association between SUA levels and atherosclerotic lesions in type 2 diabetes [4].
Furthermore, although a number of studies have assessed the relationships between SUA levels and CKD/atherosclerosis, the associations of urine uric acid excretion (UUAE) with CKD and atherosclerosis have not been investigated in general or diabetic populations. To date, epidemiological data are absent regarding these relationships in patients with type 2 diabetes.
Therefore, the aim of the present study was to examine the associations of UUAE with CKD and carotid atherosclerosis in hospitalized Chinese patients with type 2 diabetes. To our knowledge, this is the first study to investigate the associations of UUAE with CKD and atherosclerosis in type 2 diabetes.
Methods
Subjects and study design
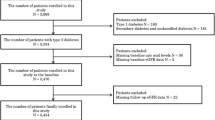
The present study was cross-sectional and was partly based on data from our previous studies [4-8]. In brief, from January 2007 to June 2009, 3598 patients with type 2 diabetes who were consecutively hospitalized in our department were observed. All patients received a diabetic diet and avoided excessive purine intake and alcohol consumption after admission to the hospital. Of the 3598 subjects, the UUAE data from 3007 patients were available. The following patients were excluded from the study: those taking any drug that interfered with uric acid metabolism, such as losartan, allopurinol and furosemide; patients with underlying renal disease inconsistent with the diabetes, such as glomerulonephritis and autoimmune kidney disease; and patients who had not undergone carotid ultrasound examination or had incomplete clinical data. Ultimately, 2627 patients were included in the final analyses.
All subjects were interviewed to obtain their histories of hypertension (HTNs), cardio-cerebrovascular events (CCEs), use of lipid-lowering drugs (LLDs), use of antihypertensive agents (AHAs), alcohol consumption and smoking habits. The HTNs, CCEs and smoking and alcohol drinking histories were defined according to the criteria utilized in our previous research [4-9]. This study was approved by the ethics committee of the Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. All subjects completed a written informed consent form before entering the study.
Physical examination and laboratory measurements
The physical examinations and laboratory measurements have been described in our previous studies [4-9]. Briefly, height, weight, waist circumference, hip circumference, and blood pressure were examined in all patients. Blood samples were obtained after an overnight fast and 2 h after breakfast measurements of glycosylated hemoglobin A1C (HbA1c), fasting plasma glucose (FPG), 2-h postprandial plasma glucose (2 h PPG), fasting C-peptide (FCP), 2-h postprandial C-peptide (2 h PCP), total triglycerides (TTG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), creatinine (Cr), serum uric acid (SUA), and C-reactive protein (CRP). Based on the plasma glucose and serum C-peptide levels, the homeostasis model assessment for insulin resistance (HOMA2-IR) and insulin sensitivity (HOMA2 %S) values were calculated using the HOMA2 calculator version 2.2.3 [10,11].
Currently, the simplest and most common approach that is applied in clinical practice to determine the renal excretion of uric acid is the measurement of urinary uric acid output over a 24-h period [12]. Therefore, a single 24-h urine sample was collected, and the 24-h UUAE was determined by enzymatic methods. The 24-h urinary albumin excretion (UAE) was calculated as the mean of the values obtained from three separate early morning urine samples during the period of hospitalization. The estimated glomerular filtration rate (eGFR) was calculated using the following equation for Chinese individuals: eGFR = 175× (serum creatinine)-1.234× (age)-0.179 (×0.79 if female) [13]. CKD was defined as an eGFR less than 60 mL/min/1.73 m2 and/or an UAE ≥ 300 mg/24 h [14] .
Ultrasonography measurements
Carotid atherosclerotic lesions including CIMT, atherosclerotic plaque and stenosis were measured by Doppler ultrasound as described previously in detail [5,7,8]. The definitions of CIMT, carotid atherosclerotic plaque and stenosis have also been described in detail in our previous study [5-8]. The reproducibilities of the measurements of the carotid arteries have also been reported in our previous studies [7,8].
Statistical analyses
SPSS 15.0 for Windows software was used for the statistical analyses. P values <0.05 were considered to be statistically significant. The data are expressed as either the means ± the S.D. or as percentages or medians (interquartile range 25%-75%). One-way ANOVAs with LSDs were used for continuous variables with normal distributions for comparisons across multiple groups. For continuous variables with skewed distributions, Mann–Whitney U tests and Kruskal-Wallis H tests were used. The χ2 test was used to compare the prevalence data. The partial correlations were used to determine the relationships between the UUAE and the variables. The general linear model was used to compare the means of the continuous variables and the associations between the UUAE quartiles and CIMT when controlling for other factors. Binary logistic regression analysis was performed to examine the associations of the UUAE quartiles with the presence of CKD, carotid plaque and stenosis.
Results
Characteristics of the subjects according to UUAE quartiles
The clinical characteristics of the subjects grouped according to the UUAE quartiles are presented in Table 1. The patients were stratified into quartiles based on the UUAE levels with the cutoff limits of <2235, 2235–2848, 2849–3606, and >3606 μmol/24 h. After controlling for age and sex, the diabetics in the higher UUAE quartiles were more likely to be male, younger, smokers and drinkers; have shorter durations of diabetes (DD); have higher DBP, BMI, WHR, FPG, 2 h PPG, FCP, 2 h PCP, HOMA2-IR, TTG, ALT, and eGFR; and have lower HOMA2-%S, HDL-C and Cr.
Associations between UUAE levels and clinical parameters
The associations between UUAE levels and the clinical parameters are shown in Table 2. After adjusting for age, sex and DD, the partial correlations analyses demonstrated strongly positive correlations of UUAE levels with SBP, DBP, BMI, WHR, FPG, 2 h PPG, FCP, 2 h PCP, HOMA2-IR, TTG, TC, ALT, eGFR, and CRP, and significantly negative correlations between UUAE levels and age (controlling for sex and DD), DD (controlling for age and sex), HOMA2 %S, HDL-C, Cr, and UAE in diabetics.
Comparison of CKD among the UUAE quartiles
Figure 1 demonstrates the comparison of CKD among the UUAE quartiles in patients. After controlling for age, sex, and DD, there was a significantly decreasing trend in the prevalence of CKD in patients across the UUAE quartiles (16.9%, 8.5%, 5.9%, and 4.9%, respectively, p < 0.001 for the trend; Figure 1A). Furthermore, the UUAE levels were obviously reduced in the diabetics with CKD compared to those without CKD (p < 0.001) (Figure 1B). Interestingly, the UUAE levels gradually decreased with the decreases in eGFR (p < 0.001; Figure 1C), and the UUAE levels were significantly decreased in the diabetics with UAEs ≥ 300 mg/24 h compared to those with UAEs <30 mg/24 h and those with UAEs in the range of 30–299 mg/24 h (p < 0.001; Figure 1D).
Comparison of CKD among the UUAE quartiles. (A) Comparison of the prevalence of CKD among the UUAE quartile groups after adjusting for age, sex, and DD. The P values for the trends were <0.001. (B) Comparison of the UUAE levels between the diabetics with and without CKD after adjusting for age, sex, and DD. The P value was <0.001. (C) Comparison of UUAE levels among the different eGFR levels after adjusting for age, sex, and DD. The P values for the trends were <0.001. (D) Comparison of UUAE levels among the different UAE levels after adjusting for age, sex, and DD. The P values for the trends were <0.001.
Comparison of the carotid atherosclerotic lesions among the UUAE quartiles
A comparison of the atherosclerotic lesions among the UUAE quartile groups after adjustments for age, sex, and DD is shown in Figure 2. There were no statistical associations between the UUAE quartiles and the carotid IMT values (p = 0.736) and the prevalence of carotid plaques (p = 0.938) and stenosis (p = 0.171) in type 2 diabetes (Figure 2A, B and C).
Comparison of carotid atherosclerotic lesions among the UUAE quartiles. (A) Comparison of the CIMT values among the UUAE quartile groups after adjusting for age, sex, and DD. (B) Comparison of the prevalence of carotid atherosclerotic plaques among the UUAE quartile groups after adjusting for age, sex, and DD. (C) Comparison of the prevalence of carotid atherosclerotic stenosis among the UUAE quartile groups after adjusting for age, sex, and DD.
Associations of UUAE quartiles with CKD
Table 3 presents the associations between the UUAE quartiles and the presence of CKD in type 2 diabetes. After controlling for age, sex, DD, HTN, smoking, alcohol drinking, and the use of LLDs and AHAs (model 1), the UUAE quartiles were independently associated with a decreased prevalence of CKD (p < 0.001 for trend). After additional adjustments for SBP, DBP, WHR and BMI (model 2) and for ALT, TC, TTG, LDL-C, HDL-C, CRP, HbA1C, FPG, 2 h PPG, FCP, 2 h PCP, HOMA2-IR, and HOMA %S (model 3) and for SUA (model 4), the UUAE quartiles retained an independent association with a decreased prevalence of CKD (all p <0.001 for the trends in model 2, model 3, and model 4).
Comparisons of carotid atherosclerotic lesions between the diabetics with and without CKD
The comparisons of carotid atherosclerotic lesions between the diabetics with and without CKD are illustrated in Figure 3. After adjusting for age, sex, and DD, the CIMT values (0.91 ± 0.22 mm for the diabetics with CKD and 0.82 ± 0.20 mm for the diabetics without CKD, p = 0.001) and the prevalence of carotid plaque (62.1% for the diabetics with CKD and 41.8% for the diabetics without CKD, p = 0.025) were significantly higher in the diabetics with CKD than in those without CKD.
Comparison of carotid atherosclerotic lesions between the diabetics with and without CKD. (A) Comparison of the CIMT values between the diabetics with and without CKD after adjusting for age, sex, and DD. (B) Comparison of the prevalence of carotid atherosclerotic plaques between the diabetics with and without CKD after adjusting for age, sex, and DD. (C) Comparison of the prevalence of carotid atherosclerotic stenosis between the diabetics with and without CKD after adjusting for age, sex, and DD.
Discussion
Recent evidence has supported uric acid as a potential mediator that is associated with CKD; however, the independence of the association between uric acid and atherosclerosis has been controversial. Furthermore, although a number of studies have focused on the associations of SUA with CKD and atherosclerosis, few investigators have investigated the relationships between UUAE and CKD/atherosclerosis in nondiabetic and diabetic populations.
Therefore, we performed this cross-sectional study of the associations between UUAE and CKD/atherosclerosis in hospitalized type 2 diabetics. Our results strongly suggested that decreased UUAE is an independent risk factor for CKD but not for carotid atherosclerotic lesions in type 2 diabetes.
The factors controlling UUAE in type 2 diabetes
The mechanisms of controlling UUAE are largely unknown in type 2 diabetes. Generally, UUAE levels are mainly dependent on tubular secretion and postsecretory reabsorption [15]. The renal excretion of uric acid is a complex process regulated by genetic and environmental factors. The primary capacity of the renal excretion of uric acid might be of genetic origin. For example, Emmerson et al. found that both urate clearance and the fractional excretion of uric acid were more similar in monozygotic twins than in dizygotic twins, which indicates that genetic factors exert a key control on the renal excretion of uric acid [16]. Furthermore, a previous study demonstrated that polymorphisms in the N-terminus of the hURAT1 gene are obviously associated with reduced renal uric acid excretion in a German Caucasian population [17]. Therefore, decreased UUAE in type 2 diabetics with CKD might be associated with a genetic predisposition.
In addition to genetic factors, other factors might influence the renal excretion of uric acid. For example, in healthy humans, physiological hyperinsulinemia can reduce urinary uric acid secretion and increase uric acid reabsorption at the tubular levels, which might lead to an increase in SUA levels [15,18]. Therefore, in healthy subjects, UUAE has been shown to be inversely related to insulin resistance and SUA [18]. However, in contrast to healthy subjects, in our present study, the diabetic patients in the higher UUAE quartiles were associated with reduced insulin sensitivity and increased insulin resistance, which theoretically should result in decreased UUAE. Indeed, patients with decreased UUAE are typically associated with impaired renal function and decreased rates of renal filtration, which might be attributed to uric acid-mediated renal damage. Consistent with our hypothesis, we observed an inreased eGFR among the UUAE quartiles. Additionally, because of the inhibitory effects of uric acid reabsorption in the kidney due to high glucose levels, UUAE levels are often increased in patients with diabetes, particularly in diabetes patients with poor glucose control [19,20]. In accordance with this perspective, we observed a positive correlation between UUAE and blood glucose levels, and the blood glucose levels were gradually elevated across the UUAE quartiles in type 2 diabetes.
Therefore, we speculated that decreased UUAE might initially originate from genetic factors in type 2 diabetics with CKD. Reduced UUAE led to impaired renal function, which further aggravated the decreases in UUAE. Thus, diminished UUAE was a risk factor for the development and progression of CKD rather than a consequence of impaired renal function.
The association between UUAE and CKD
More importantly, we observed a significant inverse association between UUAE and the presence of CKD in type 2 diabetics. A number of investigations have shown that an elevated SUA level is an independent risk factor for CKD in both the general population and the population with type 2 diabetes [1,21-23]. For example, a 5-year follow-up study demonstrated that every 1-SD increment in the SUA level was obviously associated with a 21% increase in the CKD in type 2 diabetic patients [23]. Furthermore, two observational studies that were performed with type 2 diabetes patients revealed that even high-normal SUA was also associated with albuminuria and impaired renal function [24,25]. Consistent with the results of these studies, our results revealed that the prevalence of CKD substantially increased with a decrease in UUAE in type 2 diabetes. Compared with the diabetics in the highest UUAE quartile, the diabetics in the lower UUAE quartiles exhibited a significantly higher risk of CKD. Moreover, the patients with either lower eGFR or higher UAE exhibited lower UUAE levels, which indicated that decreased UUAE might be related to the severity of CKD in type 2 diabetes.
The mechanisms underlying the association between decreased UUAE and CKD remain to be elucidated. However, it has been speculated that the local accumulation of uric acid in the kidney might be one of the mechanisms that causes CKD. Decreased UUAE in type 2 diabetes might indicate that uric acid accumulates in the kidney rather than entering the blood circulation. In an uricase gene-knockout mouse models, Wu et al. found that mouse develop pronounced hyperuricemia, renal tubular crystal deposition and renal failure [26], and these findings indicate the a key role of uric acid in the pathogenesis of CKD. More importantly, uric acid can exert direct effects on renal tubules. A recent experimental study found that uric acid can induce a phenotypic transition termed the epithelial-to-mesenchymal transition (EMT) in renal tubular cells and in the renal tubules of rats with hyperuricemia [27], and this transition plays an important role in pathogenesis of CKD. Furthermore, allopurinol significantly inhibits uric acid-induced phenotypic changes and leads to the amelioration of renal fibrosis [27]. Therefore, uric acid accumulation in the kidney undoubtedly causes the progression of kidney injury and reduced UUAE. Reduced UUAE further aggravates kidney lesions.
The association of UUAE/CKD with carotid atherosclerotic lesions
In contrast, there are no significant associations between UUAE and carotid atherosclerotic lesions in type 2 diabetes. Although no data about the association between UUAE and atherosclerosis are currently available, debate continues regarding the relationship between SUA levels and atherosclerosis in healthy individuals and patients with diabetes. Several studies have reported that high levels of SUA are associated with carotid atherosclerosis in both healthy and type 2 diabetic subjects [2,28-31]. For example, Fukui et al. found a positive correlation between SUA concentration and CIMT [28]. Similarly, Erdogan et al. also found that increased SUA levels, even within the physiological range, are significantly correlated with increased CIMT independently of other cardiovascular risk factors in healthy adults [29].
However, several cross-sectional and epidemiological follow-up studies, including our own study, have concluded that SUA is not independently associated with atherosclerosis or coronary heart disease [3,4,32-36]. For example, Iribarren et al. reported that the statistically significant association between SUA and CIMT was lost after further adjustments for known risk factors for atherosclerosis [3]. Our own study also found that SUA levels are associated with HTN and metabolic syndrome but not with atherosclerosis in type 2 diabetes [4]. Thus, the observed association between elevated SUA levels and atherosclerosis might be a consequence of concomitant other cardiovascular risk factors, such as HTN and metabolic syndrome [4,37]. Similar to the above studies, we failed to observe an independent association between UUAE and carotid atherosclerosis in type 2 diabetes in this study.
It has been demonstrated that atherosclerotic lesions are accelerated in patients with CKD even in early stages of renal dysfunction primarily due to a wide variety of accompanying traditional and non-traditional cardiovascular risk factors in the CKD population, such as aging, HTN, dyslipidemia, increased oxidative stress, inflammation and vascular calcification [38]. Therefore, it is difficult to determine whether CKD itself is independently related to the increased prevalence of atherosclerosis in patients with CKD. However, recent studies have strongly indicated that CKD itself might be an independent risk factor for atherosclerosis [39-42]. For example, one investigation enrolled 4297 asymptomatic subjects undergoing coronary CT angiography and reported that, after adjustment for confounding factors, early CKD was an independent risk factor for coronary atherosclerosis [42]. Furthermore, a study performed in Chinese patients with type 2 diabetes demonstrated that hypertension, dyslipidemia, and CKD have cumulative effects on the burden of carotid plaques [43]. Given the absence of an association between UUAE and carotid atherosclerosis, we believe that in selected populations, such as those with type 2 diabetes, the pathogenetic roles of uric acid in the development and progression of atherosclerosis might be indirect and mediated by other cardiovascular risk factors, such as CKD, HTN and metabolic syndrome. Therefore, the role of uric acid in the development and progression of atherosclerosis is ambiguous and requires further clarification.
Although multiple risk factor interventions and some medicines, such as liraglutide, can mitigate atherosclerotic lesions in type 2 diabetes, none of the biomarkers that are utilized, such as endothelial function parameters, can predict changes in the progression of atherosclerosis [44,45]. Additional risk factors related to atherosclerosis, such as CKD, should be identified, and early interventions will be useful for reducing future cardiovascular events in type 2 diabetes.
Limitations
Several limitations of this study must be mentioned. Because the participants were Chinese patients with type 2 diabetes, the results might not be generalizable to other populations. Additionally, due to the cross-sectional nature of this study, we are not able to establish the causal relationship between decreased UUAE and CKD. Prospective studies are needed to determine the relationship between UUAE and CKD. Finally, the examination of UUAE was determined by a single 24-h urine collection rather than three successive collections. Thus, the fluctuations of UUAE could not be minimized. However, when a careful protocol is followed, and the patients strictly follow a standard diet, the difference in 24-h uric acid excretion is very minor [46]. Furthermore, it might be impractical to collect three urinary samples in clinical practice.
Conclusions
Our results suggest that decreased UUAE is independently associated with the presence of CKD but not with carotid atherosclerosis in type 2 diabetes. Decreased UUAE levels might serve as a simple and useful marker for predicting the occurrence and progression of CKD in type 2 diabetes. In selected populations, such as patients with type 2 diabetes, the role of uric acid in atherosclerosis might result from other concomitant atherosclerotic risk factors, such as CKD. Further study is required to determine whether promoting UUAE represents a new therapeutic target for the prevention of CKD in type 2 diabetes.
Abbreviations
- UUAE:
-
Urine uric acid excretion
- CKD:
-
Chronic kidney disease
- CIMT:
-
Carotid intima- media-thickness
- BMI:
-
Body mass index
- WHR:
-
Waist-to-hip ratio
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- DD:
-
Duration of diabetes
- CCEs:
-
Cardio- cerebrovascular events
- LLDs:
-
Lipid-lowering drugs
- AHAs:
-
Antihypertensive agents
- SUA:
-
Serum uric acid
- UAE:
-
Urinary albumin excretion
- eGFR:
-
Estimated glomerular filtration rate
- ALT:
-
Alanine aminotransferase
- Cr:
-
Creatinine
- CRP:
-
C-reactive protein
- LDL-C:
-
Low-density lipoprotein cholesterol
- FPG:
-
Fasting plasma glucose
- 2-h PPG:
-
2-h Postprandial plasma glucose
- HbA1c:
-
Glycated hemoglobin A1C
- FCP:
-
Fasting C-peptide
- 2-h PCP:
-
2-h Postprandial C-peptide
- TTG:
-
Total triglycerides
- TC:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
References
Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169(4):342–50.
Li Q, Yang Z, Lu B, Wen J, Ye Z, Chen L, et al. Serum uric acid level and its association with metabolic syndrome and carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2011;10:72.
Iribarren C, Folsom AR, Eckfeldt JH, McGovern PG, Nieto FJ. Correlates of uric acid and its association with asymptomatic carotid atherosclerosis: the ARIC Study. Atherosclerosis Risk in Communities. Ann Epidemiol. 1996;6(4):331–40.
Li LX, Dong XH, Li MF, Zhang R, Li TT, Shen J, et al. Serum uric acid levels are associated with hypertension and metabolic syndrome but not atherosclerosis in Chinese inpatients with type 2 diabetes. J Hypertens. 2015;33(3):482–90.
Li L, Yu H, Zhu J, Wu X, Liu F, Zhang F, et al. The combination of carotid and lower extremity ultrasonography increases the detection of atherosclerosis in type 2 diabetes patients. J Diabetes Complications. 2012;26(1):23–8.
Li LX, Li MF, Lu JX, Jia LL, Zhang R, Zhao CC, et al. Retinal microvascular abnormalities are associated with early carotid atherosclerotic lesions in hospitalized Chinese patients with type 2 diabetes mellitus. J Diabetes Complications. 2014;28(3):378–85.
Li LX, Zhao CC, Ren Y, Tu YF, Lu JX, Wu X, et al. Prevalence and clinical characteristics of carotid atherosclerosis in newly diagnosed patients with ketosis-onset diabetes: a cross-sectional study. Cardiovasc Diabetol. 2013;12:18.
Li LX, Wu X, Lu JX, Tu YF, Yu LB, Li MF, et al. Comparison of carotid and lower limb atherosclerotic lesions in both previously known and newly diagnosed type 2 diabetes mellitus. J Diabetes Investig. 2014;5(6):734–42.
Li MF, Ren Y, Zhao CC, Zhang R, Li LX, Liu F, et al. Prevalence and clinical characteristics of lower limb atherosclerotic lesions in newly diagnosed patients with ketosis-onset diabetes: a cross-sectional study. Diabetol Metab Syndr. 2014;6:71.
Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2.
Matthews DR. Insulin resistance and beta-cell function - a clinical perspective. Diabetes Obes Metab. 2001;3 Suppl 1:28–33.
Perez-Ruiz F, Calabozo M, Erauskin GG, Ruibal A, Herrero-Beites AM. Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum. 2002;47(6):610–3.
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–44.
Sakaguchi Y, Shoji T, Hayashi T, Suzuki A, Shimizu M, Mitsumoto K, et al. Hypomagnesemia in type 2 diabetic nephropathy: a novel predictor of end-stage renal disease. Diabetes Care. 2012;35(7):1591–7.
Quinones GA, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol. 1995;268(1 Pt 1):E1–5.
Emmerson BT, Nagel SL, Duffy DL, Martin NG. Genetic control of the renal clearance of urate: a study of twins. Ann Rheum Dis. 1992;51(3):375–7.
Graessler J, Graessler A, Unger S, Kopprasch S, Tausche AK, Kuhlisch E, et al. Association of the human urate transporter 1 with reduced renal uric acid excretion and hyperuricemia in a German Caucasian population. Arthritis Rheum. 2006;54(1):292–300.
Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008–11.
Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G. Hypouricemia and hyperuricemia in type 2 diabetes: two different phenotypes. Eur J Clin Invest. 2001;31(4):318–21.
Tuomilehto J, Zimmet P, Wolf E, Taylor R, Ram P, King H. Plasma uric acid level and its association with diabetes mellitus and some biologic parameters in a biracial population of Fiji. Am J Epidemiol. 1988;127(2):321–36.
Sheikhbahaei S, Fotouhi A, Hafezi-Nejad N, Nakhjavani M, Esteghamati A. Serum uric Acid, the metabolic syndrome, and the risk of chronic kidney disease in patients with type 2 diabetes. Metab Syndr Relat Disord. 2014;12(2):102–9.
Cain L, Shankar A, Ducatman AM, Steenland K. The relationship between serum uric acid and chronic kidney disease among Appalachian adults. Nephrol Dial Transplant. 2010;25(11):3593–9.
Zoppini G, Targher G, Chonchol M, Ortalda V, Abaterusso C, Pichiri I, et al. Serum uric Acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care. 2012;35(1):99–104.
Cai XL, Han XY, Ji LN. High-normal serum uric acid is associated with albuminuria and impaired glomerular filtration rate in Chinese type 2 diabetic patients. Chin Med J (Engl). 2011;124(22):3629–34.
Kim WJ, Kim SS, Bae MJ, Yi YS, Jeon YK, Kim BH, et al. High-normal serum uric acid predicts the development of chronic kidney disease in patients with type 2 diabetes mellitus and preserved kidney function. J Diabetes Complications. 2014;28(2):130–4.
Wu X, Wakamiya M, Vaishnav S, Geske R, Montgomery Jr C, Jones P, et al. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci U S A. 1994;91(2):742–6.
Ryu ES, Kim MJ, Shin HS, Jang YH, Choi HS, Jo I, et al. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol Renal Physiol. 2013;304(5):F471–80.
Fukui M, Tanaka M, Shiraishi E, Harusato I, Hosoda H, Asano M, et al. Serum uric acid is associated with microalbuminuria and subclinical atherosclerosis in men with type 2 diabetes mellitus. Metabolism. 2008;57(5):625–9.
Erdogan D, Gullu H, Caliskan M, Yildirim E, Bilgi M, Ulus T, et al. Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clin Pract. 2005;59(11):1276–82.
Ishizaka N, Ishizaka Y, Toda E, Nagai R, Yamakado M. Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese individuals. Arterioscler Thromb Vasc Biol. 2005;25(5):1038–44.
Neogi T, Ellison RC, Hunt S, Terkeltaub R, Felson DT, Zhang Y. Serum uric acid is associated with carotid plaques: the National Heart, Lung, and Blood Institute Family Heart Study. J Rheumatol. 2009;36(2):378–84.
Ong G, Davis WA, Davis TM. Serum uric acid does not predict cardiovascular or all-cause mortality in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2010;53(7):1288–94.
Panero F, Gruden G, Perotto M, Fornengo P, Barutta F, Greco E, et al. Uric acid is not an independent predictor of cardiovascular mortality in type 2 diabetes: a population-based study. Atherosclerosis. 2012;221(1):183–8.
Oikonen M, Wendelin-Saarenhovi M, Lyytikainen LP, Siitonen N, Loo BM, Jula A, et al. Associations between serum uric acid and markers of subclinical atherosclerosis in young adults. The cardiovascular risk in Young Finns study. Atherosclerosis. 2012;223(2):497–503.
Hashemi M, Yavari M, Amiri N, Taheri H, Shaigannia I, Moghadas L, et al. Uric acid: a risk factor for coronary atherosclerosis. Cardiovasc J S Afr. 2007;18(1):16–9.
De Luca G, Secco GG, Santagostino M, Venegoni L, Iorio S, Cassetti E, et al. Uric acid does not affect the prevalence and extent of coronary artery disease. Results from a prospective study. Nutr Metab Cardiovasc Dis. 2012;22(5):426–33.
Rich MW. Uric acid: is it a risk factor for cardiovascular disease. Am J Cardiol. 2000;85(8):1018–21.
Balla S, Nusair MB, Alpert MA. Risk factors for atherosclerosis in patients with chronic kidney disease: recognition and management. Curr Opin Pharmacol. 2013;13(2):192–9.
Kaisar M, Isbel N, Johnson DW. Cardiovascular disease in patients with chronic kidney disease. A clinical review. Minerva Urol Nefrol. 2007;59(3):281–97.
Roy SK, Cespedes A, Li D, Choi TY, Budoff MJ. Chronic kidney disease is associated with increased coronary artery atherosclerosis as revealed by multidetector computed tomographic angiography. Tex Heart Inst J. 2012;39(6):811–6.
Shakeri A, Abdi M, Khosroshahi HT, Fouladi RF. Common carotid artery intima-media thickness and atherosclerotic plaques in carotid bulb in patients with chronic kidney disease on hemodialysis: a case–control study. Pak J Biol Sci. 2011;14(17):844–8.
Cho I, Min HS, Chun EJ, Park SK, Choi Y, Blumenthal RS, et al. Coronary atherosclerosis detected by coronary CT angiography in asymptomatic subjects with early chronic kidney disease. Atherosclerosis. 2010;208(2):406–11.
Yuan C, Lai CW, Chan LW, Chow M, Law HK, Ying M. Cumulative effects of hypertension, dyslipidemia, and chronic kidney disease on carotid atherosclerosis in Chinese patients with type 2 diabetes mellitus. J Diabetes Res. 2014;2014:179686.
Tripolt NJ, Narath SH, Eder M, Pieber TR, Wascher TC, Sourij H. Multiple risk factor intervention reduces carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2014;13:95.
Rizzo M, Chandalia M, Patti AM, Di BV, Rizvi AA, Montalto G, et al. Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8-month prospective pilot study. Cardiovasc Diabetol. 2014;13:49.
Puig JG, Torres RJ, de Miguel E, Sanchez A, Bailen R, Banegas JR. Uric acid excretion in healthy subjects: a nomogram to assess the mechanisms underlying purine metabolic disorders. Metabolism. 2012;61(4):512–8.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81170759). We thank the other investigators, the staff, and the participants of the present study for their valuable contributions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Li LX and Jia WP designed the study, supervised the work, and reviewed and edited the manuscript. Li LX researched the data, performed the statistical analysis and wrote the manuscript. Zhang R, Li TT, Wang JW, and Bao YQ researched the data and reviewed the manuscript. All authors have read and approved the final manuscript.
Lian-Xi Li and Ai-Ping Wang contributed equally to this work.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Li, LX., Wang, AP., Zhang, R. et al. Decreased urine uric acid excretion is an independent risk factor for chronic kidney disease but not for carotid atherosclerosis in hospital-based patients with type 2 diabetes: a cross-sectional study. Cardiovasc Diabetol 14, 36 (2015). https://doi.org/10.1186/s12933-015-0199-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-015-0199-y