Abstract
Background
Bronchial thermoplasty regulates structural abnormalities involved in airway narrowing in asthma. In the present study we aimed to investigate the effect of bronchial thermoplasty on histopathological bronchial structures in distinct asthma endotypes/phenotypes.
Methods
Endobronchial biopsies (n = 450) were collected from 30 patients with severe uncontrolled asthma before bronchial thermoplasty and after 3 sequential bronchial thermoplasties. Patients were classified based on blood eosinophils, atopy, allergy and smoke exposure. Tissue sections were assessed for histopathological parameters and expression of heat-shock proteins and glucocorticoid receptor. Proliferating cells were determined by Ki67-staining.
Results
In all patients, bronchial thermoplasty improved asthma control (p < 0.001), reduced airway smooth muscle (p = 0.014) and increased proliferative (Ki67 +) epithelial cells (p = 0.014). After bronchial thermoplasty, airway smooth muscle decreased predominantly in patients with T2 high asthma endotype. Epithelial cell proliferation was increased after bronchial thermoplasty in patients with low blood eosinophils (p = 0.016), patients with no allergy (p = 0.028) and patients without smoke exposure (p = 0.034).
In all patients, bronchial thermoplasty increased the expression of glucocorticoid receptor in epithelial cells (p = 0.018) and subepithelial mesenchymal cells (p = 0.033) and the translocation of glucocorticoid receptor in the nucleus (p = 0.036). Furthermore, bronchial thermoplasty increased the expression of heat shock protein-70 (p = 0.002) and heat shock protein-90 (p = 0.001) in epithelial cells and decreased the expression of heat shock protein-70 (p = 0.009) and heat shock protein-90 (p = 0.002) in subepithelial mesenchymal cells. The effect of bronchial thermoplasty on the expression of heat shock proteins -70 and -90 was distinctive across different asthma endotypes/phenotypes.
Conclusions
Bronchial thermoplasty leads to a diminishment of airway smooth muscle, to epithelial cell regeneration, increased expression and activation of glucocorticoid receptor in the airways and increased expression of heat shock proteins in the epithelium. Histopathological effects appear to be distinct in different endotypes/phenotypes indicating that the beneficial effects of bronchial thermoplasty are achieved by diverse molecular targets associated with asthma endotypes/phenotypes.
Similar content being viewed by others
Background
Asthma is now considered an umbrella diagnosis for pathologies with distinct mechanistic pathways (endotypes) and clinical presentations (phenotypes) [1]. These endotypes and phenotypes are key elements for precision medicine in the heterogeneous asthma profiles.
Bronchial thermoplasty is a non-pharmacological treatment for severe asthma. It is based on selective heating of the airways using a bronchoscope-inserted catheter that ends in 4 electrodes and generates a temperature of 65 °C for 10 s [2]. This procedure aims to improve asthma symptoms by targeting structural components of the airways.
In previous clinical trials, it was demonstrated that bronchial thermoplasty was associated with a reduction in the number of exacerbations in severe asthmatics, a step-down in treatment and an improved quality of life without improvement of lung function [3, 4]. Integrated in vitro and in silico modelling suggested that the reduction in airway smooth muscle cells (ASMC) after bronchial thermoplasty cannot be fully explained by acute heating and furthermore, it could not confer the great improvement in asthma control [5]. Tο this end, it was suggested that the heat energy that is produced during bronchial thermoplasty can alter airway structural components other than ASMC that are involved in airway narrowing and bronchial reactivity such as neuroendocrine epithelial cells and nerve endings [6]. Histological analysis of endobronchial biopsies is an important tool to ascertain the constitution of the epithelial and mesenchymal bronchial compartments that may reflect underlying inflammatory processes (endotypes) which translate in diverse clinical presentations (phenotypes) [7, 8].
Response to inhaled glucocorticoids depends on the presence of the glucocorticoid receptor (GR) in the correct conformation, that is only achieved when GR forms a complex with heat shock proteins (HSP) 70 and HSP90 [9]. HSPs comprise a family of stress-response proteins that are induced under threatening alterations of the cellular environment [10]. An important role of HSPs is that they bind to GR intracellularly and change its structure into a conformation that exhibits a high-affinity for steroid binding. We have previously shown that the expression of HSP60 is altered in bronchoalveolar lavage of patients with severe asthma after bronchial thermoplasty and that specific HSPs are involved in intracellular pathways associated with airway remodeling [11]. In addition, exposure to heat modified the expression of HSP70 and HSP90 in a cell-type specific manner, indicating that the regenerative potential of the epithelium is increased by bronchial thermoplasty, while that of ASMC is reduced [12].
The current exploratory, hypothesis-generating study aimed to evaluate the effect of bronchial thermoplasty on histopathological findings in asthma patients with different endotypes such as eosinophilia and phenotypes such as atopy, allergy and relevant smoke exposure. We hypothesized that histopathological alterations after bronchial thermoplasty differ between asthma patients with distinct endotypes/phenotypes.
Methods
Patients, bronchial thermoplasty
This is a prospective, monocentric, observational study including 30 patients diagnosed with severe asthma based on ERS/ATS guidelines and GINA 2017 criteria, who fulfilled the indication for bronchial thermoplasty as they all had a symptomatic disease with severe, persistent, poorly-controlled symptoms, recurrent exacerbations, emergency department visits and hospitalizations despite maximal medical treatment. Exclusion criteria were: (a) pulmonary condition other than asthma as the main respiratory disease, e.g., bronchiectasis; (b) decrease in diffusion capacity (as defined by a cDLCO < 50%); (c) rapid lethal disease, e.g., bronchial carcinoma, advance heart failure, end-stage renal failure; (d) severe immunosuppression including manifested AIDS, organ transplantation or neutropenia (< 500 × 109/L).
All patients were prospectively classified in the following endotypes/phenotypes: (1) patients with high (≥ 300/μl) and low (< 300/μl) eosinophils; (2) patients with atopy (IgE ≥ 100 U/ml) and without atopy (IgE < 100 U/ml); (3) patients with allergy (positive prick test) and without allergy (negative prick test); (4) patients with relevant smoke exposure (≥ 15 pack-years) and without relevant smoke exposure (< 15 pack-years).
The study was performed according to the GCP guidelines and was approved by the local Institutional Review Board (EKNZ 2016-01057). All patients provided written informed consent to participate in the study.
All patients underwent three sessions of bronchial thermoplasty separated by at least 1-month intervals [13,14,15,16]. During the procedure, EBB specimens were obtained from first- and second- generation bronchi using 2.2 mm wide single use biopsy forceps with Endo-Glide Sheath (Radial Jaw, Boston Scientific). All EBB specimens were washed in PBS, fixed in formalin and transferred to pathology (Additional file 1: Figure S1).
Histological evaluation, immunohistochemistry
To evade dissimilarities in the histology between different lobes, [17] we performed analysis of EBB (n = 3–5) of individual patients obtained from the right lower lobe before and after each bronchial thermoplasty. For each specimen, 5 sequential sections were stained with Hematoxylin/Eosin and Elastica van Gieson and were evaluated blindly by 2 senior pathologists. Only specimens with tangential sections were evaluated, so that the orientation of the sections would not affect the measurements (Additional File 1: Figure S1).
Inflammation in the stroma, tissue lymphocyte/ eosinophil/ granulocyte infiltration and thickening of reticular basement membrane (BM), were appraised using a 0–3 scale: 0 = absence/normal, 1 = mild-moderate, 2–3 = severe. The median value of all assessments for these categorical measurements was assigned to each patient as follows: 0 < 0.5 = (absence/normal), 0.5 < 1.50 = mild-moderate, 1.5–3 = severe.
Airway smooth muscle (ASM) mass was evaluated as the total percentage of the submucosal area occupied by ASMC. The distance between BM and ASM in μm, was measured from the parenchymal site of the BM towards the ASM, without including the thickness of the BM. The mean values of all assessments for these numerical measurements were assigned to each patient.
EBB tissue sections obtained before and after bronchial thermoplasty were stained using antigen-specific antibodies for Ki67, GR, HSP70 and HSP90 (Additional file 1: Figure S1).
Statistical analysis
Patient’s characteristics were summarized as means and standard errors if continuous and as counts and percentages if categorical. Comparisons between endotypes/phenotypes were carried out using the Fisher’s exact test or Mann–Whitney U-test.
Comparison of outcomes obtained before and after bronchial thermoplasty were performed using rank mixed linear regression models, if continuous, and mixed multinomial (ordinal) logistic regression models if categorical. In both cases, the factor patient was included as a random effect.
Statistics were calculated using the softwares SAS (v. 9.4, 2012, SAS Institute Inc. Cary, NC, USA) and SPSS (Windows version 23.0).
Results
Patients, asthma control after thermoplasty
The mean age of the 30 asthma patients was 57.8 years. The majority (70%) of the patients were classified as GINA 5 and were under treatment with oral corticosteroids (Table 1). Among the 30 asthma patients, seven patients had blood eosinophils ≥ 300/μl and classified to eosinophilic asthma endotype; 10 patients had relevant smoke exposure (≥ 15 PY) and classified to smoking phenotype; 16 patients had positive prick test and classified to allergic phenotype (Fig. 1). However, there were overlaps between the different groups as shown in Fig. 2. Patients of distinct endotypes and phenotypes had similar baseline characteristics (Table 1). Yet, patients with eosinophils < 300/μl had significantly lower FEV1/FVC as compared with patients with eosinophils ≥ 300/μl (p = 0.042). Furthermore, as compared to patients without smoke exposure, patients with relevant smoke exposure had lower, FEV1/FVC (p = 0.022), and higher RV% predicted (p = 0.028) and TLC% predicted (p = 0.007).
The CONSORT flow diagram of the study. All 30 asthma patients fulfilled the criteria for bronchial thermoplasty as they all had persistent, poorly-controlled symptoms, recurrent exacerbations, emergency department visits and hospitalizations despite maximal medical treatment. PY pack years, BM basement membrane, ASM airway smooth muscle, ASMC airway smooth muscle cells, SMC subepithelial mesenchymal cells, EBB endobronchial biopsies
Lung function parameters were assessed before the 1st bronchial thermoplasty, 1 month after each sequential bronchial thermoplasty and at a follow-up visit, 49.65 ± 35.25 days after the 3rd bronchial thermoplasty. There were no significant changes in lung function parameters after serial bronchial thermoplasties (Additional file 2: Table S1). However, Asthma Control Test (ACT) revealed a significant improvement of disease control after bronchial thermoplasty in all patients (p < 0.001) [mean change (SEM) in ACT total score between before bronchial thermoplasty and 3 months after last bronchial thermoplasty of 3.8 (3.9)]. Improvement of ACT total score was similar across distinct asthma endotypes/phenotypes.
Histological evaluation of endobronchial biopsies
Comparisons using mixed multinomial logistic regression models revealed that inflammation in the stroma, tissue infiltration with lymphocytes, eosinophils and granulocytes and BM thickening were not altered significantly after bronchial thermoplasty in EBB of asthma patients (Table 2A). However, after bronchial thermoplasty, ASM mass was significantly decreased (p = 0.014), while there was no significant difference in the distance between BM and ASMC (Table 2A). Representative microphotographs depicting the decrease of ASM mass in EBB of asthma patients after bronchial thermoplasty are shown in Additional File 1: Figure S1.
Across distinct endotypes/phenotypes, patients presented a significant decrease in ASM mass after bronchial thermoplasty were the ones with T2 high asthma endotype, as patients with high blood eosinophils (p = 0.009), atopy (p = 0.012), allergy (p = 0.001) and without smoke exposure (p = 0.019) (Table 2B–E).
When patients with T2 positive markers (allergy, atopy, high blood eosinophils) were stratified in subtypes according to the number of T2 positive markers, a significant decrease in ASM mass after bronchial thermoplasty (p = 0.049), was observed in patients with T2 subtype II, that is patients with any two of the T2 positive markers (Table 2F).
Using linear regression models, we observed that there was no significant association between blood eosinophils and tissue eosinophilic infiltration before bronchial thermoplasty (p = 0.474), after the first (p = 0.487) and after the second bronchial thermoplasty (p = 0.135) (Additional file 3: Table S2). Furthermore, there was no difference in the effect of bronchial thermoplasty on any of the histological parameters evaluated between patients with (n = 16) and without (n = 14) tissue eosinophilic infiltration (Additional file 4: Table S3).
Thermoplasty stimulates proliferation of epithelium
Cells undergoing active proliferation were assessed in EBB obtained before and 2 months after the 3rd bronchial thermoplasty by immunostaining for Ki67 protein (Fig. 3A). Quantitation of Ki67 + cells revealed that after bronchial thermoplasty, the number of proliferative (Ki67 +) epithelial cells was significantly increased (p = 0.014) (Fig. 3B, Table 3).
Proliferative epithelial cells and subepithelial mesenchymal cells in endobronchial biopsies (EBB) obtained before and after BT was assessed by staining with antibodies for Ki67, a nuclear protein that is expressed only in proliferating cells. A Representative microphotographs showing epithelial cells (red arrows) and subepithelial mesenchymal cells (blue arrows) stained positive for Ki67 (brown color). Photographs were captured by Olympus IX83 microscope, using a DS-Ri2 color imaging camera. Right panels show enlargement of the areas in black boxes. B Counting of proliferative cells that stained positive for Ki67 was performed in 3 randomly selected areas on each EBB under the × 200 magnification of the microscope (Nikon Eclipse Ti2 inverted microscope system). Results were expressed as number of Ki67 positive cells per 100 nuclei (%). Upper panels show paired data (from the same patient) before and after BT. In lower panels, horizontal lines in box plots represent median values. Comparisons were made by the Wilcoxon signed rank test. BT bronchial thermoplasty
Across distinct asthma endotypes/phenotypes (Table 3), proliferative epithelial cells were significantly increased after bronchial thermoplasty in asthma patients with low blood eosinophils (p = 0.016), without allergy (p = 0.028) and no smoke exposure (p = 0.034). The number of proliferative subepithelial mesenchymal cells was significantly decreased only in patients without smoke exposure (p = 0.001).
Thermoplasty increases glucocorticoid receptor expression
Immunohistochemistry in EBB before and after bronchial thermoplasty revealed that immunoreactivity for GR was observed in both epithelial cells and subepithelial mesenchymal cells both in the cytoplasm and in the nuclei (Figs. 4A, B). After bronchial thermoplasty, there was a significant increase in the expression of GR in epithelial cells (p = 0.018) and in subepithelial mesenchymal cells (p = 0.033) (Fig. 4C). Furthermore, the accumulation of GR in the nuclei of all cells was significantly increased (p = 0.036) after bronchial thermoplasty, indicating its activation (Fig. 4C).
Expression of glucocorticoid receptor (GR) in endobronchial biopsies obtained from severe asthma patients using a specific monoclonal antibody. A Representative microphotographs. Lower panels show enlargement of the areas in red boxes. B Representative microphotographs showing increased localization of GR in the nuclei after BT. C expression score in epithelial cells, subepithelial mesenchymal cells and in the nuclei of all cells from patients with severe asthma before and after thermoplasty. The p value was calculated using mixed multinomial (ordinal) logistic regression models, where the factor patient was included as a random effect. BT bronchial thermoplasty
Across distinct asthma endotypes/phenotypes the group of patients with low blood eosinophils (< 300/μl), presented a significant increase of GR expression in epithelial cells (p = 0.044) and accumulation of GR in the nuclei (p = 0.044) after bronchial thermoplasty (Table 4).
Thermoplasty alters HSPs expression
Endobronchial biopsies obtained from severe asthma patients before and after bronchial thermoplasty stained positively for HSP70 and HSP90 antigens (Figs. 5A, 6A). Quantitation of HSP70 + and HSP90 + cells revealed that tissue expression of HSP70 and HSP90 was significantly increased in epithelial cells after bronchial thermoplasty (p = 0.002 and p = 0.001, respectively) (Figs. 5B, 6B). In subepithelial mesenchymal cells, there was a significant decrease in the expression of HSP70 (p = 0.009) and HSP90 (p = 0.002) after bronchial thermoplasty (Figs. 5B, 6B).
Expression of heat shock protein 70 (HSP70) in endobronchial biopsies obtained from severe asthma patients using a specific monoclonal antibody. A Representative microphotographs showing the expression of HSP70 before and after bronchial thermoplasty (BT). B HSP70 expression score in epithelial cells and subepithelial mesenchymal cells from 30 asthma patients before and after BT. The p value was calculated using mixed multinomial (ordinal) logistic regression models, where the factor patient was included as a random effect
Expression of heat shock protein 90 (HSP90) in endobronchial biopsies obtained from severe asthma patients using a specific monoclonal antibody. A Representative microphotographs showing the expression of HSP90 before and after bronchial thermoplasty (BT). B HSP90 expression score in epithelial cells and subepithelial mesenchymal cells from 30 asthma patients before and after BT. The p value was calculated using mixed multinomial (ordinal) logistic regression models, where the factor patient was included as a random effect
Across distinct asthma endotypes/phenotypes, comparison between different groups suggested differences in the expression of HSP70 after bronchial thermoplasty in patients with atopy, in epithelial cells (increased expression, p = 0.011 vs p = 0.117 for patients without atopy), and subepithelial mesenchymal cells (decreased expression, p = 0.018 vs p = 0.063 for patients without atopy) (Table 5). However, when comparisons were made between the groups only smoke exposure was associated with significant differences in HSP70 expression in epithelial cells and subepithelial mesenchymal cells (p = 0.045 and p = 0.003, respectively, for comparisons between patients with and without smoke exposure).
Similarly, after bronchial thermoplasty, a significant increase was observed in the expression of HSP90 in epithelial cells in patients with low blood eosinophils (p = 0.006 vs. p = 0.976 for patients with high blood eosinophils) and atopy (p = 0.008 vs. p = 0.570 for patients without atopy) and a significant decrease in the expression of HSP90 in subepithelial mesenchymal cells in patients with atopy (p = 0.008 vs. p = 0.093 for patients without atopy) (Table 6). However, when comparisons were made between the groups only smoke exposure was associated with significant differences in HSP90 expression in subepithelial mesenchymal cells (p = 0.003, for comparisons between patients with and without smoke exposure).
Discussion
In the present study, we evaluated the effect of bronchial thermoplasty on histological parameters in 30 well-characterized patients with severe asthma, including various endotypes/phenotypes. The main novel finding of our study is that depending on the asthma endotype/phenotype, bronchial thermoplasty results in distinct histopathological changes including decreased bronchial ASM mass, regeneration of bronchial epithelial cells, increased expression and activation of GR in the airways and increased expression of HSPs in bronchial epithelium. Thus, bronchial thermoplasty targets alternative molecular pathways associated with asthma endotypes/phenotypes.
An important goal of asthma management strategies is to improve patients’ daily activities and to control asthma symptoms. In line with previous studies, [3, 4, 6, 18] bronchial thermoplasty did not alter postbronchodilator lung function parameters, however, significantly improved asthma control, as assessed by daily symptoms using ACT. This has been a validated method to reflect asthma control [19,20,21]. It has been suggested that a difference of 3 points for the ACT is a clinically meaningful change in asthma control in an individual patient overtime [22]. In the present study, patients achieved a difference of 3.8 points for ACT total score after bronchial thermoplasty, indicating a relevant improvement in asthma symptoms. Changes in ACT total score after bronchial thermoplasty were similar across distinct endotypes/phenotypes implying that the procedure has a beneficial effect on patients’ asthma symptoms control, irrespective their asthma endotype/phenotype.
Histopathologic examination of bronchial biopsy specimens collected at each sequential bronchial thermoplasty showed a significant reduction of ASM mass by 68.4% after the first bronchial thermoplasty and by 81.2% after the second bronchial thermoplasty, confirming the results obtained in previous studies [6, 23]. The decrease of ASM after bronchial thermoplasty was significant for patients with high blood eosinophils, atopy, allergy, and no smoke exposure, implying that patients with T2 high asthma may profit more from the procedure.
In the present study, the number of proliferating, (Ki67 +) epithelial cells, was significantly increased in asthma patients after bronchial thermoplasty, an observation that is in line with the rapid reconstruction of the epithelium after bronchial thermoplasty shown in previous studies [6, 24, 25]. Across distinct asthma endotypes/phenotypes, proliferating epithelial cells were significantly increased after bronchial thermoplasty in patients with low blood eosinophils, in patients without allergy and in patients without smoke exposure, indicating that the procedure activates similar mechanisms for epithelial cell repair in these groups of patients.
On the contrary, after bronchial thermoplasty, the number of Ki67 + subepithelial mesenchymal cells was numerically decreased, however, no significantly. This is not surprising, since the number of actively proliferating ASMC is low in subjects with asthma of varying severity, including subjects with severe asthma as those included in the present study [26]. Moreover, this observation is in line with the reduction of ASM mass observed after bronchial thermoplasty. Across distinct asthma endotypes/phenotypes, proliferating subepithelial mesenchymal cells were significantly decreased after bronchial thermoplasty only in patients without smoke exposure. However, patients without smoke exposure were presented with higher amount of ASM before bronchial thermoplasty [median (IQR): 17.2 (9.2–41.2) versus 9.2 (1.2–22.5) for patients with smoke exposure].
Although ASM mass was significantly decreased after bronchial thermoplasty there was no change in BM thickness or in the distance between BM and ASMC before and after bronchial thermoplasty in asthma patients of any endotype/phenotype. It has been suggested that besides a direct beneficial effect of bronchial thermoplasty in reducing ASMC, the therapeutic outcome of this procedure goes beyond the treated areas and influences more peripheral airways, resulting in a reduction of small airway narrowing and air-trapping [27, 28]. Therefore, it is tempting to hypothesize that the lack of significant differences in BM thickening and in the distance between BM and ASMC before and after bronchial thermoplasty, may reflect a moderate local modification of these parameters, however, distributed to distal areas of the bronchial tree and resulting in an overall significant improvement in airway remodeling and patients’ symptoms.
We have previously shown that a potential mechanism for the effect of bronchial thermoplasty on airway remodeling involves the impediment of epithelium-derived HSP60 secretion and PRMT1 in fibroblasts [11]. HSPs belong to a family of proteins that are induced in all cells when there is a threat of the cellular environment, such as inflammation. Based on our previous findings that HSPs mediate cellular pathways associated with airway remodeling, [12] we assessed tissue expression of HSP70 and HSP90 in EEB obtained before and after bronchial thermoplasty. HSP70 is a molecular chaperon and has been associated with a possible role in antigen processing and presentation [29]. Thus, HSP70 may enhance a protective immune response or a harmful allergic response. It is still unclear if overexpression of HSP70 in asthma represents increased stress or an autoprotective mechanism. In vivo experiments have shown that HSP70 protects against pulmonary inflammation and sepsis [30] and decrease mortality in an animal model of acute lung injury [31]. Furthermore, HSP70 has been reported to prevent cell apoptosis [32, 33]. The above evidence suggests that the increased expression of HSP70 in epithelial cells after bronchial thermoplasty shown in the present study, inhibits epithelial cell apoptosis and thereby helps to rebuild a functional epithelium. This hypothesis is confirmed by the increased number of proliferating (Ki67 +) epithelial cells that we observed in EBB after bronchial thermoplasty. On the other hand, HSP70 expression in subepithelial mesenchymal cells is decreased after bronchial thermoplasty and this may lead to increased apoptosis and a lower number of proliferative (Ki67 +) subepithelial mesenchymal cells that we report in the present study.
HSP90 is the most abundant HSP in eukaryotic cells with two clearly signed roles both of which are important for the cells to cope with environmental changes such as tissue injury and heat. One of these roles is to act as an extracellular tissue-repairing factor as it has been shown that HSP90 promotes re-epithelialization and cell motility [34, 35]. Thus, the observation that after bronchial thermoplasty there is an increased expression of HSP90 in epithelial cells and a decreased expression in subepithelial mesenchymal cells indicates a possible cell-type specific mechanism of epithelial cell regeneration after bronchial thermoplasty that explains the beneficial outcomes of the procedure [12].
The second role of HSP90 is to act as an intracellular chaperone that forms heterocomplexes with GR, a process that also requires HSP70 [36, 37]. A critical role of the HSPs is to facilitate the folding of the hormone-binding domain of the receptor into a high-affinity steroid binding conformation. The main function of the GR/HSP complex is to keep the receptor in an inactive, yet potential ligand-active state. Therefore, the increased expression of HSP70 and HSP90 in epithelial cells after bronchial thermoplasty reported in the present study, facilitates GR function by increasing its binding to glucocorticoids, resulting in improved pharmacological action in asthma patients across all endotypes/phenotypes. These findings also provide a reasonable explanation for the clinically documented reduced need for corticosteroids after bronchial thermoplasty [38, 39].
Another novel finding of our study that also justifies the improved response of asthma patients to glucocorticoid treatment after bronchial thermoplasty, is the increased expression of GR in epithelial cells and in subepithelial mesenchymal cells after the procedure. In a model of chronic injury of mucociliated human bronchial epithelial cells, it has been shown that glucocorticoids increase repair potential [40]. In bronchial tissue sections of asthma patients, the expression of several proproliferative proteins in the epithelium correlated with the dosage of therapeutic glucocorticoids [41]. During lung maturation the GR is the key regulator of mesenchymal cell differentiation [42]. Furthermore, it has been shown that glucocorticoids inhibit the proliferation of ASMC, through the activation of the GR [43, 44]. However, the anti-proliferative effect of glucocorticoids depends on the presence of various co-factors such as C/EBP-alpha or insulin growth factor binding protein-1 [45, 46]. Thus, the increased expression of GR in epithelial cells and in subepithelial mesenchymal cells after bronchial thermoplasty, that we report in this study, may be a key regulator of the increased proliferation of epithelial cells and the decreased proliferation of subepithelial mesenchymal cells after the procedure. Overall, the observed increased expression of the GR and HSP90 in epithelial cells and subepithelial mesenchymal cells may explain the lasting beneficial effect of bronchial thermoplasty on the airway wall structure, as well as the reduced necessity of inhaled glucocorticoids after bronchial thermoplasty.
A limitation of our study is that it is not controlled as healthy individuals could not be subjected to such an invasive procedure and sham bronchial thermoplasty would also raise ethical issues. However, a major strength of our study is the assessment of biopsies that were obtained longitudinally after sequential bronchial thermoplasties from the same location allowing precise comparisons.
Conclusions
Our study implies that bronchial thermoplasty is associated with epithelial cell regeneration, decrease of ASM, increased expression and activation of GR, and increased expression of HSPs in the airways of asthma patients. Histopathological effects appear to be distinct in different endotypes/phenotypes indicating that the beneficial effects of bronchial thermoplasty are achieved by diverse molecular targets associated with asthma endotypes/phenotypes.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACT:
-
Asthma Control Test
- ASM:
-
Airway smooth muscle
- ASMC:
-
Airway smooth muscle cells
- BM:
-
Basement membrane
- EBB:
-
Endobronchial biopsies
- GR:
-
Glucocorticoid receptor
- HSP70:
-
Heat shock protein 70
- HSP90:
-
Heat shock protein 90
- PY:
-
Pack-years
References
Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–33.
Wilhelm CP, Chipps BE. Bronchial thermoplasty: a review of the evidence. Ann Allergy Asthma Immunol. 2016;116:92–8.
Pavord ID, Cox G, Thomson NC, RISA Trial Study Group, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185–91.
Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma. Am J Respir Crit Care Med. 2010;181:116–24.
Chernyavsky IL, Russell RJ, Saunders RM, et al. In vitro, in silico and in vivo study challenges the impact of bronchial thermoplasty on acute airway smooth muscle mass loss. Eur Respir J. 2018;51:1701680.
Pretolani M, Bergqvist A, Thabut G, et al. Effectiveness of bronchial thermoplasty in patients with severe refractory asthma: clinical and histopathologic correlations. J Allergy Clin Immunol. 2017;139(4):1176–85.
Mauad T, Dolhnikoff M. Pathologic similarities and differences between asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2008;14:31–8.
Papakonstantinou E, Savic S, Siebeneichler A, et al. A pilot study to test the feasibility of histological characterisation of asthma-COPD overlap. Eur Respir J. 2019;53(6).
Hodge G, Roscioli E, Jersmann H, et al. Steroid resistance in COPD is associated with impaired molecular chaperone Hsp90 expression by pro-inflammatory lymphocytes. Respir Res. 2016;17:135.
Polla BS, Bachelet M, Dall’ava J, Vignola AM. Heat schock proteins in inflammation and asthma: Dr Jekyll or Mr Hyde? Clin Exp Allergy. 1998;28(5):527–9.
Sun Q, Fang L, Roth M, et al. Bronchial thermoplasty decreases airway remodelling by blocking epithelium-derived heat shock protein-60 secretion and protein arginine methyltransferase-1 in fibroblasts. Eur Respir J. 2019;54(6).
Fang L, Li J, Papakonstantinou E, Karakioulaki M, Sun Q, Schumann D, Tamm M, Stolz D, Roth M. Secreted heat shock proteins control airway remodeling: evidence from bronchial thermoplasty. J Allergy Clin Immunol. 2021. https://doi.org/10.1016/j.jaci.2021.02.022.
Grendelmeier P, Tamm M, Jahn K, Pflimlin E, Stolz D. Flexible bronchoscopy with moderate sedation in COPD: a case-control study. Int J Chron Obstruct Pulmon Dis. 2017;12:177–87.
Grendelmeier P, Tamm M, Pflimlin E, Stolz D. Propofol sedation for flexible bronchoscopy: a randomised, noninferiority trial. Eur Respir J. 2014;43(2):591–601.
Schlatter L, Pflimlin E, Fehrke B, Meyer A, Tamm M, Stolz D. Propofol versus propofol plus hydrocodone for flexible bronchoscopy: a randomised study. Eur Respir J. 2011;38(3):529–37.
Duhamel DR, Hales JB. Bronchial thermoplasty: a novel therapeutic approach to severe asthma. J Vis Exp. 2010;(45): 2428.
Karakioulaki M, Koletsa T, Papakonstantinou E, et al. Histopathological comparison of endobronchial biopsies from different pulmonary lobes of severe asthmatic patients. Chest. 2020. https://doi.org/10.1016/j.chest.2020.04.029.
Pavord ID, Thomson NC, Niven RM, Research in Severe Asthma Trial Study Group, et al. Safety of BT in patients with severe refractory asthma. Ann Allergy Asthma Immunol. 2013;111:402–7.
Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–56.
Schatz M, Mosen DM, Kosinski M, et al. Validity of the Asthma Control Test completed at home. Am J Manag Care. 2007;13:661–7.
Schatz M, Zeiger R, Drane A, et al. Reliability and predictive validity of the Asthma Control Test administered by telephone calls using speech recognition technology. J Allergy Clin Immunol. 2007;119:336–43.
Schatz M, Kosinski M, Yarlas A, Hanlon J, Watson M, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124:719–23.
Chakir J, Haj-Salem I, Gras D, et al. Effects of bronchial thermoplasty on airway smooth muscle and collagen deposition in asthma. Ann Am Thorac Soc. 2015;12:1612–8.
Haj Salem I, Gras D, Joubert P, et al. Persistent reduction of mucin production after bronchial thermoplasty in severe asthma. Am J Respir Crit Care Med. 2019;199(4):536–8.
Thomson NC. Recent Developments in bronchial thermoplasty for severe asthma. J Asthma Allergy. 2019;12:375–87.
Benayoun L, Letuve S, Druilhe A, et al. Regulation of peroxisome proliferator-activated receptor gamma expression in hu- man asthmatic airways: relationship with proliferation, apoptosis, and airway re- modeling. Am J Respir Crit Care Med. 2001;164(8 pt 1):1487–94.
Konietzke P, Weinheimer O, Wielpütz MO, et al. Quantitative CT detects changes in airway dimensions and air-trapping after bronchial thermoplasty for severe asthma. Eur J Radiol. 2018;107:33–8.
Pretolani M, Dombret MC, Thabut G, et al. Reduction of airway smooth muscle mass by bronchial thermoplasty in patients with severe asthma. Am J Respir Crit Care Med. 2014;190(12):1452–4.
Polla BS, Bachelet M, Dall’ava J, Vignola AM. Heat shock proteins in inflammation and asthma. Clin Exp Allergy. 1998;28(5):527–9.
Ribeiro SP, Villar J, Downey GP, Edelson JD, Slutsky AS. Sodium arsenite induces heat shock protein-72 kilodalton expression in the lungs and protects rats against sepsis. Crit Care Med. 1994;22:922–9.
Villar J, Edelson JD, Post M, Brendan J, Mullen M, Slutsky AS. Induction of heat stress proteins is associated with decreased mortality in an animal model of acute lung injury. Am Rev Resp Dis. 1993;147:177–81.
Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163–70.
Liu L, Huang Y, Feng X, Chen J, Duan Y. Overexpressed Hsp70 alleviated formaldehyde-induced apoptosis partly via PI3K/Akt signaling pathway in human bronchial epithelial cells. Environ Toxicol. 2019;34(4):495–504.
Li W, Sahu D, Tsen F. Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim Biophys Acta. 2012;1823(3):730–41.
Cheng F, Sahu D, Tsen F, et al. A fragment of secreted Hsp90alpha carries unique properties to accelerate acute and chronic wound healing. J Clin Invest. 2011;121(11):4348–61.
Czar MJ, Owens-Grillo JK, Dittmar KD, et al. Characterization of the protein-protein interactions determining the heat shock protein (hsp90.hsp70.hsp56) heterocomplex. J Biol Chem. 1994;269:11155–61.
Hutchison KA, Dittmar KD, Czar MJ, Pratt WB. Proof that hsp70 is required for assembly of the glucocorticoid receptor into a heterocomplex with hsp90. J Biol Chem. 1994;269:5043–9.
Eisenmann S, Schütte W, Funke F, Oezkan F, Islam S, Darwiche K. Bronchial thermoplasty including the middle lobe bronchus significantly improves lung function and quality of life in patients suffering from severe asthma. Lung. 2019;197(4):493–9.
Langton D, Ing A, Bennetts K, Wang W, Farah C, Peters M, Plummer V, Thien F. Bronchial thermoplasty reduces gas trapping in severe asthma. BMC Pulm Med. 2018;18(1):155.
Wadsworth SJ, Nijmeh HS, Hall IP. Glucocorticoids increase repair potential in a novel in vitro human airway epithelial wounding model. J Clin Immunol. 2006;26(4):376–87.
Vignola AM, Chiappara G, Siena L, et al. Proliferation and activation of bronchial epithelial cells in corticosteroid-dependent asthma. J Allergy Clin Immunol. 2001;108(5):738–46.
Bridges JP, Sudha P, Lipps D, et al. Glucocorticoid regulates mesenchymal cell differentiation required for perinatal lung morphogenesis and function. Am J Physiol Lung Cell Mol Physiol. 2020;319(2):L239–55.
Roth M, Johnson PR, Borger P, et al. Dysfunctional interaction of C/EBPalpha and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N Engl J Med. 2004;351(6):560–74.
Fernandes D, Guida E, Koutsoubos V, et al. Glucocorticoids inhibit proliferation, cyclin D1 expression, and retinoblastoma protein phosphorylation, but not activity of the extracellular-regulated kinases in human cultured airway smooth muscle. Am J Respir Cell Mol Biol. 1999;21(1):77–88.
Roth M, Johnson PR, Rüdiger JJ, et al. Interaction between glucocorticoids and beta2 agonists on bronchial airway smooth muscle cells through synchronised cellular signalling. Lancet. 2002;360(9342):1293–9.
Bui H, Amrani Y, Deeney B, Panettieri RA, Tliba O. Airway smooth muscle cells are insensitive to the anti-proliferative effects of corticosteroids: the novel role of insulin growth factor binding Protein-1 in asthma. Immunobiology. 2019;224(4):490–6.
Funding
Clinic of Pneumology of the University Hospital Basel and the Department of Biomedicine, University of Basel, Switzerland; Unrestricted research grant from Boston scientific.
Author information
Authors and Affiliations
Contributions
DS, MT, EP conceived and designed the study; DS, EP, LF, MK, LZ, MR, MT data collection, accuracy of data; TK, SS, evaluation of histological parameters; LG, statistical analysis. All authors contributed to and approved the final manuscript taking complete responsibility for the integrity of the work from initiation until the publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was performed according to the GCP guidelines and was approved by the local Institutional Review Board (EKNZ 2016-01057). All patients provided written informed consent to participate in the study.
Competing interests
Dr. Stolz reports grants from Astra-Zeneca AG, Curetis AG, Boston Scientific, Novartis AG, GSK AG, Roche AG, Zambon, Pfizer, Schwabe Pharma AG, Vifor AG. The other authors declare that they have no competing interests related to the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
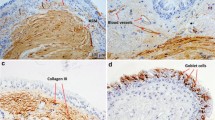
Additional file 1: Figure S1.
Representative microphotographs of endobronchial biopsies obtained from asthma patients before and after thermoplasty, at different magnifications (X100-X400).
Additional file 2: Table S1.
Lung function parameters in patients with severe asthma after bronchial thermoplasty.
Additional file 3: Table S2.
Association between blood eosinophils and tissue eosinophilic infiltration before BT using generalized linear models.
Additional file 4: Table S3.
Histopathological evaluation of endobronchial biopsies before and after bronchial thermoplasty.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Papakonstantinou, E., Koletsa, T., Zhou, L. et al. Bronchial thermoplasty in asthma: an exploratory histopathological evaluation in distinct asthma endotypes/phenotypes. Respir Res 22, 186 (2021). https://doi.org/10.1186/s12931-021-01774-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-021-01774-0