Abstract
Background
The Evaluating Respiratory Symptoms in Chronic Obstructive Pulmonary Disease (E-RS:COPD) is a patient-reported diary that assesses respiratory symptoms in stable COPD.
Methods
This post hoc analysis of a randomized, double-blind, parallel-arm trial (GSK ID: 200699; NCT02164539) assessed the structure, reliability, validity and responsiveness of the E-RS, and a separate wheeze item, for use in patients with a primary diagnosis of asthma or COPD, but with spirometric characteristics of both (fixed airflow obstruction and reversibility to salbutamol; a subset of patients referred to as spirometric asthma-COPD overlap [ACO]; N = 338).
Results
Factor analysis demonstrated that E-RS included Cough and Sputum, Chest Symptoms, and Breathlessness domains, with a Total score suitable for quantifying overall respiratory symptoms (comparative fit index: 0.9), consistent with the structure shown in COPD. The wheeze item did not fit the model. Total and domain scores were internally consistent (Cronbach’s alpha: 0.7–0.9) and reproducible (intra-class correlations > 0.7). Moderate correlations between RS-Total and RS-Breathlessness scores were observed with St George’s Respiratory Questionnaire (SGRQ) Total and Activity domain scores at baseline (r = 0.43 and r = 0.48, respectively). E-RS scores were sensitive to change when a patient global impression of change and SGRQ change scores were used to define responders, with changes of ≥ − 1.4 in RS-Total score interpreted as clinically meaningful.
Conclusions
E-RS:COPD scores were reliable, valid and responsive in this sample, suggesting the measure may be suitable for evaluating the severity of respiratory symptoms and the effects of treatment in patients with asthma and COPD that exhibit spirometric characteristics of both fixed airflow obstruction and reversibility. Further study of this instrument and wheeze in new samples of patients with ACO is warranted.
Similar content being viewed by others
Background
Patients with features of both asthma and chronic obstructive pulmonary disease (COPD) may present with chronic airflow obstruction and a reversible component. Asthma-COPD overlap (ACO) [1, 2] was first considered in 2009 [3] before appearing in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategic document in 2014. [4] Its prevalence can vary depending on the definition used. [5,6,7] Clinical studies have used varying diagnostic criteria for ACO, [8] and while recent efforts to identify recognized criteria for the condition have been made by GOLD/Global Initiative for Asthma (GINA) in 2015 [9], GINA in 2018 [1], and an international panel of experts in 2016, [10] there is no definitive consensus definition for ACO.
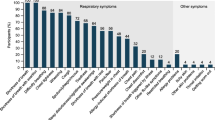
Although pharmacologic treatments have been approved in both asthma and COPD, little is known about their effects in patients with ACO. In addition, there are currently no ACO-specific patient-reported outcome (PRO) measures available to measure treatment effects on respiratory symptoms in ACO. Patients with ACO report similar symptoms to patients with COPD or asthma, [5, 11,12,13] including shortness of breath, cough, wheezing, difficulty breathing, mucus/phlegm and chest tightness. [5, 11,12,13] A reliable and valid PRO measure will provide important information on the respiratory symptom burden of ACO and the effects of treatment in this patient population. Based on the similarities in symptoms between COPD and ACO and their shared underlying airflow limitation, symptom measures developed for COPD may be appropriate in ACO. Among the most frequently utilized PROs in COPD is the St George’s Respiratory Questionnaire (SGRQ), which is a 50-item, self-administered measure for evaluating health status, with a subscale measuring the severity and impact of symptoms. [14] This instrument was not developed to evaluate respiratory symptoms per se, but includes an assessment of this as part of an overall health status score. Further, the SGRQ was designed for periodic rather than daily administration, with the latter considered optimal to capture patient symptoms more precisely. Other health status measures, such as the COPD Assessment Test [15] and the Chronic Respiratory Questionnaire [16] include symptom questions but are not scored to evaluate each of the cardinal symptoms of COPD. Questionnaires such as the University of California San Diego Shortness of Breath Questionnaire [17] and the Cough and Sputum Assessment Questionnaire [18] evaluate specific symptoms, i.e. dyspnea and cough and sputum, respectively. However, none of these instruments measure all of the cardinal symptoms, and with a daily recall to support insight into daily symptom fluctuations. The Evaluating Respiratory Symptoms in COPD (E-RS:COPD) (Evidera, Bethesda, MD, USA) measure is a patient-reported diary used to assess the cardinal symptoms of COPD overall and through three symptom-specific domains: Cough and Sputum, Chest Symptoms and Breathlessness. [19, 20] The E-RS includes 11 items from the Exacerbations of Chronic Pulmonary Disease Tool (EXACT) (Evidera, Bethesda, MD, USA) [21] and has shown evidence of content validity, reliability, validity and responsiveness in stable COPD. [19,20,21] It has also been qualified by the Food and Drug Administration and European Medicines Agency for use as an exploratory endpoint in drug development trials of COPD. [22, 23]
This post hoc analysis of clinical trial data (GSK: 200699, NCT02164539) [24] aimed to assess the factor structure, reliability, validity and responsiveness of the E-RS in patients with a primary diagnosis of asthma or COPD, and spirometric characteristics of both conditions (fixed airflow obstruction and reversibility to salbutamol). The term “spirometric ACO” is used, and in this study it reflects an ACO population inclusive of patients with and without exposure to smoking or biomass fuels. [24] As wheeze is not part of the E-RS, [20] a previously developed wheeze item (GSK, Research Triangle Park, NC, USA) was used to explore the relationship between the E-RS and wheeze scores and determine if a modification to the E-RS:COPD would be needed to use the measure in spirometric ACO.
Methods
Trial design
This was an exploratory post hoc analysis of a double- blind, parallel-arm trial conducted in Argentina, Germany, Poland, Romania, Russia, Ukraine and the USA during 2014–2015 (GSK: 200699, NCT02164539). [24] Following a 4-week run-in period (Day − 28 to Day − 1), patients with spirometric ACO were randomized on Day 1 to receive fluticasone furoate 100 mcg alone or in combination with either umeclidinium (15.6, 62.5, 125 or 250 mcg) or vilanterol 25 mcg, once daily for 4 weeks (Day 1 to Day 28) using an Ellipta inhaler (ELLIPTA is owned by or licensed to the GSK group of companies).
The trial was approved by an ethics committee or institutional review board in each country and conducted in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice guidelines [25] and the Declaration of Helsinki, 2013. [26] All patients provided written, informed consent.
Patient sample
This trial enrolled patients with a primary diagnosis of asthma or COPD, and who had spirometric characteristics of both conditions (fixed airflow obstruction and reversibility to salbutamol). [24] Patients were ≥ 18 years of age and had sufficient medical history (either smokers or non-smokers) to receive a diagnosis (via signs and symptoms) of COPD (American Thoracic Society/European Respiratory Society definition) [27] and an asthmatic component evidenced by spirometric criteria described in Additional File 1, which were consistent with the spirometric criteria for ACO available at time of study. [9] Therefore, the patients enrolled in this study were considered as having spirometric ACO. Patients were assigned a primary diagnosis (clinical) of either asthma or COPD, based on medical history and clinical judgement, at the beginning of the run-in period. This reflected the primary component of the patient’s disease. Patients also received a historical diagnosis by a healthcare professional (HCP) based on their medical records and determined by the investigator by asking the questions ‘do you have asthma?’ or ‘do you have COPD?’ at Visit 1.
Measures
Patients completed the 14-item EXACT [21] and wheeze item using electronic daily diaries throughout the run-in and treatment periods (Day − 28 to Day 28). For this analysis, only the 11 items comprising the E-RS instrument were included. For the wheeze item, patients were also asked “Did you wheeze today?” with response options of ‘Not at all’, ‘Rarely’, ‘Occasionally’, ‘Frequently’ and ‘Almost constantly’.
Morning and evening peak expiratory flow (PEF), morning and evening patient-initiated spirometry and rescue medication use were recorded using electronic daily diaries. Other variables from clinic visits included forced expiratory volume in 1 s (FEV1) % predicted (Day − 28, Day 1 and Day 28); the PRO measures of the SGRQ (Day 1 and Day 28); Patient Global Impression of Change (PGIC) in lung condition (overall disease) using a 7-point Likert scale ranging from much better to much worse (Day 28); and modified Medical Research Council (mMRC) dyspnea status (Day − 28 and Day 1).
Statistical analyses
Analyses were performed on the intent-to-treat population, including all patients who received ≥1 dose of the randomized treatment. [24] Data from the run-in period and the first 4 weeks of treatment were used, pooled across treatment groups. The baseline week was the last week of the run-in period (Day − 7 to Day − 1) and the final treatment week was the last week of the treatment period (Day 22 to Day 28). Exploratory analyses stratified by primary diagnosis were also conducted to assess the extent to which the E-RS and wheeze scores performed differently in asthma- predominant versus COPD-predominant disease.
Item analysis
Descriptive statistics for the E-RS items, as well as the Total and domain scores (using the E-RS item- and domain-level scoring algorithm [20]) and the wheeze item, were calculated for Day − 1 (prior to randomization) and Day 28 (end of study treatment). Inter-item correlations were analyzed at Day − 1 using Spearman correlation coefficients. Correlations > 0.40 and > 0.70 were defined as moderate and strong, respectively. [28]
Factor analysis
Using data from Day − 1, a confirmatory factor analysis (CFA) using structural equation modeling (higher order three-factor model) was conducted to test the factor structure of the E-RS in relation to that shown in COPD [19, 20], and to identify which factor (domain) each item was most strongly associated with (factor loading). Factor loadings > 0.40 were considered acceptable. An exploratory factor analysis was performed with the wheeze item to assess whether it was associated with one or more of the E-RS domains, suggesting a new E-RS scoring algorithm would be needed for use in spirometric ACO.
Reliability
With the factor structure in place, internal consistency (the extent to which individual items within an instrument or its domains are inter-related) of the E-RS was evaluated using Cronbach’s alpha at Day − 1 and Day 28. Values > 0.70 were considered acceptable for aggregate data. To assess the reproducibility of scores over time, test-retest reliability of the E-RS scores and wheeze item were analyzed using intra-class correlations (ICC) and paired t-tests between Days − 2 and − 1 and between Days − 7 and − 6. ICC values > 0.70 were considered acceptable. [28] Test-retest analyses were also conducted for patients with stable PEF over 2 days (change in daily morning PEF of < 15%).
Construct validity
To determine if the E-RS and wheeze item measure the constructs they were designed to measure, correlations between these instruments and conceptually-related measures were assessed. Using scores averaged across the baseline week and the final treatment week, Spearman’s correlation coefficients were calculated between E-RS or wheeze scores and the following criterion variables: SGRQ Total and domain scores, average daily rescue medication use, average morning PEF, average FEV1 from patient-initiated spirometry and clinic-collected FEV1% predicted. Moderate–strong correlations (r > 0.4) were expected for the SGRQ scores, while weak correlations were expected for pulmonary function, consistent with previous findings. [19, 20]
Known-groups validity was tested using an analysis of variance to evaluate the relationship between the mean of daily E-RS and wheeze item scores during the baseline week and categories commonly used in the criterion variables: exacerbation history (≥1 or 0) prior to Day − 28, FEV1% predicted (GOLD guidelines: ≥80%, 50–80, < 50%) at Day − 28 and Day 1, mMRC dyspnea status (0–1, 2, 3–4) at Day 1 and primary diagnosis (asthma or COPD) at Day − 28.
Responsiveness
The responsiveness of the E-RS and wheeze item, which refers to the ability of these measures to detect change over time, was examined using an analysis of covariance among patients considered to be responders from the baseline week to the final treatment week. Patients were assigned to responder groups based on their PGIC score (better/much better, no change/slightly better, slightly worse/worse), and changes in SGRQ Total scores (responder thresholds: <− 4 [better], − 4 to 4 [no change] and > 4 [worse]). [29] The E-RS and wheeze item were considered responsive if scores improved or declined in these responder groups (results in the no-change group provided evidence of reproducibility). To understand the magnitude of change, effect sizes were calculated; effect sizes of 0.20, 0.5 and 0.8 were interpreted as small, moderate and large, respectively. [28]
Within-patient change thresholds
An anchor-based approach was used to identify the threshold of within-patient change considered meaningful for E-RS (Total and domain) and wheeze item scores. Meaningful score improvements were identified by a PGIC score of 1 (slightly better) or a decrease (improvement) in SGRQ Total score of 4.0–5.0 points inclusive. E-RS and wheeze scores representing meaningful deterioration were defined by a PGIC score of − 1 (slightly worse) or an increase in SGRQ Total score of 4.0–5.0 points inclusive.
Results
Patient population
A total of 338 patients were randomized to receive trial treatment, of whom 97% completed the trial. Most patients were ≥ 40 years of age (95%), white (98%), 53% were male, and 54 and 46% had a primary diagnosis (according to patient medical records) of asthma or COPD, respectively (Table 1). A total of 227 (67%) and 273 (81%) of patients had a diagnosis by a HCP of asthma and COPD, respectively. All patients met the spirometric ACO criteria at baseline. Spirometric analyses by primary diagnosis are included in Additional file 1: Table S1. Most (63%) patients were current or former smokers, with an overall mean of 24.4 pack-years at screening. For patients with a primary diagnosis of asthma or COPD, 56 and 15% of patients indicated that they had never smoked and 45 and 85% were current or former smokers, respectively (data not shown). The mean (standard deviation [SD]) baseline E-RS Total (RS-Total) and SGRQ Total scores were 10.7 (6.1) and 44.0 (15.3) respectively, across treatment groups.
Item analysis
Inter-item correlation
Descriptive statistics for the E-RS and wheeze item are summarized in Additional file 1: Table S2. In the overall sample, correlations between items within the same domain were stronger (0.4–1.0) than those across different domains (0.2–0.6) at Day − 1 (Table 2). There was a moderate correlation (all > 0.5 and < 0.7) between ‘chest symptoms’ (items 5 and 6 [Chest domain]) and ‘chest congested’ (item 1 [Cough and Sputum domain]) and ‘breathless’ (item 7 [Breathlessness domain]).
Factor analysis
In the CFA, the 11 E-RS items loaded clearly onto the domains of Cough and Sputum, Chest Symptoms and Breathlessness (Fig. 1). Model fit was strong (comparative fit index: 0.9, root mean square error of approximation: 0.1). In the exploratory factor analysis (EFA; Additional file 1: Table S3), the wheeze item did not load onto any of the E-RS domains (promax rotation factor loading across domains: < 0.37). The factor analysis results confirmed the factor structure of the E-RS, excluding wheeze, and including a Total score and three domain scores, for use in patients with spirometric ACO. Thus, the E-RS structure was retained for tests of reliability, validity and responsiveness and the wheeze item was analyzed separately.
Reliability
RS-Total and domain scores were internally consistent at Day − 1 (Cronbach’s alpha: 0.7–0.9) and Day 28 (Cronbach’s alpha: 0.8–0.9). All scores exceeded the 0.7 threshold at Day − 1 (Table 3) and Day 28 (data not shown).
Over the Day − 2 to Day − 1 test-retest, E-RS and wheeze item scores were reproducible, with all ICC scores reaching the 0.7 threshold (Table 3). There were no significant differences between Day − 2 and Day − 1 tests for E-RS scores or wheeze item scores (p = 0.3–0.7). From Day − 7 to Day − 6, E-RS scores were reproducible (p = 0.4–0.9), although the ICC score for the wheeze item did not reach 0.7 (0.67; p = 0.56; Additional file 1: Table S4). E-RS scores were also reproducible in patients who exhibited < 15% change from the previous day in morning PEF for both test-retests (Table 3 and Additional file 1: Table S4).
Construct validity
RS-Total and RS-Breathlessness scores were correlated with SGRQ Total and Activity domain scores (r = 0.43 to 0.48) during the baseline week (Table 4). Correlations between the RS-Total and RS-Breathlessness scores and the SGRQ Impacts and Symptoms domain scores were lower (r = 0.35 to 0.39) (Table 4). The correlations between RS-Total and domain scores and pulmonary function variables were low (morning PEF: r = − 0.19 to − 0.33; FEV1: r = − 0.06 to − 0.24; FEV1% predicted: r = − 0.05 to − 0.22). For the wheeze item during the baseline week, correlation with the SGRQ Symptom domain score was moderate (r = 0.41) and correlations with pulmonary function variables were weak (morning PEF: -0.12; FEV1: r = 0.01; FEV1% predicted: r = − 0.11). Correlations during the final treatment week are shown in Table 4.
In the known-groups validity analysis, RS-Total scores during the baseline week differentiated between patients grouped according to FEV1% predicted at Day − 28 (p = 0.0323) and Day 1 (p = 0.0154), mMRC dyspnea status (p < 0.0001) and primary diagnosis of asthma versus COPD (p = 0.0033), but not exacerbation history (p = 0.3378; Fig. 2). The wheeze item during the baseline week differentiated between patients grouped according to mMRC dyspnea status (p = 0.0002) but not exacerbation history (p = 0.081), FEV1% predicted at Day − 28 (p = 0.1986) or Day 1 (p = 0.147) or primary diagnosis (p = 0.45).
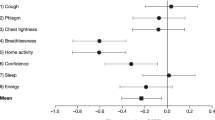
Mean (SD) known groups validity of E-RS Total and domain scores and wheeze item at the baseline week. The baseline week was defined as the last week of run-in (Day − 7 to Day − 1). *p < 0.05; **p < 0.01; ***p < 0.001; Error bars show standard deviations. COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms in COPD; FEV1, forced expiratory volume in 1 s; mMRC, Modified Medical Research Council; SD, standard deviation
Responsiveness
For patients rated as better/much better from baseline to Day 28 on the PGIC scale for lung condition (overall disease), mean (SD) change from baseline to final week in RS-Total was − 2.2 (3.6), with an effect size of − 0.4, representing a small to moderate improvement (Fig. 3). Similarly, for patients with a SGRQ Total score improvement from baseline, mean (SD) change in RS-Total from baseline to final week was − 2.1 (3.3) with an effect size of − 0.4. Mean (SD) change in RS-Total for patients with relatively stable disease, as indicated by no change on the PGIC scale or − 4 to 4 on the SGRQ scale, was − 1.2 (3.55) and − 1.3 (3.43), respectively, each with small effect sizes of − 0.2. Responsiveness of E-RS domain scores is shown in Fig. 3.
Responsiveness for E-RS scores and wheeze item (mean change from baseline week to final week). The baseline week was defined as the last week of run-in (Day − 7 to Day − 1). The final week was defined as the final week of the 4-week treatment period (Day 21 to Day 28). E-RS, Evaluating Respiratory Symptoms in chronic obstructive pulmonary disease; SGRQ, St George’s Respiratory Questionnaire. Of the 160 patients categorized as no change/slightly better, 31 were categorized as no change and 129 as slightly better. Of the 17 patients categorized as slightly worse/worse, 14 were categorized as slightly worse and 3 as worse
For the wheeze item, mean (SD) change in wheeze scores from baseline to final week was − 0.22 (0.54) for patients rated as better/much better on the PGIC scale and − 0.18 (0.48) for patients with an improvement on the SGRQ scale, both with an effect size of − 0.3 (Fig. 3). In stable patients, according to PGIC and SGRQ scales, the mean (SD) change in wheeze scores was − 0.1 (0.52) and − 0.2 (0.56), with effect sizes of − 0.1 and − 0.2, respectively.
Within-patient change thresholds
Patients reporting slightly better lung condition (overall disease) on the PGIC (1 point) in the final week relative to baseline had a mean (SD) change in RS-Total score of − 1.4 (3.4), while those with a meaningful improvement in health status based on a change in SGRQ Total score of 4 to 5 points had a mean change score in RS-Total of − 2.7 (3.4) (Table 5). RS-Cough and Sputum, RS-Chest Symptoms and RS-Breathlessness values in patients rated as slightly better (n = 129) on the PGIC scale were − 0.3 (1.2), − 0.4 (1.1) and − 0.7 (1.6), respectively, while those with health status improvements (n = 8) as assessed by SGRQ had values of − 0.7 (0.6), − 0.8 (1.4), and − 1.2 (1.6), respectively (Table 5). Values for the wheeze item are shown in Table 5.
Impact of primary diagnosis
The psychometric properties of the E-RS and wheeze item were consistent between patients with a primary diagnosis of asthma and COPD (Additional file 1: Tables S5–S13). Further details are described in the Additional File 1.
Discussion
These post hoc analyses indicate that E-RS scores were reliable, valid and responsive in patients with spirometric ACO (fixed airflow obstruction and reversibility to salbutamol), including patients whose underlying respiratory disease was classified as primarily asthma or COPD who were enrolled in a clinical trial to test the effects of pharmaceutical agents on change from baseline in clinic trough (pre-dose) FEV1. [24] The higher-order factor structure of the E-RS in the present study excluded wheeze and supported three domain and Total scale scores, consistent with the structure of the E-RS in COPD. [19, 20] Total and domain scores showed high levels of internal consistency, similar to those reported in patients with COPD (0.70–0.90), indicating a high degree of precision with low measurement error. [19, 20] Although the strength and pattern of relationships observed between E-RS scores and both SGRQ scores and pulmonary function variables supported the validity of the instrument in this sample, the correlations between the E-RS and SGRQ scores were not as strong as those shown in COPD. [20] For example, the correlation between the E-RS and SGRQ Total scores was 0.75 in COPD compared with 0.46 in this study. [20] The weak correlations between RS-Total and SGRQ domain scores and pulmonary function variables in the present study were consistent with those reported in COPD. [20, 21] Meaningful improvements in RS-Total score were determined based on a PGIC score of 1 (slightly better) or a decrease (improvement) in SGRQ Total score of greater than − 4 points. As these corresponded with mean changes in RS-Total scores of − 1.4 and − 2.7, respectively, scores greater than − 1.4 may be used as a starting point for interpreting change scores in clinical trials evaluating the effect of treatment on respiratory symptoms in patients with spirometric ACO. This estimate is smaller than the estimate of − 2.0 in COPD. [19] Patient numbers for some of the subgroups assessed in the within-patient change threshold analyses were low (n = 8–14), and therefore, care should be taken when drawing conclusions from these data.
Findings for the wheeze item were less clear. The item did not correlate with E-RS items, nor did it fit into the factor structure of the E-RS. The wheeze item did correlate with SGRQ scores but known-groups validity was poor and limited to differentiating only mMRC dyspnea levels. Responsiveness analyses demonstrated limited sensitivity of the wheeze item to change, which failed to show a difference between groups stratified by PGIC or change in SGRQ. Although patients with spirometric ACO have described ‘wheeze’ as part of their symptom experience, their descriptions of its precise nature vary, [12] making standardized assessment of wheeze from a patient perspective difficult. This variation is consistent with qualitative descriptions of wheeze in patients with COPD. [20] Wheeze may be best reclassified as a sign of disease and assessed by clinicians through auditory evaluation (lung sounds), rather than patient self-report. Alternatively, self-report of wheeze with varied terminology could be explored in future studies.
In this study, patients reported a mean (SD) RS-Total score of 10.7 (6.1) at Day − 1, which is lower than the mean scores (11.0–18.2) reported in studies of mild to very severe COPD across different trial populations. [19, 30,31,32] This suggests that the patients in this study were less symptomatic than typical patients with stable COPD. [19] In contrast, the health status of this sample, as assessed by SGRQ (mean [SD] Total scores: 44.0 [15.3]), was comparable to patients with mild to very severe COPD (mean SGRQ: 47.6, range: 29.2–55.2 across subgroups by symptom), [14] moderate to severe COPD (mean SGRQ: 47.8–49.6 across treatment groups), [33] severe COPD (mean SGRQ: 53.5–54.8 across treatment groups) [34] and severe asthma (41.2). [35] This suggests that factors other than symptoms may be limiting health status in patients with spirometric ACO.
Limitations of this study include that the standardized diagnostic criteria used for ACO are debatable. [9, 10] Therefore, the study sample may not fulfill all of the elements contained in the recently proposed ACO criteria. [10] For example, a recent consensus statement suggests patients with ACO usually present with persistent airflow limitation, at least 10 pack-years of smoking, and documentation of asthma before 40 years of age OR a bronchodilator response of > 400 mL (major criteria). [10] Minor criteria include history of atopy or allergic rhinitis, a bronchodilator response of FEV1 ≥ 200 mL and 12% and a peripheral blood eosinophil count of ≥300 cells per μL. [10] In this study, although most patients were current or former smokers, 37% of patients had never smoked, of whom, 56 and 15% had a primary diagnosis of asthma and COPD, respectively. In addition, only 46% of patients demonstrated FEV1 reversibility of ≥400 mL. However, consistent with the recent consensus definition of ACO, most patients had received a diagnosis of asthma (primary diagnosis: 54%, diagnosis by a HCP: 67%), most were ≥ 40 years of age (95%), and all exhibited persistent airflow limitation (post-bronchodilator FEV1/forced vital capacity < 0.70) and a post-bronchodilator increase in FEV1 of ≥12% and ≥ 200 mL. This indicates that the sample generally represents patients with ACO and ACO-like illness sufficient to test the performance properties of the E-RS as a measure of respiratory symptoms in studies of ACO. Of note, smoking is not included as standard across all definitions of ACO, [36, 37] and although smoke and/or biomass exposure are compatible with an ACO diagnosis, the condition is present in up to 60% of non-smokers when diagnosis is based on lung function criteria [38], supporting the relevance of the population reported in this study. These results are also the first to suggest a suitable tool for measuring symptoms in spirometric ACO regardless of primary diagnosis. While we acknowledge that our spirometric ACO population may differ slightly from other ACO populations in terms of its clinical characteristics, we stress that none of the features of asthma or COPD is pathognomonic, as discussed by Bateman, et al., [38] and our results are relevant in a spirometric ACO population as defined herein. As our analysis included patients from a single clinical trial and was defined by criteria based on spirometric outcomes, these results may not be generalizable to all patients with ACO.
Conclusion
Results of this study suggest that the E-RS:COPD may be useful for quantifying treatment effects on respiratory symptoms in clinical trials of patients with spirometric ACO. Further study in patients with ACO is warranted as the characteristic features of this clinical population become more clearly defined.
Abbreviations
- ACO:
-
Asthma-COPD overlap
- CFA:
-
Confirmatory factor analysis
- COPD:
-
Chronic obstructive pulmonary disease
- EFA:
-
Exploratory factor analysis
- E-RS:COPD:
-
Evaluating Respiratory Symptoms in COPD
- EXACT:
-
Exacerbations of Chronic Pulmonary Disease Tool
- FEV1 :
-
Forced expiratory volume in one second
- GINA:
-
Global Initiative for Asthma
- GOLD:
-
Global Initiative for Chronic Obstructive Lung Disease
- ICC:
-
Intra-class correlations
- mMRC:
-
modified Medical Research Council
- PEF:
-
Peak expiratory flow
- PGIC:
-
Patient Global Impression of Change
- PRO:
-
Patient-reported outcome
- SD:
-
Standard deviation
- SGRQ:
-
St George’s Respiratory Questionnaire
References
Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. https://ginasthma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf. [Accessed May 2019]. 2018.
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of Chronic Obstructive Pulmonary Disease. https://goldcopd.org/gold-reports/ [Accessed December 2018]. 2019.
Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64:728–35.
Chronic Obstructive Pulmonary Disease (GOLD). Global strategy for the diagnosis, management and prevention of Chronic Obstructive Pulmonary Disease. https://goldcopd.org/gold-reports/ [Accessed Dec 13, 2018]. 2014.
de Marco R, Pesce G, Marcon A, Accordini S, Antonicelli L, Bugiani M, Casali L, Ferrari M, Nicolini G, Panico MG, Pirina P, Zanolin ME, Cerveri I, Verlato G. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One. 2013;8:e62985.
Gibson PG, McDonald VM. Asthma-COPD overlap 2015: now we are six. Thorax. 2015;70:683–91.
Montes de Oca M, Victorina Lopez Varela M, Laucho-Contreras ME, Casas A, Schiavi E, Mora JC. Asthma-COPD overlap syndrome (ACOS) in primary care of four Latin America countries: the PUMA study. BMC Pulm Med. 2017;17:69.
Alshabanat A, Zafari Z, Albanyan O, Dairi M, FitzGerald JM. Asthma and COPD overlap syndrome (ACOS): a systematic review and Meta analysis. PLoS One. 2015;10:e0136065.
Global Initiative for Chronic Obstructive Lung Disease (GOLD)/Global Initiative for Asthma (GINA). Diagnosis of diseases of chronic airflow limitation: asthma, COPD and acthma-COPD overlap syndrome (ACOS). https://goldcopd.org/asthma-copd-asthma-copd-overlap-syndrome/ [Accessed December 2018]. 2015.
Sin DD, Miravitlles M, Mannino DM, Soriano JB, Price D, Celli BR, Leung JM, Nakano Y, Park HY, Wark PA, Wechsler ME. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J. 2016;48:664–73.
Menezes AMB, Montes de Oca M, Perez-Padilla R, Nadeau G, Wehrmeister FC, Lopez-Varela MV, Muino A, Jardim JRB, Valdivia G, Talamo C. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145:297–304.
Nelsen L, Gater A, Panter C, Tolley C, Lee L, Pascoe S. Understanding and measuring symptoms and health status in asthma COPD overlap: content validity of the EXACT and SGRQ. J Patient Rep Outcomes. 2017;2:18.
Pascoe SJ, Wu W, Collison KA, Nelsen LM, Wurst KE, Lee LA. Use of clinical characteristics to predict spirometric classification of obstructive lung disease. Int J Chron Obstruct Pulmon Dis. 2018;13:889–902.
Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: The St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–7.
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–54.
Williams JE, Singh SJ, Sewell L, Guyatt GH, Morgan MD. Development of a self-reported Chronic Respiratory Questionnaire (CRQ-SR). Thorax. 2001;56:954–9.
Eakin EG, Sassi-Dambron DE, Ries AL, Kaplan RM. Reliability and validity of dyspnea measures in patients with obstructive lung disease. Int J Behav Med. 1995;2:118–34.
Crawford B, Monz B, Hohlfeld J, Roche N, Rubin B, Magnussen H, Nivens C, Ghafouri M, McDonald J, Tetzlaff K. Development and validation of a cough and sputum assessment questionnaire. Respir Med. 2008;102:1545–55.
Leidy NK, Murray LT, Monz BU, Nelsen L, Goldman M, Jones PW, Dansie EJ, Sethi S. Measuring respiratory symptoms of COPD: performance of the EXACT- Respiratory Symptoms Tool (E-RS) in three clinical trials. Respir Res. 2014;15:124.
Leidy NK, Sexton CC, Jones PW, Notte SM, Monz BU, Nelsen L, Goldman M, Murray LT, Sethi S. Measuring respiratory symptoms in clinical trials of COPD: reliability and validity of a daily diary. Thorax. 2014;69:443–9.
Leidy NK, Wilcox TK, Jones PW, Roberts L, Powers JH, Sethi S. Standardizing measurement of chronic obstructive pulmonary disease exacerbations. Reliability and validity of a patient-reported diary. Am J Respir Crit Care Med. 2011;183:323–9.
European Medicines Agency. Draft qualification opinion of qualification of exacerbations of chronic pulmonary disease tool (EXACT), and EXACT-respiratory symptoms measure (E-RS) for evaluating treatment outcomes in clinical trials in COPD (EMA/CHMP/SAWP/178465/2015). https://www.ema.europa.eu/documents/regulatory-procedural-guideline/draft-qualification-opinion-qualification-exacerbations-chronic-pulmonary-disease-tool-exact-exact_en.pdf [Accessed November 2018]. 2015.
Food and Drug Administration. Attachment to Guidance on Quantification Process for Drug Development Tools: Evaluating Respiratory Symptoms in Chronic Obstructive Disease, a Patient-reported Outcome Instrument for the Measurement of Severity of Respiratory Symptoms in Stable Chronic Obstructive Pulmonary Disease: Qualification for Exploratory Use. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM489526.pdf [Accessed November 2018]. 2016.
Lee L, Kerwin E, Collison K, Nelsen L, Wu W, Yang S, Pascoe S. The effect of umeclidinium on lung function and symptoms in patients with fixed airflow obstruction and reversibility to salbutamol: a randomised, 3-phase study. Respir Med. 2017;131:148–57.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human use. ICH harmonised tripartite guideline: guideline for good clinical practice E6(1). 1996.
World Medical Association. WMA Declaration of Helsinki - ethical principles for medical research involving human subjects. 2013. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed May 2019.
Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19:398–404.
Beier J, Mroz R, Kirsten AM, Chuecos F, Gil EG. Improvement in 24-hour bronchodilation and symptom control with aclidinium bromide versus tiotropium and placebo in symptomatic patients with COPD: post hoc analysis of a phase IIIb study. Int J Chron Obstruct Pulmon Dis. 2017;12:1731–40.
D'Urzo AD, Rennard SI, Kerwin EM, Mergel V, Leselbaum AR, Caracta CF, AUGMENT COPD study investigators. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res. 2014;15:123.
Tabberer M, Lomas DA, Birk R, Brealey N, Zhu CQ, Pascoe S, Locantore N, Lipson DA. Once-Daily Triple Therapy in Patients with COPD: Patient-Reported Symptoms and Quality of Life. Adv Ther. 2018;35:56–71.
Kerwin E, Donohue JF, Goodin T, Tosiello R, Wheeler A, Ferguson GT. Efficacy and safety of glycopyrrolate/eFlow® CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trials. Respir Med. 2017;132:238–50.
Papi A, Dokic D, Tzimas W, Meszaros I, Olech-Cudzik A, Koroknai Z, McAulay K, Mersmann S, Dalvi PS, Overend T. Fluticasone propionate/formoterol for COPD management: a randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2017;12:1961–71.
Bae YJ, Kim YS, Park CS, Lee YS, Chang YS, Cho YS, Jang AS, Cho SH, Choi BW, Kim SG, Moon HB, Kim TB. Reliability and validity of the St George's Respiratory Questionnaire for asthma. Int J Tuberc Lung Dis. 2011;15:966–71.
Kankaanranta H, Harju T, Kilpelainen M, Mazur W, Lehto JT, Katajisto M, Peisa T, Meinander T, Lehtimaki L. Diagnosis and pharmacotherapy of stable chronic obstructive pulmonary disease: the Finnish guidelines. Basic Clin Pharmacol Toxicol. 2015;116:291–307.
Koblizek V, Chlumsky J, Zindr V, Neumannova K, Zatloukal J, Zak J, Sedlak V, Kocianova J, Zatloukal J, Hejduk K, Pracharova S. Chronic Obstructive Pulmonary Disease: official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological Society; a novel phenotypic approach to COPD with patient-oriented care. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:189–201.
Bateman ED, Reddel HK, van Zyl-Smit RN, Agusti A. The asthma-COPD overlap syndrome: towards a revised taxonomy of chronic airways diseases? Lancet Respir Med. 2015;3:719–28.
Acknowledgments
Editorial support in the form of initial preparation of the outline based on input from all authors and collation and incorporation of author feedback to develop subsequent drafts, assembling tables and figures, copyediting and referencing was provided by Rachael Baylie, PhD, of Fishawack Indicia Ltd., UK, and was funded by GSK.
Funding
This post hoc analysis (GSK ID: HO-15-16166) of data from GSK study 200699 (NCT02164539), including the work presented here, the conduct of the trial, data analysis and interpretation, was funded by GSK. The sponsor was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Availability of data and materials
Information on GSK’s data sharing commitments and requesting access to anonymized individual participant data and associated documents can be found at www.clinicalstudydatarequest.com. The data generated or analyzed during this study are included in this published article and Additional File 1.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this analysis as well as analysis and interpretation of the data. All authors were involved in preparation and review of the manuscript and approved the final version to be submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethics approval was required for this study as data were taken from a previously conducted clinical trial (GSK: 200699, NCT02164539) that had obtained ethical approval. [24]
Consent for publication
Not applicable.
Competing interests
The authors met the criteria for authorship as recommended by the International Committee of Medical Journal Editors. LMN and LAL are employees of GSK and own stocks/shares in GSK. XL, WW and SJP were employees of GSK at the time the study was performed and own GSK stocks/shares. WW is currently employed by PAREXEL International. LM and NKL are employees of Evidera, which provides consulting and other research services to pharmaceutical, device, government and non-government organizations. Evidera holds the copyright to the EXACT, E-RS:COPD and all derivative measures. All authors were not paid for the development of this manuscript. Ellipta is owned by or licensed to the GSK group of companies.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Includes additional text describing the patient sample, the descriptive statistics for the E-RS and wheeze item, and the results of the inter-item correlations, factor analysis and known-groups validity by primary diagnosis. Descriptive statistics for the E-RS and wheeze item scores for Day − 1 and Day 28 and the EFA at Day − 1 for E-RS with and without the wheeze item in the overall population and by primary diagnosis of asthma and COPD are also described. (DOCX 87 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Nelsen, L.M., Lee, L.A., Wu, W. et al. Reliability, validity and responsiveness of E-RS:COPD in patients with spirometric asthma-COPD overlap. Respir Res 20, 107 (2019). https://doi.org/10.1186/s12931-019-1070-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-019-1070-6