Abstract
Background
Obstructive sleep apnea (OSA) is highly prevalent in patients with coronary artery disease (CAD) and is associated with recurrent cardiovascular risk. However, whether treatment with continuous positive airway pressure (CPAP) reduces this risk remains unclear. We performed a systematic review and meta-analysis to assess the effect of CPAP on long-term cardiovascular outcomes in patients with concomitant CAD and OSA.
Methods
We searched the PubMed, EMBASE, and Cochrane library from their inceptions to October 7, 2017. We included observational studies and randomized controlled trials (RCTs) that described the association of CPAP treatment with cardiovascular events in patients with CAD and OSA. The primary outcome of interest was major adverse cardiovascular event (MACE), including all-cause or cardiovascular death, myocardial infarction, stroke, repeat revascularization, or hospitalization for heart failure. Outcomes data were pooled using random effects models and heterogeneity assessed with the I2 statistic.
Results
We identified 9 studies (2 RCTs and 7 observational studies) with 1430 participants. The median follow-up duration was from 36 to 86.5 months. Treatment with CPAP was associated with a significantly lower risk of MACE in 6 observational studies (RR 0.61, 95% CI: 0.39–0.94, P = 0.02), but this was not reproduced in 2 RCTs (RR 0.57, 95% CI: 0.32–1.02, P = 0.06). Similarly, CPAP significantly reduced the risk of all-cause death (4 observational studies) and cardiovascular death (3 observational studies), which were also not confirmed in RCTs.
Conclusions
The use of CPAP in patients with CAD and OSA might prevent subsequent cardiovascular events, which was only demonstrated in observational studies, but not in RCTs. The value of CPAP therapy as second prevention for CAD needs further investigation.
Similar content being viewed by others
Background
Obstructive sleep apnea (OSA) is highly prevalent in patients with cardiovascular diseases. Compared to the general population, OSA is more common in patients with coronary artery disease (CAD), with a reported prevalence of 38% to 65% [1]. Observational studies have shown OSA was associated with increased risk of subsequent cardiovascular events in various subsets of CAD patients [2,3,4,5,6]. Continuous positive airway pressure (CPAP) is recommended for symptomatic patients with OSA, but multiple observational studies [7,8,9,10,11,12,13] and randomized controlled trials (RCTs) [14, 15] have shown inconsistent results of CPAP therapy in reducing cardiovascular events in patients with established CAD. Moreover, there is no meta-analysis focusing on patients with concomitant CAD and OSA and evaluating the role of CPAP in preventing recurrent adverse events. Therefore, we conducted a systematic review and meta-analysis to assess whether adding CPAP therapy would improve long-term cardiovascular outcomes in patients with CAD and OSA.
Methods
Search strategies
This meta-analysis was conducted in accordance to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement [16]. The searches included the PubMed, EMBASE, and Cochrane library from their inceptions to October 7, 2017, without language restrictions. We used Medical Subject Heading terms “Continuous Positive Airway Pressure”, “Sleep Apnea Syndromes”, “Myocardial Ischemia”, and related text words including CPAP, sleep apnea, and coronary disease. We also checked the reference lists of all included studies, relevant review articles, and conference abstracts manually for potential citations. An example search strategy is presented in Additional file 1: Table S1.
Study selection and eligibility criteria
Two authors (X.W. and Y.Z., both cardiologists) assessed the eligibility of articles by initially screening the titles and abstracts. Articles that reported the impact of CPAP versus standard therapy (control group) among patients with OSA and CAD were considered for inclusion. Each full-text article was then reviewed in duplicate by these authors. Studies that were not performed in patients with CAD, studies that did not report on outcomes of interest (cardiovascular events), and studies with less than 1-year follow-up were excluded. Any disagreement was resolved by consensus through referral to a third reviewer (S.N.).
Data extraction and validity assessment
Data extraction was performed independently and in duplicate by two reviewers (X.W. and Y.Z.) using a standardized electronic form, and verified by a senior author (S.N.). Any discrepancies were resolved by consensus. We recorded the following information: study design, location, and time span, number of participants, inclusion criteria for OSA, demographic characteristics, methods of OSA assessment, duration and completeness of follow-up, cardiovascular events, and potential confounders included in adjusted analysis.
The potential risk of bias of RCTs was appraised according to the Cochrane Collaboration guidelines [17]. The quality items included random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias, which were each classified as low, unclear, or high.
The quality of observational studies was evaluated using the Newcastle-Ottawa Scale for cohort studies [18]. A quality score was calculated according to a maximum of one star for each item upon selection (4 items: representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, demonstration that outcome was not present at study start), comparability (2 items: controls for the most important factor and any additional factor), and outcomes (3 items: assessment, duration, and adequacy of follow-up) categories.
The primary outcome of interest was major adverse cardiovascular events (MACE), defined as a composite of all-cause or cardiovascular death, myocardial infarction (MI), stroke, repeat revascularization, or hospitalization for heart failure. Secondary endpoints included all-cause death, cardiovascular death, MI, stroke, and repeat revascularization. Definitions of events were in accordance to guidelines during each study period. Endpoints were assessed at the longest follow-up.
Data synthesis and analysis
In general, we collected multivariable-adjusted hazard ratio (HR) or risk ratio (RR) from original studies. In case of unreported HR or RR of the outcomes of interest, we calculated unadjusted RR using crude values. Summary RR with 95% confidence interval (CI) were estimated for primary and secondary outcomes by DerSimonian and Laird random-effects model. We used the Cochran Q test and I2 statistic to assess heterogeneity across studies with a significance level of p < 0.10. All analyses were performed with Cochrane Review Manager software (version 5.3). A 2-sided p value < 0.05 was deemed significant.
Results
Study selection and characteristics
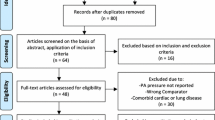
The literature search yielded 1452 citations of which 21 were retained for full-text review (Fig. 1). We subsequently excluded 12 studies, of which 7 studies did not specify patients with CAD, and 4 studies did not report on the outcomes of interest, and 1 study had less than 1-year follow-up.
Finally, a total of 9 studies [7,8,9,10,11,12,13,14,15] with 1430 participants were included in this meta-analysis. Study characteristics are listed in Tables 1 and 2. Seven studies were prospective cohort [7, 9,10,11, 13], 2 studies were retrospective cohort [8, 12], and 2 studies were RCTs [14, 15]. All studies enrolled patients with CAD and OSA. OSA was assessed primarily by overnight polysomnography in 7 studies [7,8,9, 11, 12, 14, 15], and by validated portable diagnostic devices in 2 studies [10, 13]. The definition of OSA was based on standardized assessment of apnea-hypopnea index (AHI) in all studies, with AHI ≥ 15 as cut-off value in most studies [7, 8, 10, 12, 14, 15]. In 2 studies, either CPAP (84% to 98%) or upper airway surgery (2% to 16%) was used, and effect measures were assessed for the entire modality [7, 8], whereas patients were exclusively treated with CPAP in others studies. Two retrospective cohort identified those who refused CPAP therapy or were not adherent to CPAP as untreated group [8, 12], whereas the others considered all patients receiving CPAP as being treated regardless of adherence. CPAP adherence data were available in 2 cohort studies [7, 9] (5.7 h [7] and 6.1 h [9] per night) and in 2 RCTs (4.5 h per night in Huang’s study [14] and 4.4 to 6.9 h per night during 6 years follow-up in Peker’s study [15]).
The median duration of follow-up was from 36 months to 86.5 months, and a small proportion of patients were lost to follow-up (up to 4.9%). Most of the studies reported adjusted risk estimates for the primary endpoint except 3 cohort studies [8, 11, 13] and 1 RCT [14], thus contributing to potential bias.
The 2 RCTs were open-label study and did not include blinding of participants and personnel to the intervention, but all did blinded assessment of cardiovascular outcomes [14, 15] (Additional file 1: Table S2). All the observational studies except 1 retrospective cohort [8] showed moderate-to-high quality (Newcastle-Ottawa Scale score > 6) (Additional file 1: Table S3).
Association of CPAP with MACE
Eight studies (6 observational studies and 2 RCTs) with 1307 patients reported outcome of MACE. Treatment with CPAP was associated with a significantly lower risk of MACE in 6 observational studies (RR 0.61, 95% CI: 0.39–0.94, P = 0.02). However, this result was not confirmed in 2 RCTs (RR 0.57, 95% CI: 0.32–1.02, P = 0.06) (Fig. 2). There was evidence of statistical heterogeneity for the composite endpoint in the observational studies (Q statistic P = 0.01; I2 = 66%). We further did subgroup analysis and showed that the decreased risk of MACE remained significant in 4 prospective cohort studies (RR 0.39, 95% CI: 0.21–0.74, P = 0.003; I2 = 34%), but was not significant in 2 retrospective cohort studies (RR 0.92, 95% CI: 0.78–1.07, P = 0.29; I2 = 0%), and the heterogeneity was attenuated in both subgroups (Additional file 1: Figure S1).
Association of CPAP with all-cause and cardiovascular death
There were 5 studies (1080 participants) that reported outcomes of all-cause death. CPAP significantly reduced the risk of all-cause death in 4 observational studies (RR 0.60, 95% CI 0.39–0.94, P = 0.03; I2 = 0%). However, the only RCT did not show significant risk reduction (RR 0.78, 95% CI 0.30–2.02, P = 0.61) (Fig. 3).
Cardiovascular death was evaluated in 5 studies with 866 participants. Similarly, CPAP therapy significantly reduced the risk of cardiovascular death in 3 observational studies (RR 0.28, 95% CI 0.12–0.68, I2 = 0%), which were also not reproduced in 2 RCTs (RR 0.41, 95% CI 0.12–1.41, I2 = 0%) (Fig. 4).
Association of CPAP with individual cardiovascular events
We also evaluated the effect of CPAP on outcomes of MI in 5 studies (3 observational and 2 RCTs; 781 participants), stroke in 3 studies (1 observational and 2 RCTs; 612 participants), and repeat revascularization in 6 studies (5 observational and 1 RCTs; 886 participants). There was no association of CPAP with all individual cardiovascular events in both observational studies and RCTs (Figs. 5, 6, and 7).
Discussion
In the present meta-analysis, the associations of CPAP use with risk reduction of composite cardiovascular events, all-cause and cardiovascular death in patients with concomitant CAD and OSA were only demonstrated in observational studies, but not RCTs. There were also no significant associations between CPAP treatment with individual cardiovascular outcomes. Based on these results, there is still no clear evidence to prescribe CPAP with the purpose of preventing future cardiovascular events in patients with OSA and established CAD.
OSA was linked to a series of cardiovascular risk factors and outcomes. CPAP is effective in reversing upper airway obstruction and hypoxemia. Randomized trials have demonstrated that CPAP treatment improves cardiovascular surrogate endpoints, such as blood pressure [14, 19] and insulin resistance [20]. However, in the trials evaluating MACE, no significant beneficial effects of CPAP were shown in patients with OSA [15, 21, 22]. In the most recently SAVE (Sleep Apnea Cardiovascular Endpoints) trial that randomized 2717 participants with coronary or cerebrovascular disease and moderate-to-severe OSA, CPAP did not result in a lower rate of the composite cardiovascular events at a median follow-up of 3.7 years [21]. Furthermore, several meta-analyses of randomized trials also showed no effect of CPAP therapy on MACE for OSA with or without cardiovascular morbidities [23,24,25]. However, the study populations of included studies are diverse, from general population to patients with severe CAD (such as MI), thus precluding definitive conclusions.
To the best of our knowledge, the present meta-analysis is the first attempt to focus on a relatively homogenous group of patients with established CAD. Our findings suggested adding CPAP as a secondary prevention for patients with CAD and concomitant OSA might be beneficial in the long-term follow-up, but this was only shown in observational studies, and not verified in RCTs. The enrolled studies in the prospective and retrospective cohorts were usually conducted more than 10 years ago and had a wide range of follow-up, therefore they do not represent contemporary medical and interventional therapy. Also, the results could be underpowered due to a relatively small sample size and variations in study populations and definitions of events. The negative results of RCTs were mainly derived from the RICCADSA (Randomized Intervention With CPAP in Coronary Artery Disease and Sleep Apnea) trial, which enrolled 224 patients with OSA and revascularized CAD. There is no significant difference in the composite endpoint of repeat revascularization, MI, stroke, or cardiovascular death between CPAP and untreated OSA patients [15]. It should be noted that CPAP therapy tended to be associated with a reduced risk of MACE in the 2 RCTs (RR 0.57, 95% CI: 0.32–1.02), although there was no significant difference. Due to the small number of included RCTs, this result should be interpreted with caution. In the RICCADSA trial, adjusted on-treatment analysis exhibited better outcomes among patients who were adherent to CPAP therapy (≥4 h per night). In addition, patients in the RICCADSA trial were heterogeneous with variable risk profiles, including both percutaneous coronary intervention (PCI) and CABG, and both acute or elective PCI, thus attenuating the anticipated treatment effect. In case of second prevention of CAD patients, the treatment effects of CPAP are still needed to be evaluated in a high-risk group with homogenous CAD populations (ACS, MI, or PCI, etc.).
In the contemporary era, with the extensive use of lipid-lowering and blood pressure lowering agents, antiplatelet therapy, and drug-eluting stents, treatment of OSA with CPAP might not add more benefits for CAD patients based on current evidence. As OSA is highly prevalent and is associated with subsequent cardiovascular risk, we should still pay more attention to this condition when assessing patients with CAD in clinical practice. Whether increased compliance to CPAP or novel treatment options can lead to better cardiovascular outcomes needs further investigation.
Study limitations
First, we observed significant statistical heterogeneity in the outcome measure of MACE in the observational studies, which could be partly explained by different study design, small sample size, and study quality according to whether adjustment for confounders was performed. We did subgroup analysis based on prospective or retrospective cohorts, and the heterogeneity was attenuated in both subgroups. Second, the study population varies across studies, from general CAD patients to MI with or without revascularization (PCI or CABG). The treatment effects of CPAP need to be further investigated in more homogenous patients. Third, there are significant differences in the definitions of the use and adherence of CPAP, which could impact the treatment effects compared to the control group. Fourth, there are not enough studies (less than 10) to test for publication bias for the primary endpoint. Fifth, the risk estimates of individual cardiovascular events could be underpowered due to a small number of included studies and variations in events definition.
Conclusions
Compared to standard therapy alone, the use of CPAP in patients with OSA and concomitant CAD was associated with a reduced risk of major cardiovascular events, all-cause and cardiovascular mortality, which was only observed in observational studies, but not in RCTs. There is a need for large-scale RCTs to further explore the value of CPAP therapy as a second prevention in a high-risk and homogenous CAD population.
Abbreviations
- ACS:
-
Acute coronary syndrome
- AHI:
-
Apnea-hypopnea index
- AMI:
-
Acute myocardial infarction
- BMI:
-
Body mass index
- CABG:
-
Coronary artery bypass grafting
- CAD:
-
Coronary artery disease
- CI:
-
Confidence interval
- CPAP:
-
Continuous positive airway pressure
- HR:
-
Hazard ratio
- MACE:
-
Major adverse cardiovascular event
- MI:
-
Myocardial infarction
- NR:
-
Not reported
- OSA:
-
Obstructive sleep apnea
- PCI:
-
Percutaneous coronary intervention
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis
- RCTs:
-
Randomized controlled trials
- RICCADSA:
-
Randomized Intervention With CPAP in Coronary Artery Disease and Sleep Apnea
- RR:
-
Risk ratio
- SAVE:
-
Sleep Apnea Cardiovascular Endpoints
- STEMI:
-
ST-segment elevation myocardial infarction
References
Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–58.
Lee CH, Sethi R, Li R, Ho HH, Hein T, Jim MH, et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation. 2016;133(21):2008–17.
Xie J, Sert Kuniyoshi FH, Covassin N, Singh P, Gami AS, Wang S, et al. Nocturnal hypoxemia due to obstructive sleep apnea is an independent predictor of poor prognosis after myocardial infarction. J Am Heart Assoc. 2016;5(8):e003162.
Mazaki T, Kasai T, Yokoi H, Kuramitsu S, Yamaji K, Morinaga T, et al. Impact of sleep-disordered breathing on long-term outcomes in patients with acute coronary syndrome who have undergone primary percutaneous coronary intervention. J Am Heart Assoc. 2016;5(6):e003270.
Lee CH, Khoo SM, Chan MY, Wong HB, Low AF, Phua QH, et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med. 2011;7(6):616–21.
Yumino D, Tsurumi Y, Takagi A, Suzuki K, Kasanuki H. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol. 2007;99(1):26–30.
Milleron O, Pilliere R, Foucher A, de Roquefeuil F, Aegerter P, Jondeau G, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25(9):728–34.
Cassar A, Morgenthaler TI, Lennon RJ, Rihal CS, Lerman A. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50(14):1310–4.
Garcia-Rio F, Alonso-Fernandez A, Armada E, Mediano O, Lores V, Rojo B, et al. CPAP effect on recurrent episodes in patients with sleep apnea and myocardial infarction. Int J Cardiol. 2013;168(2):1328–35.
Capodanno D, Milazzo G, Cumbo M, Marchese A, Salemi A, Quartarone L, et al. Positive airway pressure in patients with coronary artery disease and obstructive sleep apnea syndrome. J Cardiovasc Med (Hagerstown). 2014;15(5):402–6.
Nakashima H, Kurobe M, Minami K, Furudono S, Uchida Y, Amenomori K, et al. Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2015;4(1):75–84.
Wu X, Lv S, Yu X, Yao L, Mokhlesi B, Wei Y. Treatment of OSA reduces the risk of repeat revascularization after percutaneous coronary intervention. Chest. 2015;147(3):708–18.
Leao S, Conde B, Fontes P, Calvo T, Afonso A, Moreira I. Effect of obstructive sleep apnea in acute coronary syndrome. Am J Cardiol. 2016;117(7):1084–7.
Huang Z, Liu Z, Luo Q, Zhao Q, Zhao Z, Ma X, et al. Long-term effects of continuous positive airway pressure on blood pressure and prognosis in hypertensive patients with coronary heart disease and obstructive sleep apnea: a randomized controlled trial. Am J Hypertens. 2015;28(3):300–6.
Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunstrom E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–20.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. London: The Cochrane Collaboration; 2011. Available from http://handbook.cochrane.org.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Martinez-Garcia MA, Capote F, Campos-Rodriguez F, Lloberes P, Diaz de Atauri MJ, Somoza M, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–15.
Martinez-Ceron E, Barquiel B, Bezos AM, Casitas R, Galera R, Garcia-Benito C, et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes. A randomized clinical trial. Am J Respir Crit Care Med. 2016;194(4):476–85.
McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–31.
Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, Martinez-Alonso M, Carmona C, Barcelo A, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–8.
Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156–66.
Abuzaid AS, Al Ashry HS, Elbadawi A, Ld H, Saad M, Elgendy IY, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure therapy in patients with obstructive sleep apnea. Am J Cardiol. 2017;120(4):693–9.
Guo J, Sun Y, Xue LJ, Huang ZY, Wang YS, Zhang L, et al. Effect of CPAP therapy on cardiovascular events and mortality in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath. 2016;20(3):965–74.
Acknowledgements
Not applicable.
Funding
This study was funded by grants from International Science & Technology Cooperation Program of China (2015DFA30160), Beijing Municipal Science & Technology Commission (Z141100006014057), National Natural Science Foundation of China (81600209), Beijing Municipal Administration of Hospitals’ Youth Program (QML20160605), Beijing Municipal Administration of Hospitals Incubating Program (PX2016048), and Beijing Municipal Organization Department (2016000021469G194).
Availability of data and materials
Data are available from the authors upon request.
Author information
Authors and Affiliations
Contributions
SN and YW conceived the study. XW, SN, and YW designed the study. XW, YZ, and SN conducted the literature search and data extraction. XW and YZ performed the meta-analysis. XW, YZ, ZD, and JF interpreted the data. XW and YZ wrote the draft of the manuscript, and all authors critically revised the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Supplemental Material. (DOCX 353 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, X., Zhang, Y., Dong, Z. et al. Effect of continuous positive airway pressure on long-term cardiovascular outcomes in patients with coronary artery disease and obstructive sleep apnea: a systematic review and meta-analysis. Respir Res 19, 61 (2018). https://doi.org/10.1186/s12931-018-0761-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-018-0761-8