Abstract
Background
Thrombocytosis has been associated with COPD prevalence and increased all-cause mortality in patients with acute exacerbation of COPD (AECOPD); but whether it is associated with morbidity in stable COPD is unknown. This study aims to determine the association of thrombocytosis with COPD morbidity including reported AECOPD, respiratory symptoms and exercise capacity.
Methods
Participants with COPD were included from two multi-center observational studies (SPIROMICS and COPDGene). Cross-sectional associations of thrombocytosis (platelet count ≥350 × 109/L) with AECOPD during prior year (none vs. any), exertional dyspnea (modified Medical Research Council (mMRC) score ≥ 2), COPD Assessment Test (CAT) score ≥ 10, six-minute-walk distance (6MWD), and St. George Respiratory questionnaire (SGRQ) were modeled using multivariable logistic or linear regression. A pooled effect estimate for thrombocytosis was produced using meta-analysis of data from both studies.
Results
Thrombocytosis was present in 124/1820 (6.8%) SPIROMICS participants and 111/2185 (5.1%) COPDGene participants. In meta-analysis thrombocytosis was associated with any AECOPD (adjusted odds ratio [aOR] 1.5; 95% confidence interval [95% CI]: 1.1–2.0), severe AECOPD (aOR 1.5; 95% CI: 1.1–2.2), dyspnea (mMRC ≥ 2 aOR 1.4; 95% CI: 1.0–1.9), respiratory symptoms (CAT ≥ 10 aOR 1.6; 95% CI: 1.1–2.4), and higher SGRQ score (β 2.7; 95% CI: 0.5, 5). Thrombocytosis was also associated with classification into Global Initiative for Chronic Obstructive Lung Disease (GOLD) group D (aOR 1.7 95% CI: 1.2–2.4).
Conclusions
Thrombocytosis was associated with higher likelihood of prior exacerbation and worse symptoms. Platelet count, a commonly measured clinical assay, may be a biomarker for moderate-severe COPD symptoms, guide disease classification and intensity of treatment. Future longitudinal studies investigating the role of platelets in COPD progression may be warranted.
Trial registration
ClinicalTrials.gov: NCT01969344 (SPIROMICS) and NCT00608764 (COPDGene).
Similar content being viewed by others
Background
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the United States with multiple systemic manifestations and a disease trajectory affected by comorbid conditions, including cardiovascular (CV) disease [1, 2]. Platelets, long implicated in CV disease [3], are multifunctional and play a role in many pathophysiologic processes beyond hemostasis [4]. The pathogenesis of sustained platelet count elevation (thrombocytosis) involves either a myeloproliferative disorder or occurs as a reactive process secondary to an underlying neoplasm, chronic infection, inflammation, or physiologic stress [5]. Reactive thrombocytosis is a consequence of inappropriately elevated levels of thrombopoietin, which is up-regulated by the inflammatory cytokine interlukin-6, and has been associated with increased soluble markers of platelet activation [5, 6]. Previous animal studies have documented the role of platelets and platelet activation in bronchoconstriction, bronchial reactivity, airway inflammation, and remodeling and have corroborated clinical studies suggesting a role of increased platelet activity in allergic and non-allergic asthmatics [7].
In COPD, thrombocytosis and markers of platelet activation have been associated with disease prevalence and, recently, thrombocytosis has been associated with increased all-cause mortality in patients with acute exacerbation of COPD (AECOPD) [8,9,10,11,12]. Notably, prior research suggests that the platelet-mortality association is independent of cardiovascular outcomes suggesting an alternate pathophysiologic mechanism [10]. However, the role of platelets in COPD morbidity has not been explored. This study investigates the association of thrombocytosis with AECOPD, exercise capacity and patient reported respiratory symptoms and quality of life. The main hypothesis of this study is that thrombocytosis is associated with worse COPD morbidity. The large, well-characterized cohorts of the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) and the Genetic Epidemiology of COPD study (COPDGene®) are ideal populations in which to test this hypothesis.
Methods
Participants with COPD (post-bronchodilator ratio of forced expiratory volume in 1 s to forced vital capacity [FEV1/FVC] < 70%) were selected from the SPIROMICS and COPDGene studies, two multi-center observational cohorts with mutually exclusive recruitment. SPIROMICS enrolled current and former smokers (≥20 pack-years) and nonsmokers (≤1 pack-year) aged 40–80 years from twelve clinical sites in the US with the goal of identifying intermediate outcome measures that predict long-term clinical endpoints of morbidity [13]. COPDGene enrolled self-identified non-Hispanic whites or African-Americans aged 45–80 years from twenty-one clinical sites throughout the US with ≥10 pack-year smoking history with the goal of identifying genetic factors associated with COPD [14].
In SPIROMICS, cross-sectional analysis of baseline data on complete blood count (CBC), symptoms, and recall of events from the year prior to the baseline visit were ascertained. In COPDGene, CBC was collected at a five-year follow-up visit and thus outcome data collected at the 5-year visit (current as of September 2016) were analyzed cross-sectionally. Thrombocytosis was defined as platelet count of ≥350 × 109/L, a definition previously used in the literature [15,16,17]. All CBCs were performed at certified laboratories at respective sites.
Outcomes
The primary outcome of interest was patient-reported AECOPD during the previous 12 months. Any AECOPD included events treated with antibiotics or oral corticosteroids as well as severe AECOPD, defined as symptoms requiring an emergency department visit or hospitalization. Secondary outcomes of interest included dyspnea (modified Medical Research Council questionnaire, mMRC) [18], COPD health status (COPD Assessment Test™, CAT) [19], exercise capacity (6-min walk distance in meters, 6MWD) [20], respiratory-specific quality of life (St George’s Respiratory Questionnaire, SGRQ) [21], and COPD disease burden based on Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria for symptom categories A-D [22]. Classification into GOLD group D, indicative of the largest symptom burden and highest risk for future exacerbations, was defined as at least one severe exacerbation or ≥2 exacerbations during the prior year and mMRC score ≥ 2 or CAT score ≥ 10.
Statistical analysis
Differences in participant demographics, respiratory function, comorbid conditions, and univariate outcomes by presence of thrombocytosis were assessed using chi-squared test or Fisher’s exact test for proportions and t-test for continuous variables. Cross-sectional analyses were performed using multivariable logistic regression to model the relationship of thrombocytosis (dichotomized at platelet count ≥350 × 109/L) with dichotomous outcomes including at least one AECOPD within the prior year, at least one severe AECOPD within the prior year, mMRC score ≥ 2, CAT score ≥ 10 and classification into GOLD group D. Multivariable linear regression was used to model the relationship of thrombocytosis with 6MWD and SGRQ score. SPIROMICS and COPDGene were analyzed separately then combined by meta-analysis using inverse variance weighting to produce a pooled effect estimate.
Potential covariates were tested including age (continuous), gender, race (African American vs other), highest level of educational achievement (less than high school vs high school or above), percentile of post-bronchodilator FEV1 (continuous) [23], smoking status (current vs former smokers), ambulatory hypoxemia (oxygen saturation after 6-min walk ≤90%), inhaled corticosteroid use (ICS), body mass index (BMI; categorical: underweight <18.5 kg/m2 vs normal/overweight 18.5 - <30 kg/m2 vs obese ≥30 kg/m2), anemia (dichotomous using gender-specific cutoffs of hemoglobin), and CV comorbidities (hypertension, coronary artery disease, congestive heart failure, diabetes, and stroke) using a change in the effect estimate ≥10% between the crude and bivariate models of the association between thrombocytosis and each outcome as criteria for inclusion in models [24, 25]. Age, gender, education, FEV1, smoking status, ambulatory hypoxemia, ICS use, anemia, diabetes, congestive heart failure, and hypertension met criteria for at least one outcome in either study and were included as covariates in all models. Because there is no standard definition for reactive thrombocytosis, sensitivity analysis was performed dichotomizing platelet count at 400 × 109/L to test the robustness of the analysis. In a subset of SPIROMICS participants who had biomarkers measured concurrently with platelet count, the relationship of C-reactive protein (CRP) with thrombocytosis was explored using the Kruskal-Wallis test.
All analyses were conducted using SAS 9.4 (Carey, NC) except for the meta-analysis which was performed using Stata version 13/SE (College Station, TX). SPIROMICS and COPDGene were approved by the institutional review boards at each center and all participants provided written informed consent (ClinicalTrials.gov: NCT01969344 (SPIROMICS) and NCT00608764 (COPDGene)).
Results
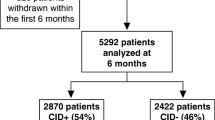
In SPIROMICS, Thrombocytosis was detected in 124 (6.8%) of 1820 SPIROMICS participants and 111 (5.1%) of 2185 COPDGene participants with COPD (Fig. 1). Severity of lung disease, smoking status, supplemental oxygen use, and BMI were similarly distributed between COPDGene and SPIROMICS, although COPDGene participants were slightly older with higher prevalence of some comorbidities including anemia, hypertension, congestive heart failure, and diabetes. In both cohorts, compared to participants with platelet count <350 × 109/L, participants with thrombocytosis were more likely to be female, have anemia and less likely to have coronary artery disease. In SPIROMICS, participants with thrombocytosis were also more likely to have less than a high school education and use supplemental oxygen while in COPDGene they were younger and more likely to be current smokers. Each of these trends was present but non-statistically significant in the other cohort (Table 1).
In unadjusted analysis in both cohorts, participants with thrombocytosis were significantly more likely to report an AECOPD (SPIROMICS: 43.6% vs. 30.7%, p = 0.003; COPDGene: 37.8% vs. 27.5%, p = 0.02) or a severe AECOPD (SPIROMICS: 24.2% vs. 15.5%, p = 0.01; COPDGene: 23.4% vs. 14.6%, p = 0.01) in the past year compared to the group with lower platelet count. Those with thrombocytosis were also significantly more likely to have worse dyspnea (mMRC score ≥ 2), functional status, quality of life (CAT score ≥ 10 and higher St. George Respiratory Questionnaire score), and more likely to be classified as GOLD group D compared to the group with lower platelet count (Table 2). Six-minute walk distance was reduced among participants with thrombocytosis in both cohorts but was statistically significant only in SPIROMICS.
In adjusted analysis, compared with platelet count <350 × 109/L, participants with thrombocytosis were 1.6 times more likely to report any AECOPD (95% confidence interval [CI]: 1.0–2.4) and severe AECOPD (95% CI: 1.0–2.7) in SPIROMICS, while results lost statistical significance in COPDGene for any AECOPD (adjusted odds ratio [aOR] 1.3; 95% CI: 0.8–2.1) and severe AECOPD (aOR 1.4; 95% CI: 0.9–2.4). Thrombocytosis was significantly associated with GOLD group D classification in COPDGene and with borderline significance in participants from SPIROMICS. Thrombocytosis was also significantly associated with mMRC ≥2 and reduced 6MWD in SPIROMICS. Among those with thrombocytosis, poorer health status and worse quality of life were present in both SPIROMICS and COPDGene, but differences between groups did not reach statistical significance (Figs 2 and 3).
Association of thrombocytosis (platelet count ≥350 × 109/L) with COPD morbidity (dichotomous outcomes). aOR: adjusted odds ratio; mMRC: modified medical research council questionnaire; CAT: COPD assessment test; GOLD: Global Initiative for Chronic Obstructive Lung Disease. Statistical significance (p < 0.05) denoted by asterisk
In meta-analysis combining both cohorts, participants with thrombocytosis had a 1.5-fold increased odds of reporting any AECOPD (95% CI: 1.1–2.0) or severe AECOPD (95% CI: 1.1–2.2) during the preceding year, 1.4-fold increased odds of moderate-severe dyspnea (mMRC ≥2; 95% CI: 1.0–1.9), 1.6-fold increased odds of reporting worse COPD health status (CAT score ≥ 10; 95% CI: 1.1–2.4), and were more likely to be classified as GOLD group D (aOR 1.7; 95% CI: 1.2–2.4) (Fig. 2). Thrombocytosis was associated with worse respiratory-specific quality of life (2.7 points higher SGRQ score; 95% CI: 0.5, 5) but not 6MWD (Fig. 3).
Sensitivity analysis defining thrombocytosis as ≥400 × 109/L (SPIROMICS n = 38, COPDGene n = 39) produced similar results for any and severe AECOPD, CAT ≥10, GOLD group D classification, and SGRQ, with results for mMRC ≥2 of similar magnitude to the main analysis but losing statistical significance (Table 3). Among SPIROMICS participants who had platelet count and CRP measured at baseline (n = 1041) participants with thrombocytosis had significantly higher median CRP (4.6 vs. 3.0, p = 0.01).
Discussion
This appears to be the first study assessing the association of thrombocytosis with exacerbations and patient-reported respiratory outcomes in a large, stable COPD cohort. In meta-analysis of two observational cohort studies (SPIROMICS and COPDGene), thrombocytosis was associated with increased odds of having AECOPD or severe AECOPD during the prior year, increased dyspnea, poorer health status, and worse respiratory-specific quality of life. Participants with thrombocytosis were also more likely to be classified in GOLD symptom category D. These findings, which persisted after adjustment for degree of airflow obstruction, CV disease, and other comorbid chronic diseases, suggest that platelet count, a routinely measured clinical assay, represents an objective measure associated with recent AECOPD and respiratory morbidity that may help guide treatment intensity in patients with stable COPD.
Elevated platelet levels have previously been shown to be more prevalent in patients with stable COPD [8], correlated with increasing airflow obstruction [26], all-cause mortality [10], and may be a surrogate for platelet activation [27]. In a study of 109 patients with stable COPD, platelet count was significantly higher than that of 51 healthy controls and did not differ by smoking status [8]. A strong correlation between platelet count and elevated levels of P-selectin, a glycoprotein expressed and secreted by activated platelets, has been reported [27], and a few studies have reported increased platelet activation in stable COPD measured directly as platelet-monocyte aggregates or as soluble markers of platelet activation [9, 28,29,30]. A recent study of patients hospitalized for AECOPD found that thrombocytosis was associated with increased risk of in-hospital and 1-year mortality independent of cardiovascular events and correlated with respiratory failure and exacerbation severity [10]. Among 452 individuals with stable COPD, mean platelet count increased with increasing severity of airflow obstruction [26]. Despite these few studies, there has been limited data to date evaluating whether platelet levels are associated with other measures of COPD morbidity.
Findings from this study show that thrombocytosis is linked to several respiratory outcomes in patients with COPD, including increased prevalence of exacerbations and worse dyspnea and respiratory-specific quality of life. Inclusion of CV comorbidities as a covariate in models did not eliminate or substantially change the association between platelet count and outcomes and thus is likely not a major mediator of the association. These findings further support the likelihood that the pathophysiologic mechanism underlying the association of platelets and COPD morbidity is independent of increased cardiac risk. In addition, some prior studies have reported higher platelet counts among smokers compared to non-smokers, however findings have been inconsistent [8, 31,32,33]. Approximately one-third of the study participants reported actively smoking and thrombocytosis was associated with smoking status only in COPDGene. However, inclusion of smoking status as a covariate in all models implies that the association of thrombocytosis with COPD morbidity does not simply reflect increased COPD morbidity among current smokers.
Several alternative mechanisms have been proposed for the role of platelets in COPD that could potentially inform the association with AECOPD. COPD is now recognized as a disease involving systemic inflammation [34], which increases further during AECOPD [35] with persistent elevation of inflammatory markers among non-responders, frequent, and recurrent exacerbators [36]. Platelets act in the inflammatory pathway by releasing pro-inflammatory mediators and activating other inflammatory cells [37]. Prior studies have shown a weak non-significant correlation between platelet count measured as a continuous variable and CRP in both stable [8] and exacerbated [10] COPD, however, in this study thrombocytosis was associated with significantly elevated CRP, as participants with thrombocytosis had significantly higher median CRP (4.6 vs. 3.0, p = 0.01), implying that systemic inflammation may play a role in the pathway between platelet elevation and COPD morbidity. It remains unclear whether AECOPD elicit a reactive thrombocytosis leading to inflammation through increased platelet activation and cytokine release or if systemic inflammation precedes reactive thrombocytosis and AECOPD.
Additionally, pulmonary endovascular abnormalities are prevalent in COPD and are present even in mild disease [38]. Studies have shown endothelial dysfunction, intimal thickening, as well as reduced and narrowed vasculature in COPD, leading to impaired relaxation and gas exchange and promotion of platelet rolling and aggregation [39,40,41]. Platelets also play a role in angiogenesis, maintaining vascular integrity, and release vasoactive substances [37]. Prior studies reported increased platelet count among hypoxic patients with airflow obstruction [42], possibly due to increased interaction and activation of platelets driven by compromised endothelium. The present study demonstrates that thrombocytosis was independently associated with AECOPD after controlling for ambulatory hypoxemia. While this may suggest that the association of platelets with morbidity is independent of endothelial dysfunction, hypoxemia is multifactorial and only one of several physiologic manifestations of endothelial abnormalities. More precise and direct measures of endothelial dysfunction are required to elucidate platelet-endothelial interactions in COPD.
There are limitations to this study. Given the cross-sectional nature of this investigation, it is not possible to determine temporality and it remains unclear whether thrombocytosis is a risk factor for or physiologic reaction to AECOPD or disease severity. Without a clear clinical cut-off for defining thrombocytosis, misclassification based upon the chosen value is possible, however, any misclassification would attenuate the associations described in this study toward the null. Furthermore, these findings were robust to a sensitivity analysis using a higher cutoff for defining thrombocytosis, even though analysis of mMRC score lost statistical significance owing to the low prevalence (approximately 2%) of platelet count greater than 400 × 109/L. In a substudy of SPIROMICS participants (n = 98) undergoing repeat baseline evaluation 2–4 weeks from the original visit a change in exacerbation history recall of the previous year occurred in 30% and 13% of participants reporting total and severe exacerbations respectively, with repeatability of other measures ranging from fair (mMRC) to excellent (SGRQ) [43].
Conclusions
Thrombocytosis was independently associated with worse COPD morbidity, including higher likelihood of prior exacerbation, worse patient reported outcomes, and GOLD group D classification. While the mechanism behind this association remains unclear and requires additional investigation, increased platelet activation perpetuating local and systemic inflammation may play a prominent role in COPD morbidity. These study results suggest that platelet count, a routinely measured clinical assay, may be an important biomarker associated with AECOPD and respiratory morbidity in patients with previously stable COPD that can aid in classification of disease severity and treatment intensity.
Abbreviations
- 6MWD:
-
Six-minute-walk distance
- 95% CI:
-
95% confidence interval
- AECOPD:
-
Acute exacerbation of chronic obstructive pulmonary disease
- aOR:
-
Adjusted odds ratio
- CAT:
-
COPD Assessment Test
- COPD:
-
Chronic obstructive pulmonary disease
- COPDGene:
-
Genetic Epidemiology of COPD study
- CRP:
-
C-reactive protein
- FEV1 :
-
Forced expiratory volume in 1 s
- GOLD:
-
Global Initiative for Chronic Obstructive Lung Disease
- mMRC:
-
Modified Medical Research Council questionnaire
- SGRQ:
-
St. George Respiratory questionnaire
- SPIROMICS:
-
Subpopulations and Intermediate Outcome Measures in COPD Study
References
Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128(4):2640–6.
Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc. 2005;2(1):8–11.
Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357(24):2482–94.
Ghoshal K, Bhattacharyya M. Overview of platelet physiology: its hemostatic and nonhemostatic role in disease pathogenesis. TheScientificWorldJOURNAL. 2014;2014:781857.
Schafer AI. Thrombocytosis. N Engl J Med. 2004;350(12):1211–9.
Viallard J-F, Solanilla A, Gauthier B, Contin C, Déchanet J, Grosset C, Moreau J-F, Praloran V, Nurden P, Pellegrin J-L. Increased soluble and platelet-associated CD40 ligand in essential thrombocythemia and reactive thrombocytosis. Blood. 2002;99(7):2612–4.
Kornerup K, Page C. The role of platelets in the pathophysiology of asthma. Platelets. 2007;18(5):319–28.
Biljak VR, Pancirov D, Cepelak I, Popovic-Grle S, Stjepanovic G, Grubisic TZ. Platelet count, mean platelet volume and smoking status in stable chronic obstructive pulmonary disease. Platelets. 2011;22(6):466–70.
Ferroni P, Basili S, Martini F, Vieri M, Labbadia G, Cordova C, Alessandri C, Gazzaniga PP, Soluble P. Selectin as a marker of platelet hyperactivity in patients with chronic obstructive pulmonary disease. J Investig Med. 2000;48(1):21–7.
Harrison MT, Short P, Williamson PA, Singanayagam A, Chalmers JD, Schembri S. Thrombocytosis is associated with increased short and long term mortality after exacerbation of chronic obstructive pulmonary disease: a role for antiplatelet therapy? Thorax. 2014;69(7):609–15.
Maclay JD, McAllister DA, Macnee W. Cardiovascular risk in chronic obstructive pulmonary disease. Respirology. 2007;12(5):634–41.
Onder I, Topcu S, Dokmetas HS, Turkay C, Seyfikli Z. Platelet aggregation size and volume in chronic obstructive pulmonary disease. Materia medica Polona Polish journal of medicine and pharmacy. 1997;29(1–4):11–3.
Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, Kanner R, Kleerup E, Martinez FJ, Woodruff PG, et al. Design of the Subpopulations and Intermediate Outcomes in COPD study (SPIROMICS). Thorax. 2014;69(5):491–4.
Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. Copd. 2010;7(1):32–43.
Hannisdal E, Tveit KM, Theodorsen L, Høst H. Host markers and prognosis in recurrent rectal carcinomas treated with radiotherapy. Acta Oncol. 1994;33(4):415–21.
Sandelin M, Holgersson G, Janson C, Ekman S, Bergqvist M. The prognostic value of anemia, thrombocytosis and leukocytosis at time of diagnosis in patients with non-small cell lung cancer. In: Eur Respiratory Soc. 2011;
Digklia A, Voutsadakis IA. Thrombocytosis as a prognostic marker in stage III and IV serous ovarian cancer. Obstetrics & gynecology science. 2014;57(6):457–63.
Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–6.
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–54.
Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, McCormack MC, Carlin BW, Sciurba FC, Pitta F, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–46.
Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's respiratory questionnaire. Am Rev Respir Dis. 1992;145(6):1321–7.
Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38.
Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–36.
Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79(3):340–9.
Cakmak G, Saglam Z, Saler T, Yenigun M, Ataoglu E, Temiz L, Demir T. Platelets: indicator of inflammation in COPD. International Journal of Medicine and Medical Sciences. 2009;1(5):227–9.
Semenov AV, Romanov YA, Loktionova SA, Tikhomirov OY, Khachikian MV, Vasil’ev SA, Mazurov AV. Production of soluble P-selectin by platelets and endothelial cells. Biochemistry Biokhimiia. 1999;64(11):1326–35.
Davi G, Basili S, Vieri M, Cipollone F, Santarone S, Alessandri C, Gazzaniga P, Cordova C, Violi F. Enhanced thromboxane biosynthesis in patients with chronic obstructive pulmonary disease. The chronic obstructive bronchitis and Haemostasis study group. Am J Respir Crit Care Med. 1997;156(6):1794–9.
Maclay JD, McAllister DA, Johnston S, Raftis J, McGuinnes C, Deans A, Newby DE, Mills NL, MacNee W. Increased platelet activation in patients with stable and acute exacerbation of COPD. Thorax. 2011;66(9):769–74.
Shigeta A, Tada Y, Tatsumi K. High concentration of plasma soluble CD40 ligand is associated with impaired lung function and alveolar structural destruction. Am J Respir Crit Care Med. 2012;185:A4555.
Erikssen J, Hellem A, Stormorken H. Chronic effect of smoking on platelet count and platelet adhesiveness in presumably healthy middle-aged men. Thromb Haemost. 1977;38(3):606–11.
Butkiewicz A, Kemona-Chetnik I, Dymicka-Piekarska V, Matowicka-Karna J, Kemona H, Radziwon P. Does smoking affect thrombocytopoiesis and platelet activation in women and men? Advances in medical sciences. 2006;51:123–6.
Suwansaksri J, Wiwanitkit V, Soogarun S. Effect of smoking on platelet count and platelet parameters: an observation. Clin Appl Thromb Hemost. 2004;10(3):287–8.
Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–80.
Hurst JR, Perera WR, Wilkinson TM, Donaldson GC, Wedzicha JA. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(1):71–8.
Perera WR, Hurst JR, Wilkinson TM, Sapsford RJ, Müllerova H, Donaldson GC, Wedzicha JA. Inflammatory changes, recovery and recurrence at COPD exacerbation. Eur Respir J. 2007;29(3):527–34.
Arman M, Payne H, Ponomaryov T, Brill A. Role of Platelets in Inflammation. In: Kerrigan, SW, Moran N, editors. The Non-Thrombotic Role of Platelets in Health and Disease. InTech; 2015. https://doi.org/10.5772/60536.
Ferrer E, Peinado VI, Díez M, Carrasco JL, Musri MM, Martínez A, Rodríguez-Roisin R, Barberà J. Effects of cigarette smoke on endothelial function of pulmonary arteries in the guinea pig. Respir Res. 2009;10(1):76.
Yu N, Wei X, Li Y, Deng L, Jin C-W, Guo Y. Computed tomography quantification of pulmonary vessels in chronic obstructive pulmonary disease as identified by 3D automated approach. Medicine. 2016;95(40)
Peinado VI, Barberà JA, Ramírez J, Gómez FP, Roca J, Jover L, Gimferrer JM, Rodriguez-Roisin R. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1998;274(6):L908–13.
Barberà JA, Riverola A, Roca J, Ramirez J, Wagner PD, Ros D, Wiggs BR, Rodriguez-Roisin R. Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;149(2):423–9.
Wedzicha JA, Cotter FE, Empey DW. Platelet size in patients with chronic airflow obstruction with and without hypoxaemia. Thorax. 1988;43(1):61–4.
Anderson WH, O'Neal WK, Doerschuk CM, Carretta EE, Couper DJ, Tashkin D, Paine I, Robert CCB, Bleecker ER, Barr R. Short-term stability of pulmonary function and clinical measures in COPD using a cohort from Spiromics (subpopulations and intermediate outcome measures in COPD study). Am J Respir Crit Care Med. 2016:A3515–5.
Acknowledgements
The authors thank the SPIROMICS participants and participating physicians, investigators and staff for making this research possible. More information about the study and how to access SPIROMICS data is at www.spiromics.org. We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: : Neil E Alexis, PhD; Wayne H Anderson, PhD; Igor Barjaktarevic, MD, PhD; R Graham Barr, MD, DrPH; Eugene R Bleecker, MD; Richard C Boucher, MD; Russell P Bowler, MD, PhD; Elizabeth E Carretta, MPH; Stephanie A Christenson, MD; Alejandro P Comellas, MD; Christopher B Cooper, MD, PhD; David J Couper, PhD; Gerard J Criner, MD; Ronald G Crystal, MD; Jeffrey L Curtis, MD; Claire M Doerschuk, MD; Mark T Dransfield, MD; Christine M Freeman, PhD; MeiLan K Han, MD, MS; Nadia N Hansel, MD, MPH; Annette T Hastie, PhD; Eric A Hoffman, PhD; Robert J Kaner, MD; Richard E Kanner, MD; Eric C Kleerup, MD; Jerry A Krishnan, MD, PhD; Lisa M LaVange, PhD; Stephen C Lazarus, MD; Fernando J Martinez, MD, MS; Deborah A Meyers, PhD; Wendy C Moore, MD; John D Newell Jr., MD; Laura Paulin, MD, MHS; Stephen Peters, MD, PhD; Cheryl Pirozzi, MD; Elizabeth C Oelsner, MD, MPH; Wanda K O’Neal, PhD; Victor E Ortega, MD, PhD; Robert Paine, III, MD; Nirupama Putcha, MD, MHS; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P Tashkin, MD; J Michael Wells, MD; Robert A Wise, MD; and Prescott G Woodruff, MD, MPH.
Funding
AF: NIH NHLBI T32HL007534. SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc..; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Nycomed GmbH; Takeda Pharmaceutical Company; Novartis Pharmaceuticals Corporation; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; and Sunovion. COPDGene was supported by Award Number R01 HL089897 and Award Number R01 HL089856 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Availability of data and materials
The data that support the findings of this study are available from the authors upon reasonable request and with permission from the SPIROMICS and COPDGene study groups.
Author information
Authors and Affiliations
Consortia
Contributions
AF, NP, and NNH contributed to the conception and design of the study, interpretation of results, drafting and revising the manuscript. MKH, RW, REK, RPB, RGB, and NNH contributed to the acquisition of the data. LMP, CPA, WWL, MKH, RW, REK, RPB, and RGB contributed to interpretation of the results and revisions of the manuscript for critically important intellectual content. All of the authors approved this version of the manuscript to be published and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
SPIROMICS and COPDGene were approved by the institutional review boards at each center and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fawzy, A., Putcha, N., Paulin, L.M. et al. Association of thrombocytosis with COPD morbidity: the SPIROMICS and COPDGene cohorts. Respir Res 19, 20 (2018). https://doi.org/10.1186/s12931-018-0717-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-018-0717-z