Abstract
Background
Idiopathic pulmonary fibrosis (IPF) is characterized by the complex interaction of cells involved in chronic inflammation and fibrosis. Global gene expression of a homogenous cell population will identify novel candidate genes.
Methods
Gene expression of fibroblasts derived from lung tissues (8 IPF and 4 controls) was profiled, and ontology and functional pathway were analyzed in the genes exhibiting >2 absolute fold changes with p-values < 0.05. CCL8 mRNA and protein levels were quantified using real-time PCR and ELISA. CCL8 localization was evaluated by immunofluorescence staining.
Results
One hundred seventy eight genes differentially expressed and 15 genes exhibited >10-fold change. Among them, 13 were novel in relation with IPF. CCL8 expression was 22.8-fold higher in IPF fibroblasts. The levels of CCL8 mRNA and protein were 3 and 9-fold higher in 14 IPF fibroblasts than those in 10 control fibroblasts by real-time PCR and ELISA (p = 0.022 and p = 0.026, respectively). The CCL8 concentrations in BAL fluid was significantly higher in 86 patients with IPF than those in 41 controls, and other interstitial lung diseases including non-specific interstitial pneumonia (n = 22), hypersensitivity pneumonitis (n = 20) and sarcoidosis (n = 19) (p < 0.005, respectively). Cut-off values of 2.29 pg/mL and 0.43 pg/mL possessed 80.2 and 70.7% accuracy for the discrimination of IPF from NC and the other lung diseases, respectively. IPF subjects with CCL8 levels >28.61 pg/mL showed shorter survival compared to those with lower levels (p = 0.012). CCL8 was expressed by α-SMA-positive cells in the interstitium of IPF.
Conclusions
Transcriptome analysis identified several novel IPF-related genes. Among them, CCL8 is a candidate molecule for the differential diagnosis and prediction of survival.
Similar content being viewed by others
Background
Idiopathic pulmonary fibrosis (IPF) is characterized by alveolar epithelial cell hyperplasia and increased myofibroblast with the interstitial deposition of extracellular matrix (ECM) [1]. The disease course is highly variable due to interactions between chronic inflammatory and fibrosis-related processes [2, 3]. Exploration of global gene expression in lung tissues may facilitate the identification of novel candidate genes to further explain the complex mechanism and to predict the clinical courses of IPF. In a human study, 164 differentially expressed genes were demonstrated in IPF lung tissues [4]. In this study, fibrotic lungs showed changes in the expression of genes involved in ECM formation and degradation. A comparison of rapid-and slow-progressor patients revealed 437 differentially expressed genes involved in morphogenesis [5]. In another study, integration of the expression levels of 134 genes enabled the discrimination of progressive and stable subjects [6]. Almost identical patterns of gene expression to those of stable IPF were reported in cases of acute exacerbation [7]. These studies have demonstrated novel candidate genes related with IPF.
The use of whole-lung tissues, however, may be a limitation of transcriptomic studies because transcriptomic changes are cell-type-specific [8]. The pathologic characteristics of IPF include mixed features with normal lungs, alveolar inflammation, interstitial fibrosis, and honeycomb changes [2]. Furthermore, the extent of fibrosis and inflammation varies markedly during the disease course. Accordingly, selective separation of homogenous cells from diseased lungs would be optimal, but is problematic. Among the various cell types present in lung tissue, fibroblasts are easily obtained and maintained, and the biologic properties of IPF fibroblasts differ from those of normal lung fibroblasts [9, 10]. To further investigate the molecular mechanisms of IPF lungs, a global transcriptome analysis was conducted using fibroblasts obtained from the lung tissues of 8 patients with IPF and normal lungs of 4 subjects with localized lung lesions. The differential expression of CCL8 was validated using an additional number of fibroblasts and bronchoalveolar lavage (BAL) fluid samples from normal controls (NC), patients with IPF and those with other interstitial lung diseases including non-specific interstitial pneumonia (NSIP), hypersensitivity pneumonitis (HP), and sarcoidosis.
Methods
Study subjects
Plasma, BAL fluids, and lung tissues of the subjects with diffuse interstitial lung disease were obtained from a biobank in Soonchunhyang University Bucheon Hospital (schbc-biobank-2014-005-1, schbc-biobank-2014-005-2) after the study protocol was approved by institutional review board (IRB) in Korea National Institute for Bioethics Policy (KoNIBP; P01-201408-BS-01-00). Control BAL fluids were obtained from general population and hospital personnel, and blood samples were obtained from spouses, general population and hospital personnel after approval by the hospital ethics committee (SCHBC 2015-08-025-005, schbc-biobank-2015-013). An informed written consent to participate was obtained from each subject. The diagnostic criteria for IPF, NSIP, HP and sarcoidosis were based on the international consensus statement [1, 11–14]. All subjects were examined by physicians to obtain their medical history and underwent a chest X-ray, pulmonary function tests, high-resolution chest computed tomography (HRCT), and blood tests to exclude collagen vascular diseases. None of the IPF patients had any evidence of underlying collagen vascular diseases through clinical manifestations or laboratory tests. IPF was diagnosed by the presence of a UIP pattern in the pathological specimen (surgical IPF) and/or by HRCT in patients who were not subjected to surgical lung biopsy (clinical IPF). Two pathologists examined each slide independently after they were informed of the patients’ age, sex, and HCRT results. The pathologic recognition of the NSIP pattern included two major aspects: (1) recognition of the characteristic histologic features and (2) exclusion of other patterns of ILD as described in the ATS/ERS 2002 classification [14, 15], previous publication of ours [16] and the modified version on the histologic definition of the NSIP pattern [17].
HP was diagnosed by the presence of compatible clinical manifestations with a non-necrotizing granulomatous interstitial bronchiolocentric pneumonitis [11]. The diagnosis of sarcoidosis was made on the basis of the compatible clinical pictures and histologic demonstration of noncaseating granulomas [12, 13] The diagnosis of HP and sarcoidosis needed exclusion of other diseases capable of producing a similar histologic picture: Biopsy tissues were subjected to special stains (acid fast bacilli stain and Gömöri methenamine silver stain) to rule out microorganisms and fungi. IPF patients were evaluated using serial FVC and DLCO measurements. The annual rate of FVC decline [dFVC(%/year)] was calculated as follows: (last FVC - baseline FVC)/baseline FVC/year. The normal controls had no respiratory symptoms, as determined by a screening questionnaire [18], had a predicted FEV1 and FVC > 80%, and had normal chest radiogram results.
Fibroblast culture
Lung fibroblasts were cultured from the surgical specimens of 14 patients with IPF and normal lungs of 10 subjects who underwent surgery to remove stage I or II lung cancer as described in the previous publication [19]. Briefly, lung specimens were finely minced and placed into 150 cm2 cell culture flasks with tissue culture media (TCM) consisting of DMEM (Lonza Walkersville, Inc., Walkersville, MD, USA), 10% fetal bovine serum (Thermo Fisher Scientific Inc., Rockford, IL, USA), 2mmol/L glutamine, and 1% penicillin-streptomycin-amphotericin (Lonza Walkersville, Inc Cells were maintained at 37 °C in a 5% CO2 incubator and serially subcultured to yield a morphologically homogeneous population of adherent fibroblasts under the microscopy and α-smooth muscle actin (SMA) immunohistochemical stain (Abcam, Cambridge, MA, USA) until the fourth passage. The cells were then stored at −170 °C. Fifth-passage fibroblasts (2.5 × 106) were seeded in 1mL TCM in 10cm2 dishes. After reaching 90% confluence, the fibroblasts were washed twice with PBS (Thermo Fisher Scientific Inc.) and used for RNA. Total RNA was extracted using TRI reagent (Ambion, Carlsbad, CA, USA). The cell pellets were prepared in RIPA buffer for immunoblot analysis, and protein concentrations were measured using a BCA kit (Thermo Fisher Scientific Inc.).
Transcriptome microarray and analysis of gene ontology and functional pathways
Total RNA was extracted from the fibroblasts and converted to cDNA, which was amplified and purified using an Illumina® Total Prep™ RNA Amplification Kit (Ambion, Carlsbad, CA, USA). A transcriptome assay was performed using a HumanHT-12 (BeadChip Illumina, San Diego, CA, USA) containing sequences representing ~47,315 probes, which covered 27,455 curated and putative genes. The quality and quantity of the extracted RNA were examined by a RNA quantification reagent (Ribogreen®, Invitrogen, Carlsbad, CA, USA). Fluorescence was determined using a fluorometer (Victor 3, Perkin Elmer, Boston, MA, USA). An IlluminaiScan scanner was used to create images of the microarrays. Intensities of the images were measured using GenomeStudio (v.2011.1, Illumina, Inc., San Diego, CA, USA) with Gene Expression Module (v1.0). The expression value of each gene was determined by calculating differences by perfect match intensity minus mismatch intensity of the probe pairs in use. Genes showing detection call p-values <0.01 were discarded to reduce the number of false positives, and the remaining 15,020 genes were analyzed. Fold change of gene expression was calculated as follows: a mean of expression levels of IPF-fibroblasts divided by that of control fibroblasts if the levels were higher in the IPF than the controls. In cases of countertrend, fold change was determined by dividing the mean value of control group by that of the IPF group and presented as minus value. The microarray data were analyzed using ScoreGenes software package (http://compbio.cs.huji.ac.il/scoregenes/). The general approach to analysis has been previously described [20]. Correction for multiple testing was performed by calculating the false discovery rate, as previously described [20]. Genes were defined as being substantially changed if they had a P-value of less than 0.05 by t-test, and a threshold number of misclassifications (TNoM) score of 0 and a t-test with a P value of less than 0.05 and absolute fold change of greater than 2, as previously described [21]. A heat map of the differentially expressed genes was constructed using the GenomeStudio software. Gene ontology enrichment was performed by the Gene Ontology (GO) database using the WebGestalt (http://www.webgestalt.org/), which was based on hypergeometric distribution to show the overrepresented gene ontology categories (p < 0.05). P-value was calculated using BINOMDIST function on the basis of overrepresentation of gene ontology categories when compared to all genes on the chip. The online program Pathway-Express (Onto-Tools, Wayne State University, Detroit, MI, USA, http://vortex.cs.wayne.edu/Projects.html) was used to explore biologically relevant pathways impacted by a list of input genes. The gene expression data were deposited in the NCBI Gene Expression Omnibus (series accession number GSE71351; (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE71351). Gene ontology and pathway predictions were performed using the Gene Ontology database (http://www.webgestalt.org/) and Pathway-Express software (Onto-Tools; http://vortex.cs.wayne.edu/Projects.html).
RT-PCR and real-time PCR of CCL8 mRNA
Total RNA purified using TRI reagent was treated with a Turbo DNA-Free™ Kit (Ambion). Total RNA (3 μg) suspended in diethylpyrocarbonate-treated water was heated at 65 °C for 5 min with 0.5 μg of oligodeoxythymidine and 10 mM dNTPs, and then cooled on ice. Amplification was performed for 30 cycles (5min at 94 °C, 30s at 94 °C, 30s at 60 °C, and 30s at 72 °C) with extension at 72 °C for 7min. The following primer sequences were used: CCL8: sense 5′-TGGAGAGCTACACAAGAATCACC-3′andantisense5′-TGGTCCAGATGCTTCATGGAA-3′; β-actin: sense 5′-GGACTTCGAGCAAGAGATGG-3′ and antisense 5′-AGCACTGTGTTGGCGTACAG-3′. PCR products were separated on a 1.0% agarose gel containing ethidium bromide in Tris-borate EDTA buffer at 100V for 40min and visualized under UV light. The CCL8 band intensities were normalized to those of β-actin. Real-time PCR was performed using the StepOneTM Real-Time PCR System (Applied Biosystems, Foster city, CA, USA). The PCR mixture (20μL) contained 1μg cDNA, 1μL 10pmol forward and reverse primers, and 10μL 2 × Power SYBR Green PCR Master Mix (Applied Biosystems). The reaction was carried out in a two-step procedure: denaturation at 95 °C for 15s and 60 °C for 1min, and melting at 95 °C for 15 s, 60 °C for 1min, and 95 °C for 15s. Data were analyzed by the 2-ΔΔCT method [22], presented as the relative fold change after normalization to β-actin.
Determination of CCL8 protein levels
BAL was performed in the mostly involved segments of IPF on HRCT without any immunosuppressive therapies or in the right middle lobe of normal control subjects as previously described [2, 23–25]. Total cell count was done using a hemocytometer. Differential count of five hundred cells was performed on slides of BAL cells prepared by a cytocentrifuge and stained with Diff-Quik. Cell pellets were separated from supernatants using centrifugation (500G, 5 min), and the supernatants were stored −80 °C. CCL8 protein concentrations in BAL fluids and plasma were measured in normal controls and IPF patients using an ELISA kit (Abnova, Taipei, Taiwan) according to the manufacturer’s recommendations. The lower limit of detection was 1.5pg/mL; values below this limit were regarded as 0 pg/mL. The coefficients of variance for inter- and intra-assays were less than 15%. CCL8 protein concentrations were measured in BAL fluids and plasma using an ELISA kit (Abnova, Taipei, Taiwan), and normalized to total protein concentration.
Double immunofluorescence staining of CCL8 and α-smooth muscle actin
Paraffin blocks of IPF and control lung tissues were cut into 4-μm-thick slices, deparaffinized, rehydrated, and stained using hematoxylin and eosin. The sections were incubated in Fc receptor blocker (InnovexBiosciences, Richmond, CA, USA) for 30 min, incubated in TBS with 5% BSA for 1 h to block non-specific binding, and then incubated with monoclonal anti–human CCL8 antibody (1:100, Origene, Rockville, MD, USA) or polyclonal anti-human α-SMA antibody (1:200, Abcam, Cambridge, MA, USA) in 5% BSA overnight at 4 °C. After washing with 1 × TBS, the sections were incubated with secondary antibodies: FITC-conjugated goat anti-rabbit antibody (1:2000, Abcam) and PE-conjugated donkey anti-mouse antibody (1:2000, Abcam). Nuclei were counterstained with DAPI (Invitrogen, Carlsbad, CA, USA). Confocal laser scanning was performed using a microscope (LSM 510 META at 100× magnification) coupled to a Photometrics Coolsnap HQ camera (Photometrics, Tucson, AZ, USA), and images were generated using the Zeiss LSM image browser.
Statistical analysis
The data were analyzed using SPSS v. 20.0. The differences in gene expression between NC and IPF groups were considered statistically significant when the absolute fold-change in the mean value was >2 and the p-value was < 0.05 using t-tests and the nonparametric TNoM scoring method [21]. Comparisons of CCL8 concentrations between the study groups were performed using the Kruskal-Wallis test and post hoc analysis (Mann-Whitney U test). A receiver operating characteristic (ROC) curve, AUC, and cut-off value were calculated using MedCalc [26]. Correlations between the CCL8 levels and other parameters were analyzed by Spearman’s correlation coefficient. The data were presented as median values with 25 and 75% quartiles for skewed variables, or as the means ± SEM for those with a normal distribution. An optimal cutoff level of CCL8 was calculated using Cutoff Finder [27] and survival rates were estimated by Kaplan-Meier’s method and compared using log-rank test. Values of p < 0.05 were considered statistical significance.
Results
Clinical characteristics of the study groups
Lung fibroblasts were cultured from 14 IPF-lungs and 10 control lungs. Among them, 8 IPF-fibroblasts and 4 controls were used for the transcriptome study (Additional file 1: Table S1). BAL samples were obtained from patients with IPF (n = 86), NSIP (n = 22), hypersensitivity pneumonitis (n = 20) and sarcoidosis (n = 19) (Table 1). The patients with IPF had significantly higher values for total cell count and numbers of macrophages, neutrophils and eosinophils in the BAL fluid, and lower FVC and DLCO values compared with those of NC (p < 0.05). The IPF group was comprised of 34 surgical IPF and 52 clinical IPF patients. There were no significant differences in the clinical and physiological parameters between the two groups (Additional file 1: Table S2). NSIP, hypersensitivity pneumonitis and sarcoidosis groups also had significantly higher values for total cell count and numbers of macrophages, neutrophils and eosinophils in the BAL fluid, and lower FVC and DLCO values compared with those of NC(p < 0.05).
Global gene expression profiling of the IPF and control groups
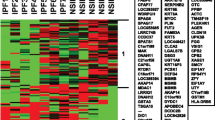
The cultured fibroblasts expressed α-SMA, but not E-cadherin on Western blotting (Additional file 2: Figure S1). The intensities of α-SMA were significantly higher in the fibroblasts from IPF lungs (p = 0.011). The expression levels of 15,020 genes were compared between the two groups. Unsupervised hierarchical clustering was done using the 15,020 genes in IPF fibroblasts and control fibroblast (Additional file 3: Figure S2). Although no gene passed the false discovery rate of less than 5% correction for multiple testing, 178 genes showed different expression levels according to the t-test and TNOM (p < 0.05 and fold changes > 2, Additional file 1: Table S3) (Fig. 1a). The expressions of 109 genes were increased, while that of 69 genes decreased in the IPF group compared to the control group. The top 15 genes showed 10-fold or greater changes: 13 genes (PF4V1, MYOC, CCL8, ROR2, HBG2, D4S234E, KCNJ2, RGS18, PITX1, EPB41L3, FGF7, LOC, and POSTN) were increased, and 2 genes (FLJ25037 and IGFBP2) were decreased (Table 2 and Fig. 1b). CCL8 expression was 22.8-fold higher in IPF-fibroblasts compared with control-fibroblasts.
Gene expression profiles of fibroblasts derived from the lung tissues of 8 patients with IPF and 4 controls. a A heat map of 178 genes differentially expressed between the two groups (p-value <0.05 and absolute fold-change ratio >2 by t-test and TNoM). The maximum value (red) of each gene was set to 3, the minimum value to −3, and the remaining values were linearly fitted in the range. b A heat map of top 15 genes differentially expressed between the two groups (p-value < 0.05 by t-test and TNoM, absolute fold-change ratio >10). c The top 8 significantly perturbed Gene Ontology nodes in the IPF patients versus the controls. Left, statistical significance of the perturbation as determined by a gene set test; right, ratio of enrichment. The significance of differences between the dataset and the canonical pathway was measured as a ratio. Solid and open bars represent upregulation and downregulation, respectively. d Biological pathway analysis of differentially expressed gene sets related to IPF (corrected gamma p-value < 0.05). P-values and impact factors are plotted on the left and right axes, respectively
Ontology and pathway analysis of the differentially expressed genes
A gene ontology analysis of the 178 genes was conducted. In comparison with those expected, the observed gene numbers were significantly higher in a total of 16 ontology categories (corrected p-values < 0.05; Additional file 1: Table S4). A ratio of enrichment of > 10 and a p-value < 0.001 was found in the following categories: regulation of fibroblast growth factor production (p = 4.6 × 10−5, ratio = 167.6) and cell adhesion molecular binding (p = 0.0005, ratio = 19.2) (Fig. 1c). The differentially expressed genes had a significant impact on pathways. Among 109 up-regulated genes, 11genes (BMP2, CCL26, CCL8, EPAS1, EPB41L3, FGF7, GAS1, LEF1, PF4V1, PRKG2 andPTGS2) were mapped to 7 significant pathways (corrected gamma p-value < 0.05), including Adherens junction, Melanoma, Cytokine-cytokine receptor interaction, Pathways in cancer, Hedgehog signaling pathway, Tight junction, and Long-term depression (Additional file 1: Table S5 and Fig. 1d). Among 68 down-regulated genes, 5 genes (CADM1, ITGA10, LLGL2, NLGN1 and PRKCB) were also mapped to 5 significant pathways (Regulation of actin cytoskeleton, Tight junction, Long-term potentiation, Cell adhesion molecules (CAMs), MAPK signaling pathway). CCL8 was included in the ontology categories of extracellular region, receptor binding, heparin binding, G-protein-coupled receptor binding, chemokine activity, carbohydrate derivative binding, and glycosaminoglycan binding and the cytokine-cytokine receptor interaction pathway.
Quantitation of CCL8 mRNA and protein levels in fibroblasts
Fibroblasts from 14 IPF lungs and 10 control lungs were used. The CCL8 mRNA level normalized to that of β-actin was 3-fold higher in the IPF fibroblasts than in the control fibroblasts by RT-PCR (p = 0.0001; Fig. 2a and b) and real-time PCR (p = 0.022; Fig. 2c). The CCL8 mRNA levels determined by the transcriptomic analysis showed a strong correlation to those determined by real-time PCR in the 12 subjects (r = 0.615, p = 0.033; Fig. 2d). In addition, we measured the CCL8 protein amount in supernatants and cell lysates of the cultured fibroblasts (1 × 106) using CCL8 ELISA kit (Abnova, Taipei, Taiwan). CCL8 levels were significantly higher in the supernatants from the IPF- fibroblasts compared those from the control-fibroblasts (p = 0.026) and cell lysates (p = 0.446) (Fig. 2e, f). The CCL8 protein levels were well correlated with the CCL8 mRNA levels (r = 0.511, p = 0.011) (Fig. 2g).
CCL8 mRNA and protein levels in lung tissue-derived fibroblasts from 14 IPF patients and 10 controls. (a) RT-PCR, (b) densitometry of the CCL8 RT-PCR band intensity normalized to that of β-actin, (c) real-time PCR, and (d) correlations of the CCL8 mRNA levels of 12 subjects determined by the transcriptome chip with those by real-time PCR. e, f CCL8 protein level of Culture media and cell lysate, and (g) correlations of the CCL8 protein levels and CCL8 mRNA levels of 24 subjects determined by the ELISA with those by real-time PCR. The data were presented as median values with 25 and 75% quartiles
CCL8 protein levels in BAL fluids
The CCL8 levels were significantly higher in the IPF patients than those in the NC [6.01(2.75–17.16pg/mL) vs. 0.90(0.00–2.07pg/mL), p = 7.62E-10], those in the NSIP [0.12(0.00–7.47pg/mL), p = 0.00079], those in the HP [1.52(0.00–7.37pg/mL), p = 0.0029] and those in the sarcoidosis patients [0.00(0.00–1.9pg/mL), p = 0.000027] (Fig. 3a). A ROC curve showed a clear difference between the IPF patients and NC (AUC = 0.857, Fig. 3b), and the other interstitial lung diseases groups (including NSIP, HP and sarcoidosis, n = 61) (AUC = 0.750, Fig. 3c). A cut-off value (2.29pg/mL) possessed 80.2% accuracy with 86.0% specificity and 65.7% sensitivity between the IPF patients and NC. A cut-off value (0.43pg/mL) possessed 70.7% accuracy with 91.9% specificity and 57.4% sensitivity between the IPF patients and the other interstitial lung diseases groups. The CCL8 levels were analyzed in 69 subjects followed up for 1 to 8 years with respect to the survival rate. When the subjects were divided into two groups with a cut-off value of 28.61 pg/mL, the survival rate was significantly lower in the group > 28.61 pg/mL compared with that in the group < 28.61 pg/mL (hazard ratio = 3.93; CI: 1.25–12.39; p = 0.012; Fig. 3d). The CCL8 protein concentrations showed a significant correlation with neutrophil (p = 0.014, r = 0.297), while no correlations with macrophage, lymphocytes and eosinophil numbers in BAL fluid and physiological parameters (Additional file 1: Table S6). There was no difference of plasma CCL8 levels between 35 NC and 66 IPF patients (p = 0.167; Additional file 4: Figure S3A), and no correlation between CCL8 concentrations in plasma and those in BAL fluids of 60 IPF patients (p = 0.169; Additional file 4: Figure S3B).
CCL8 protein concentrations in BAL fluids and ROC curves. a CCL8 protein was detected in 25 of 41 normal controls, 80 of 86 IPF patients, 11 of 22 NSIP patients, 8 of 20 HP patients and 13 of 19 sarcoidosis patients. Open and closed circles indicate CCL8 protein levels detected (>1.5pg/mL) and those below the lower limit of detection, respectively. The data were presented as median values with 25 and 75% quartiles. b ROC curve of the CCL8 protein concentration between the two groups. A cut-off value of 2.17pg/mL had 80.2% accuracy, 86.0% specificity, and 65.7% sensitivity for differentiating IPF patients from controls. c ROC curve of the CCL8 protein concentration between the other interstitial lung diseases groups. A cut-off value 0.53pg/mL had 70.7% accuracy, 91.9% specificity, 57.4% sensitivity between the IPF patients and the other interstitial lung diseases group. d A Kaplan-Meier plot of 69 subjects with IPF followed up for 1to 8 years. The percent survival rate was markedly lower in the group with a CCL8 level of >28.61 pg/mL (red line) compared with that in the group with a CCL8 level of <28.61 pg/mL (black line) (hazard ratio = 3.93, CI: 1.25–12.39, p = 0.012)
Immunofluorescence staining for CCL8
To confirm CCL8 expression by myofibroblasts in IPF lungs, CCL8 and α-SMA double immunofluorescence staining was performed in 3 IPF and 3 control lung tissues. In the control lungs, most probably smooth muscle cells were stained for both α-SMA and CCL8. In the IPF lungs, α-SMA was robustly expressed by interstitial fibroblasts, most but not all of which expressed CCL8 (Fig. 4, Additional file 5: Figure S4).
Representative double immunofluorescence-stained images of IPF and control lung tissues. CCL8 and α-smooth muscle actin (α-SMA) were stained using PE- (red) and FITC-conjugated antibodies (green), respectively. A proportion of interstitial fibroblasts (IT) and the peribronchial and vascular area (VS) showed staining for both CCL8 and α-SMA (magnification, 200×)
Discussion
In our study, 178 genes were found to be differentially expressed by the fibroblasts derived from IPF lungs. Among the top 15 genes of them, only FGF7 and POSTN have been previously reported as related with IPF. The new genes identified in our study may provide insight into the pathogenesis of IPF. Among the top genes, CCL8 expression was >20-fold higher in the IPF-fibroblasts. CCL8 gene was reported to overexpress in 26 IPF lungs compared with 11 normal lungs (p = 0.099) in the GDS1252 dataset, as well as in 6 sarcoidosis lung compared with 6 normal lungs (p = 0.016) in the GDS3580 dataset of NCBI GEO DataSet Browser (https://www.ncbi.nlm.nih.gov/geo/). CCL8 was included in the ontology categories of extracellular region, receptor binding, heparin binding, G-protein-coupled receptor binding, chemokine activity, carbohydrate derivative binding, and glycosaminoglycan binding, which are essential pathways in the pathogenesis of IPF. In validation, CCL8 levels in BAL fluids appeared useful as a candidate marker for the differential diagnosis from NC and other chronic interstitial lung diseases. Thus, we identified several novel genes, and demonstrated the clinical relevance of CCL8 as a candidate marker for the diagnosis and prognosis of IPF patients for the first time to the best of our knowledge.
CCL8 is secreted into the peripheral circulation. However, no correlation between CCL8 protein levels in BAL fluids and peripheral bloods was observed. This may be due to sources of CCL8 other than fibroblasts or individual variance in the diffusion of CCL8 from IPF-fibroblasts into the bloodstream. Among the CC chemokine ligand family, CCL26 expression was increased in IPF patients compared with controls (2.39-fold increase, p = 0.048) in our study. In other studies of IPF-patients, CCL2 are present in metaplastic epithelial cells and vascular endothelial cells [28] and CCL3, CCL4, and CCL7 expressions are elevated in BAL fluids [29, 30]. The discrepancy between our data and these reports may be due to the presence of other sources of CCL2, CCL3, CCL4, and CCL7 in the lung.
CCL8 activates various immune cells, including mast cells, eosinophils, basophils, monocytes, T cells, and NK cells [31]. Recently, diverse functions of CCL8 have been discovered in infection, immunity, and allergic inflammation. CCL8 recruits gamma/delta T cells, which preferentially express IL-17F and synergistically enhance neutrophil chemotaxis in the presence of IL-8 [32]. CCL8 is induced by fibroblasts and endothelial cells when co-stimulated with IL1-β and interferon (IFN)-γ. IFN-γ has also a synergistic effect with activation of TLR2, TLR3 or TLR4. The application of both IFN-γ and dsRNA via TLRs resulted in the synergistic induction of CCL8 expression [33]. All of them are known mediators involved in the development of IPF. Thus, CCL8 appears to be related with innate immune response in the development of IPF, but the exact role of CCL8 remains to be solved in near future.
In the ontology analysis of the 178 genes, regulation of fibroblast growth factor production, extracellular region, positive regulation of the EMT, and positive regulation of cell morphogenesis were the most relevant groups. The fibroblasts used in our study expresses markers of smooth muscle differentiation, such as α-SMA [34]. Lindahl GE and collaborators performed Gene ontology analysis using 843 differentially expressed genes between IPF and scleroderma fibroblasts and normal control fibroblasts. Enriched functional groups represent 12 broad categories as follows: anatomical structural development, regulation of cell cycle, response to stress and wounding, regulation of apoptosis, cell migration and smooth muscle contraction in upregulation and inflammatory and immune response, response to biotic stimulus, regulation of apoptosis, regulation of cell migration, regulation of cell proliferation, and regulation of I-kB/NF-kB cascade in down regulation [35]. The enriched functional groups are in part compatible with our findings. Studies using human whole lungs also showed elevated expression of genes related to tissue remodeling/reorganization and ECM formation/degradation [4, 5]. Interestingly, the expression of CCL8 was positively correlated with those of IL-8, IL-13RA2, and CCL2, ADAMTS1, ADAMTS8, MMP10, MMP2, MMP3, TIMP2, ECM1, TGFBI, and CLEC3B, and inversely correlated with FN1 (p < 0.05, respectively) (Additional file 1: Tables S7 and S8). However, the contents of the gene ontology are different between the studies. In our study, the following 18 genes were included in the extracellular region: PF4V1, NID1, PTHLH, CREG1, TFPI, FBN2, RSPO3, TSKU, EPDR1, BMP2, CCL26, POSTN, MYOC, CCL8, C1QTNF9B, CLEC18C, FGF7, and TUBA4A. Among them, FGF7 [36], POSTN [37], TSKU [38], and TFPI [39] have been suggested to be involved in the pathogenesis of IPF. Two genes (PTGS2 and RGCC) in our study were included in the fibroblast growth factor production and EMT categories. Among them, PTGS2 is known to be involved in the development of IPF [40]. These results indicate that more than half of the genes in our study are newly identified.
Our study had the following limitation: the small number of lung tissue samples available for microarray analysis. Therefor we used the t-test and nonparametric TNoM scoring method to compare the differences in gene expression between NC and IPF groups because of no gene passed of less than 5% correction for multiple testing [21]. The use of unadjusted P-values may be less problematic than omitting informative genes in studies aimed at identifying target genes responsible for biological mechanisms [41]. In addition, when the differentially expressed genes in our study was compared with those with Lindahl GE’s study using cultured fibroblasts (n = 744, 10 controls and 3 IPF subjects) [35], those with Sridhr S’s study using cultured fibroblasts (n = 1813, 4 controls and 10 IPF subjects; GSE44723), those with Ronzani C’s study using (n = 3, 5 controls and 5 IPF subjects; GES45686) and those with Emblom-Callahan’s study using uncultured fibroblast (n = 1, 6 controls and 12 IPF subjects) [8]. Only 3–10% of the differentially expressed genes were overlapped between the studies as seen in a Venn diagram in the Supplement (Additional file 6: Figure S5). This discrepancy may be due to the small numbers of fibroblasts in each study in addition to the phenotypic changes of fibroblasts during the passage in culture.
Fibroblasts derived from IPF lungs have distinct biological characteristics: a high percentage of apoptotic cells, and increased collagen, fibronectin, gelatinase B, TIMPs, β-FGF, and PDGF expression (i.e., a pro-fibrotic secretory phenotype) [42], and a reduced capacity to secrete anti-fibrotic molecules, such as prostaglandin E2 and hepatocyte growth factor [9]. Interestingly, the above-mentioned genes were not identified in our study. Recently, a genomic expression in non-cultured fibroblasts obtained from IPF-lungs demonstrated 1,813 significantly differentially expressed transcripts from those of normal fibroblasts [8]. When they were compared with the 178 genes of our study, only 9 genes, including ALDH3A2, CDC42EP3, IGFBP2, MOXD1, NBEAL2, PITX1, POSTN, TMEM51, and UBE2K, were overlapped. This may be due to biological differences between the cultured fibroblasts used in our study and the uncultured ones from IPF-lungs. However, we validated the CCL8 concentration in BAL fluid in 86 patients with IPF and those in 41 controls and CCL8 protein expression using immunohistochemical stain with antibodies to CCL8 and α-SMA-positive cells in the lung tissues of IPF. Because the BAL fluids and lung tissues were obtained in un-cultured condition, so CCL8 protein may be generated per se, not solely due to a phenotypic shift of the fibroblasts. However, replication of the result is mandatory for useful biomarkers. Another limitation was the use of control fibroblasts from the resected cancer specimens. It cannot be excluded that the gene expression in fibroblasts derived from the lungs in which cancer has developed may be different form fibroblasts derived from the normal lungs.
Epigenetic changes, such as CpG methylation and miRNA, are widespread throughout the genome in IPF-lung tissues and may regulate the expression of important genes [43–46]. Although fibroblasts have been studied mainly at the steady-state RNA level, there is evidence for the abnormal regulation of mRNA translation in the fibroblasts [47]. Furthermore, the surrounding environment influences the gene expression of fibroblasts. Recently, diseased ECM was reported to be the predominant driver of pathological gene expression, and the expression of ECM-sensitive genes is regulated primarily at the translational level [48]. Genes encoding IPF-associated ECM proteins are targets for miR-29, which is downregulated in fibroblasts grown on IPF-derived ECM. Other candidate miRNAs are localized to the chromosome 14q32 microRNA cluster, many of which belong to the miR-154 family [49]. Thus, the differentially expressed genes in our study should be analyzed together with global changes in CpG methylation and miRNA expression in the future.
Conclusion
Global gene expression profiling of fibroblasts from lung tissues from IPF patients was performed to identify novel candidate genes. CCL8 was validated as a candidate in BAL fluids and lung tissues samples. A total of 178 genes showed differential expression; among 15 genes showing ≥ 10-fold changes, 13 were newly identified in relation with IPF. The CCL8 protein concentrations in BAL fluids were significantly higher in patients with IPF, and a cut-off value of 2.29pg/mL showed a high degree of accuracy for diagnosis. The levels were also higher compared to those in other interstitial lung diseases including NSIP, HP and sarcoidosis with a cut-off value (0.43 pg/mL) possessing a high degree of accuracy for the discrimination from the other interstitial lung diseases. The subjects with IPF with CCL8 levels >28.61 pg/mL showed a reduced survival rate. In conclusion, our transcriptome analysis identified new genes that may be involved in the pathogenesis of IPF. Among them, CCL8 may be useful as a candidate molecule for the differential diagnosis of IPF and prediction of survival.
Abbreviations
- BAL:
-
Bronchoalveolar lavage
- ECM:
-
Extracellular matrix
- GO:
-
Gene Ontology
- HP:
-
Hypersensitivity pneumonitis
- IFN:
-
Interferon
- IPF:
-
Idiopathic pulmonary fibrosis
- NC:
-
Normal controls
- NSIP:
-
Non-specific interstitial pneumonia
- ROC:
-
Receiver operating characteristic
References
American_Thoracic_Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161:646–64.
Kim DS, Collard HR, King Jr TE. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:285–92.
Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–50.
Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci U S A. 2002;99:6292–7.
Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, Aziz N, Kaminski N, Zlotnik A. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2006;173:188–98.
Boon K, Bailey NW, Yang J, Steel MP, Groshong S, Kervitsky D, Brown KK, Schwarz MI, Schwartz DA. Molecular phenotypes distinguish patients with relatively stable from progressive idiopathic pulmonary fibrosis (IPF). PLoS One. 2009;4:e5134.
Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, Bisceglia M, Gilbert S, Yousem SA, Song JW, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180:167–75.
Emblom-Callahan MC, Chhina MK, Shlobin OA, Ahmad S, Reese ES, Iyer EP, Cox DN, Brenner R, Burton NA, Grant GM, Nathan SD. Genomic phenotype of non-cultured pulmonary fibroblasts in idiopathic pulmonary fibrosis. Genomics. 2010;96:134–45.
Marchand-Adam S, Fabre A, Mailleux AA, Marchal J, Quesnel C, Kataoka H, Aubier M, Dehoux M, Soler P, Crestani B. Defect of pro-hepatocyte growth factor activation by fibroblasts in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:58–66.
King Jr TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–61.
Selman M, Pardo A, King Jr TE. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186:314–24.
Parrish S, Turner JF. Diagnosis of sarcoidosis. Dis Mon. 2009;55:693–703.
Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011;183:573–81.
Travis WD, Costabel U, Hansell DM, King Jr TE, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–48.
American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2001;165:277–304.
Park CS, Chung SW, Ki SY, Lim GI, Uh ST, Kim YH, Choi DI, Park JS, Lee DW, Kitaichi M. Increased levels of interleukin-6 are associated with lymphocytosis in bronchoalveolar lavage fluids of idiopathic nonspecific interstitial pneumonia. Am J Respir Crit Care Med. 2000;162:1162–8.
Travis WD, Hunninghake G, King Jr TE, Lynch DA, Colby TV, Galvin JR, Brown KK, Chung MP, Cordier JF, du Bois RM, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med. 2008;177:1338–47.
Venables KM, Farrer N, Sharp L, Graneek BJ, Newman Taylor AJ. Respiratory symptoms questionnaire for asthma epidemiology: validity and reproducibility. Thorax. 1993;48:214–9.
Raghu G, Chen YY, Rusch V, Rabinovitch PS. Differential proliferation of fibroblasts cultured from normal and fibrotic human lungs. Am Rev Respir Dis. 1988;138:703–8.
Dave NB, Kaminski N. Analysis of microarray experiments for pulmonary fibrosis. Methods Mol Med. 2005;117:333–58.
Radom-Aizik S, Kaminski N, Hayek S, Halkin H, Cooper DM, Ben-Dov I. Effects of exercise training on quadriceps muscle gene expression in chronic obstructive pulmonary disease. J Appl Physiol (1985). 2007;102:1976–84.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–5.
Kim TH, Lee YH, Kim KH, Lee SH, Cha JY, Shin EK, Jung S, Jang AS, Park SW, Uh ST, et al. Role of lung apolipoprotein A-I in idiopathic pulmonary fibrosis: antiinflammatory and antifibrotic effect on experimental lung injury and fibrosis. Am J Respir Crit Care Med. 2010;182:633–42.
Park SW, Ahn MH, Jang HK, Jang AS, Kim DJ, Koh ES, Park JS, Uh ST, Kim YH, Park JS, et al. Interleukin-13 and its receptors in idiopathic interstitial pneumonia: clinical implications for lung function. J Korean Med Sci. 2009;24:614–20.
Soreide K. Receiver-operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J Clin Pathol. 2009;62:1–5.
Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S, Denkert C. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862.
Iyonaga K, Takeya M, Saita N, Sakamoto O, Yoshimura T, Ando M, Takahashi K. Monocyte chemoattractant protein-1 in idiopathic pulmonary fibrosis and other interstitial lung diseases. Hum Pathol. 1994;25:455–63.
Capelli A, Di Stefano A, Gnemmi I, Donner CF. CCR5 expression and CC chemokine levels in idiopathic pulmonary fibrosis. Eur Respir J. 2005;25:701–7.
Choi ES, Jakubzick C, Carpenter KJ, Kunkel SL, Evanoff H, Martinez FJ, Flaherty KR, Toews GB, Colby TV, Kazerooni EA, et al. Enhanced monocyte chemoattractant protein-3/CC chemokine ligand-7 in usual interstitial pneumonia. Am J Respir Crit Care Med. 2004;170:508–15.
Proost P, Wuyts A, Van Damme J. Human monocyte chemotactic proteins-2 and −3: structural and functional comparison with MCP-1. J Leukoc Biol. 1996;59:67–74.
Gouwy M, Struyf S, Catusse J, Proost P, Van Damme J. Synergy between proinflammatory ligands of G protein-coupled receptors in neutrophil activation and migration. J Leukoc Biol. 2004;76:185–94.
Struyf S, Proost P, Vandercappellen J, Dempe S, Noyens B, Nelissen S, Gouwy M, Locati M, Opdenakker G, Dinsart C, Van Damme J. Synergistic up-regulation of MCP-2/CCL8 activity is counteracted by chemokine cleavage, limiting its inflammatory and anti-tumoral effects. Eur J Immunol. 2009;39:843–57.
Selman M, Ruiz V, Cabrera S, Segura L, Ramirez R, Barrios R, Pardo A. TIMP-1, −2, −3, and −4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am J Physiol Lung Cell Mol Physiol. 2000;279:L562–74.
Lindahl GE, Stock CJ, Shi-Wen X, Leoni P, Sestini P, Howat SL, Bou-Gharios G, Nicholson AG, Denton CP, Grutters JC, et al. Microarray profiling reveals suppressed interferon stimulated gene program in fibroblasts from scleroderma-associated interstitial lung disease. Respir Res. 2013;14:80.
Marchand-Adam S, Plantier L, Bernuau D, Legrand A, Cohen M, Marchal J, Soler P, Leseche G, Mal H, Aubier M, et al. Keratinocyte growth factor expression by fibroblasts in pulmonary fibrosis: poor response to interleukin-1beta. Am J Respir Cell Mol Biol. 2005;32:470–7.
Okamoto M, Hoshino T, Kitasato Y, Sakazaki Y, Kawayama T, Fujimoto K, Ohshima K, Shiraishi H, Uchida M, Ono J, et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J. 2011;37:1119–27.
Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–87.
Fujii M, Hayakawa H, Urano T, Sato A, Chida K, Nakamura H, Takada A. Relevance of tissue factor and tissue factor pathway inhibitor for hypercoagulable state in the lungs of patients with idiopathic pulmonary fibrosis. Thromb Res. 2000;99:111–7.
Xaubet A, Fu WJ, Li M, Serrano-Mollar A, Ancochea J, Molina-Molina M, Rodriguez-Becerra E, Morell F, Rodriguez-Arias JM, Pereda J, et al. A haplotype of cyclooxygenase-2 gene is associated with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27:121–30.
Bender R, Lange S. Adjusting for multiple testing-when and how? J Clin Epidemiol. 2001;54:343–9.
Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24:591–8.
Rabinovich EI, Kapetanaki MG, Steinfeld I, Gibson KF, Pandit KV, Yu G, Yakhini Z, Kaminski N. Global methylation patterns in idiopathic pulmonary fibrosis. PLoS One. 2012;7:e33770.
Sanders YY, Ambalavanan N, Halloran B, Zhang X, Liu H, Crossman DK, Bray M, Zhang K, Thannickal VJ, Hagood JS. Altered DNA methylation profile in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:525–35.
Huang SK, Scruggs AM, McEachin RC, White ES, Peters-Golden M. Lung fibroblasts from patients with idiopathic pulmonary fibrosis exhibit genome-wide differences in DNA methylation compared to fibroblasts from nonfibrotic lung. PLoS One. 2014;9:e107055.
Dakhlallah D, Batte K, Wang Y, Cantemir-Stone CZ, Yan P, Nuovo G, Mikhail A, Hitchcock CL, Wright VP, Nana-Sinkam SP, et al. Epigenetic regulation of miR-17 ~ 92 contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;187:397–405.
Larsson O, Diebold D, Fan D, Peterson M, Nho RS, Bitterman PB, Henke CA. Fibrotic myofibroblasts manifest genome-wide derangements of translational control. PLoS One. 2008;3:e3220.
Carstens JL, Lovisa S, Kalluri R. Microenvironment-dependent cues trigger miRNA-regulated feedback loop to facilitate the EMT/MET switch. J Clin Invest. 2014;124:1458–60.
Milosevic J, Pandit K, Magister M, Rabinovich E, Ellwanger DC, Yu G, Vuga LJ, Weksler B, Benos PV, Gibson KF, et al. Profibrotic role of miR-154 in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;47:879–87.
Acknowledgements
The samples were generously provided by Soonchunhyang University, Bucheon Hospital Biobank, a member of the National Biobank of Korea, supported by the Ministry of Health, Welfare, and Family Affairs, Republic of Korea.
Funding
This study was supported by a grant from the National Research Foundation of Korea funded by the Korean Government (2013R1A2A2A01068077) and by a research grant from Soonchunhyang University.
Availability of data and materials
Available.
Authors’ contributions
JU Lee and CS Park conceived and designed the experiments, EY Shim and HS Chang performed the fibroblast culture and RNA isolation, HS Cheong and HD Shin performed chip assay, DJ Bae performed ELISA, ST Uh, YH Kim, and JS Park provided clinical samples, Bora Lee performed statistical analysis, JU Lee, HS Cheong, and CS Park wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors have read and approved the submission of manuscript.
Ethics approval and consent to participate
It was wrote at the materials and methods sections; as follows: The study protocol was approved by institutional review board (IRB) in Korea National Institute for Bioethics Policy (KoNIBP; P01-201408-BS-01-00) and the Soonchunhyang University hospital ethics committee (SCHBC 2015-08-025-005).
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:
Supplementary data. (DOC 379 kb)
Additional file 2: Figure S1.
Expression of epithelial, mesenchyal and myofibroblast markers of IPF fibroblasts and controls. Fibroblasts, obtained from lung biopsies of 14 IPF and normal lung sections of 10 subjects with localized lung cancer, was characterized using Western blot analysis for E-cadherin (epithelial marker), vimentin (mesenchymal cell marker) and α-smooth muscle actin (myofibroblast marker). The expression levels were normalized to β-actin as an internal control protein. (TIF 8191 kb)
Additional file 3: Figure S2.
A heat map of 15,020 genes in IPF fibroblast and control fibroblast. A gradient scale ranging between green (down-regulated) and red (up-regulated) was indicated. The maximum value (red) of each gene was set to 3, the minimum value to −3, and the remaining values were linearly fitted in the range. (TIF 18692 kb)
Additional file 4: Figure S3.
Plasma CCL8 concentrations in the study subjects and its correlation with CCL8 levels in BAL fluids. (A) CCL8 concentration in plasma from normal controls (n = 35) and IPF subjects (n = 66), and, (B) correlation of the paired samples between plasma and BAL fluids from IPF subjects (n = 60). The data were presented as median values with 25 and 75% quartiles. (TIF 3385 kb)
Additional file 5: Figure S4.
A blocking study using a recombinant CCL8 protein in Immunofluorescence staining of CCL8 protein. The mouse-anti human CCL8 antibody (1:100) were incubated with 10, 1, 0.1ng of a recombinant CCL8 (Origene, Rockville, MD, USA) for 2 h at room temperature, then the mixtures were incubated for overnight with the tissue sections of IPF lung tissues at 4 °C. The 2nd Ab (rabbit anti-mouse-PE 1:2000) was incubated for 2 h and Confocal laser scanning was performed using a microscope. As shown in the pictures, intensity of CCL8 staining was decreased as the concentration of recombinant CCL8 protein increased. (TIF 8134 kb)
Additional file 6: Figure S5.
Venn Diagram of the differentially expressed genes by 4 studies using the cultured and uncultured fibroblasts. The differentially expressed genes in our study (4 controls and 8 IPF-subjects, blue) were compared with those with Lindahl’s study using cultured fibroblasts (10 controls and 3 IPF subjects, yellow) [36], those with Sridhr’s study using cultured fibroblasts (4 controls and 10 IPF subjects, grey; GSE44723), those with Ronzani C’s study using cultured fibroblasts (5 controls and 5 IPF subjects, green; GSE45686) and those with Emblom-Callahan’s study using uncultured fibroblast (6 controls and 12 IPF subjects, red) [8]. (TIF 7160 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lee, JU., Cheong, H.S., Shim, EY. et al. Gene profile of fibroblasts identify relation of CCL8 with idiopathic pulmonary fibrosis. Respir Res 18, 3 (2017). https://doi.org/10.1186/s12931-016-0493-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-016-0493-6