Abstract
Objectives
Heart failure (HF) has been implicated in osteoporosis. However, causality remains unestablished. Here, we sought to assess causal associations of genetic liability to HF with osteoporosis using Mendelian randomization (MR) analyses.
Methods
Independent single nucleotide polymorphisms associated with HF at genome-wide significance were derived from a large genome-wide association study (GWAS) (including up to 977,323 individuals). We obtained summary statistics for forearm (FA) bone mineral density (BMD) (n = 8,143), femoral neck (FN) BMD (n = 32,735), lumbar spine (LS) BMD (n = 28,498), heel (HE) BMD (n = 426,824), and fracture (n = 1,214,434) from other GWAS meta-analyses. Inverse variance weighted (IVW) and several supplementary methods were performed to calculate the MR estimates.
Results
Genetically determined HF has no causal effect on FA-BMD (odds ratio (OR) 1.17; 95% confidence interval (CI) 0.82, 1.66; P = 0.389), FN-BMD (OR 1.01; 95% CI 0.85, 1.19; P = 0.936), LS-BMD (OR 0.96; 95% CI 0.80, 1.17; P = 0.705), HE-BMD (OR 1.01; 95% CI 0.90, 1.13; P = 0.884), and fracture risk (OR 1.00; 95% CI 0.92, 1.10; P = 0.927). Complementary analyses returned broadly consistent results.
Conclusion
This MR study provides genetic evidence that HF may not lead to an increased risk of reduced BMDs or fracture.
Similar content being viewed by others
Introduction
Osteoporosis describes a chronic skeletal condition characterized by decreased bone mineral density (BMD), microarchitecture impairment, and increased fracture risk [1]. The forearm (FA), femoral neck (FN), lumbar spine (LS), and heel (HE) are the most common skeletal sites of osteoporosis [2]. BMD is highly heritable and polygenic [3, 4]; both ultrasound and dual-energy X-ray absorptiometry (DXA)-measured BMD have been applied to predict fracture [5, 6]. As a growing public health problem and its most critical complication, hip fracture, osteoporosis has been linked to high mortality and disability rate worldwide [7]. Nowadays, the disorder is largely preventable due to a better understanding of its risk factors, including low body mass index, estrogen deficiency, and smoking [1].
Heart failure (HF) is a common health threat that affects approximately 5.7 million Americans [8]. Patients with HF typically suffer from decreased cardiac function and disturbed neurohumoral status [9]. In recent years, HF has been recognized as a multisystem disorder associated with numerous metabolic disorders [10]. In the clinical setting, disturbed bone metabolism was found to be highly prevalent in adults with HF. Observational studies found evidence that HF was associated with reduced BMD in both sexes [11,12,13]. In addition, it was reported that lower BMD might be determined by HF severity (higher New York Heart Association class and N-terminal pro-B-type natriuretic peptide levels) [12, 14]. Cohort studies further revealed that patients with HF experienced an increased fracture risk [15, 16]. However, given that observational studies are easily biased by residual confounding, misclassification, and reverse causality [17], it remains an unanswered question whether HF has a causal effect on reduced BMD and fracture, or if it is just an episodic phenomenon when some shared risk factors linked both syndromes.
Mendelian randomization (MR) is commonly used to estimate the causal effect of exposures on outcomes where single nucleotide polymorphisms (SNPs) are utilized as instrumental variables (IVs) [18, 19]. According to Mendel's law of inheritance, the genetic variants are randomly assorted, independently of the environment and remain constant after conception. Those who inherit the allele were actually assigned to a higher specific trait [18, 19]. Therefore, the approach is considered a natural genetic counterpart of randomized controlled trials that can vastly diminish the influence of residual confounders and reverse causality. In this study, we conducted a two-sample MR study and sought to illustrate the causal associations of HF with reduced BMDs and fracture risk. We present the following article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) statement (Additional file 1: Table S1) [20].
Methods
Study design
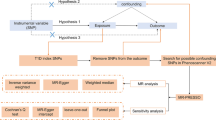
There are three key(critical) assumptions for the MR approach. First, the IVs should be associated with the exposure in a significant way. Second, the IVs are not linked to potential confounders that may affect the exposure and (or) outcomes. Third, the IVs affect the outcomes exclusively by exposure but not via other pathways. (Fig. 1) [21]
Publicly available genome-wide association study (GWAS) summary statistics were selected for the analysis. No specific ethical approval or written informed consent was necessary.
Data sources and IVs selection
Summary statistics for associations between genetic variants and FA-BMD, FN-BMD, and LS-BMD were obtained from a GWAS meta-analysis conducted by the Genetic Factors for Osteoporosis (GEFOS) consortium, in which BMD was measured by DXA-scanning [22] (Table 1). For HE-BMD estimated from ultrasound (n = 426,824) and fracture (n = 1,214,434), summary-level data were derived from another GWAS meta-analysis consisting of the UK Biobank and 23andMe cohort [23] (Table 1). Heel quantitative ultrasound can estimate BMD to the same extent as DXA-scanning, with the advantages of being mobile, inexpensive, and radiation-free [24]. Fracture cases were defined by two methods: 1) Hospital Episodes Statistics and 2) questionnaire-based self-reported fractures within the past five years [23].
From the GWAS meta-analysis conducted by the Heart Failure Molecular Epidemiology for Therapeutic Targets (HERMES) Consortium, we obtained the summary statistics for HF (Table 1), where cases were diagnosed by clinical diagnosis of any etiology, with any etiology based on left ventricular ejection fraction [25]. Initially, the GWAS meta-analysis provided 12 SNPs strongly associated with HF at genome-wide significance (P < 5 × 10−8) [25]. We next performed a strict clumping procedure (r2 < 0.001 within a 10,000 kb window) referring to the European 1000 genomes project, with one SNP (rs140570886) excluded from the study. Detailed information on the 11 independent SNPs leveraged as IVs is provided in Additional file 1: Table S2. Next, we calculated the F statistic using the formula F = R2(n − 2)/ (1 − R2) to detect any weak IVs bias [26]. Here n represents the sample size; R2 refers to the proportion of variance explained by the selected SNPs and was calculated using the method described previously [27]. Altogether, they explained 1.42% of the phenotypic variability of HF (Additional file 1: Table S2). Besides, we did not find evidence of weak IVs bias since all these SNPs have F statistics higher than 10 (Additional file 1: Table S2). IVs absent in the outcome datasets were replaced with proxies in linkage disequilibrium (r2 > 0.8) if available. For those SNPs not available in the outcome datasets, we found proxies to replace them by searching a publicly available online tool [28]. Here, we found rs2395655, rs10738607, rs2519093, and rs11065979 to replace rs4135240, rs1556516, rs600038, and rs4766578, respectively. However, no eligible proxies were found for rs11745324, rs17617337, rs4746140, and rs55730499 (Additional file 1: Table S2).
Statistical analyses
The MR estimates were calculated by combining the SNP-exposure, and SNP-outcome associations with inverse variance-weighted (IVW) used as the main method. The IVW approach can return an unbiased causal estimate when there is no horizontal pleiotropy or heterogeneity [29]. Several complementary analyses were used to test the robustness of the results: (1) Weighted median method. This method calculates the median of the instrumental variable estimates, providing unbiased results even if up to 50% of the IVs were invalid [30]; (2) Simple median method. It can be recognized as a weighted median estimator with equal weights [30]. (3) MR-Egger regression method [31]. This method provided a consistent ME estimate when the instrumental variables exhibited a pleiotropic effect. However, the result may be easily affected by outlier SNPs. (4) MR-Pleiotropy RESidual Sum and Outlier (MR-PRESSO) method. This method can identify and correct for pleiotropic outliers that may bias the results.
In the sensitivity analyses, we assessed heterogeneity among the SNPs by calculating the I2 statistic [32]. I2 statistics above 25% will be considered significantly heterogeneous [32], and then a multiplicative random effects IVW (mre-IVW) method was used [33]. Horizontal pleiotropy refers to IVs affecting outcomes through pathways other than the selected exposure. We here performed the MR-Egger regression and MR-PRESSO analyses to assess horizontal pleiotropy. Additionally, leave-one-out analyses were carried out to identify whether MR estimates were biased by any single SNP.
A Bonferroni-corrected P value of < 0.01 (0.05/5 outcomes) was considered significant. We performed the statistical analyses using TwoSampleMR [34] together with MR-PRESSO [35] packages in R software (version 4.1.0).
Results
As shown in Table 1, there was no sample overlap between Data sources for HF and BMDs and fracture. In analyses for the associations of HF with BMD at four skeletal sites, mre-IVW approach returned that HF was not causally associated with FA-BMD (odds ratio (OR) 1.17; 95% confidence interval (CI) 0.82, 1.66; P = 0.389), FN-BMD (OR 1.01; 95% CI 0.85, 1.19; P = 0.936), LS-BMD (OR 0.96; 95% CI 0.80, 1.17; P = 0.705), and HE-BMD (OR 1.01; 95% CI 0.90, 1.13; P = 0.884) (Fig. 2). In addition, mre-IVW analysis showed null causal association between HF and fracture (OR 1.00; 95% CI 0.92, 1.10; P = 0.927) (Fig. 2). Complementary analyses including weighted median, simple median, and MR-Egger regression methods yielded similar results, except for a suggestive association between HF and FN-BMD in the MR-Egger method (OR 1.71; 95% CI 1.18, 2.47; P = 0.037; Table 2).
Mendelian randomization estimates of the causal associations of HF with bone mineral density and fracture risk. CI, confidence interval; FA-BMD, forearm bone mineral density; FN-BMD, femoral neck bone mineral density; HE-BMD, heel bone mineral density; LS-BMD, lumbar spine bone mineral density; OR, odds ratio; SNP, single-nucleotide polymorphism
Heterogeneity was detected in sensitivity analyses as suggested by Cochran’s Q and I2 statistics (Additional file 1: Table S3). However, the mre-IVW that we used can provide reliable estimates even in the presence of heterogeneity [33]. The MR-Egger intercept was close to zero (Pintercept > 0.05) for all considered outcomes except for FN-BMD (Pintercept = 0.033). No pleiotropic outliers were detected in the MR-PRESSO analysis (Additional file 1: Table S3). In addition, the MR-PRESSO method identified 4 outliers (rs4135240, rs56094641, rs56094641, and rs660240) for HE-BMD; and we observed that the null causal association persisted after excluding these outliers (OR 1.02; 95% CI 0.99, 1.05; P = 0.177; Table 2). Scatter plots describing the main results are shown in Fig. 3. The leave-one-out analyses showed no SNPs could potentially drive the null causal effects of HF on BMDs and fracture (Fig. 4).
Discussion
We used summary statistics from different GWASs for the current two-sample MR study. The analyses provided little evidence that HF was causally associated with decreased BMD at different skeletal sites (including FA, FN, LS, and HE) or increased fracture risk.
Available data from observational studies over the past decade have suggested potential associations of HF with lower BMD and increased fracture risk. A meta-analysis revealed a significantly reduced BMD in patients with HF [36]. Similarly, another meta-analysis combining data from 7 cohort studies linked HF to an increased risk of fracture [37]. However, whether HF plays the role of driver or passenger in osteoporosis or fracture was largely unknown. No conclusion of causality can be drawn from the available data. The lack of causality in this MR study suggested that the association between HF and BMD and fracture observed in the observational studies may be biased by limited sample size, residual confounders, or misclassification. Our results corroborated a previous multicenter study suggesting the association between heart failure and hip fracture may be largely due to shared risk factors [38].
Aging is one of the most prominent confounders that influence both bone metabolism and cardiac function. More than 10% of older adults (70 + years) have HF [39]. Also, aging brings with it many changes in body composition and is considered a risk factor for a declined BMD [1]. As global aging progresses, both HF and osteoporosis represent major health threats. On the other hand, the rising prevalence of dementia places a heavy burden on the health care system [40]. Patients with dementia experienced higher hip fracture risk [41] and had a poor prognosis after hip fracture surgery [42]. Furthermore, prolonged use of loop diuretics in patients with HF is known for its effect on calcium homeostasis [43]. Increased plasma parathyroid hormone levels induced by loop diuretics treatment can mobilize calcium from cortical bone by enhancing turnover [44]. However, observational studies usually have difficulty controlling for these confounding factors [11, 12, 44]. Collectively, aging, dementia, and loop diuretics use that accompany heart failure, rather than heart failure itself, are likely to contribute to lower BMDs and increased risk of fracture that was observed in traditional studies.
MR methods for enhancing the reliability of the results included consensus methods (weighted median, simple median, and MR-egger method), outlier-robust methods (MR-PRESSO), modelling methods (MR-Egger intercept), and leave-one-out analysis. The no-causal results did not negate the previously observed associations of HF with BMD and fracture but provided greater insight into the mechanisms underlying these disorders.
Several strengths were notable in this study. First, the MR approach utilizing the largest GWAS to date diminished the residual confounders and inverse causality, which commonly occurs in traditional epidemiological studies. Second, several complementary analyses (Weighted median, simple median, MR-Egger regression, MR-PRESSO methods, and leave-one-out analysis) returned broadly consistent results, thus strengthening the causal inference. Third, the data sources that we used were confined to individuals of European ancestry. Therefore, our results are less susceptible to population structure bias.
There were some limitations worth noting. First, we did not explore the causality in the associations of HF with BMDs and fracture in other populations due to the lack of related GWAS datasets. This may limit the generalizability of our findings. Further studies on other populations are warranted. Second, despite the use of a set of sensitivity analyses, the bias introduced by horizontal pleiotropy and heterogeneity among the SNPs remains a concern. Finally, some of the fracture cases were identified by questionnaire-based self-reporting, which may introduce measurement bias.
Conclusion
This two-sample MR study does not provide evidence of a causal association between HF and the risk of reduced FA-BMD, FN-BMD, LS-BMD, HE-BMD, or the increase risk of fracture in the European population.
Availability of data and materials
The summary statistics of GWAS for heart failure are derived from a GWAS meta-analysis conducted by HERMES consortium (https://cvd.hugeamp.org/); Full GWAS summary statistics for FA-BMD, FN-BMD, and LS-BMD are publicly available through http://www.gefos.org/; Summary level data for HE-BMD and fracture can be accessed at https://www.ebi.ac.uk/gwas/downloads/summary-statistics.
Abbreviations
- HF:
-
Heart failure
- MR:
-
Mendelian randomization
- FA:
-
Forearm
- FN:
-
Femoral neck
- LS:
-
Lumbar spine
- HE:
-
Heel
- BMD:
-
Bone mineral density
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- GWAS:
-
Genome-wide association study
- SNP:
-
Single nucleotide polymorphism
- IV:
-
Instrumental instrument
- IVW:
-
Inverse variance weighted
- mre:
-
Multiplicative random effects
- MR-PRESSO:
-
MR-pleiotropy residual sum and outlier
References
Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285(6):785–795.
Stone KL, Seeley DG, Lui L-Y, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18(11):1947–54.
Arden NK, Baker J, Hogg C, Baan K, Spector TD. The heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: a study of postmenopausal twins. J Bone Miner Res. 1996;11(4):530–4.
Richards JB, Zheng H-F, Spector TD. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet. 2012;13(8):576–88.
Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185–94.
McCloskey EV, Kanis JA, Odén A, Harvey NC, Bauer D, González-Macias J, Hans D, Kaptoge S, Krieg MA, Kwok T, et al. Predictive ability of heel quantitative ultrasound for incident fractures: an individual-level meta-analysis. Osteoporos Int. 2015;26(7):1979–87.
Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(Suppl 2):S3–7.
Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8(1):143–64.
Zittermann A, Schleithoff SS, Koerfer R. Markers of bone metabolism in congestive heart failure. Clin Chim Acta. 2006;366(1–2):27–36.
De Jong KA, Lopaschuk GD. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol. 2017;33(7):860–71.
Majumdar SR, Ezekowitz JA, Lix LM, Leslie WD. Heart failure is a clinically and densitometrically independent risk factor for osteoporotic fractures: population-based cohort study of 45,509 subjects. J Clin Endocrinol Metab. 2012;97(4):1179–86.
Jankowska EA, Jakubaszko J, Cwynar A, Majda J, Ponikowska B, Kustrzycka-Kratochwil D, Reczuch K, Borodulin-Nadzieja L, Banasiak W, Poole-Wilson PA, et al. Bone mineral status and bone loss over time in men with chronic systolic heart failure and their clinical and hormonal determinants. Eur J Heart Fail. 2009;11(1):28–38.
Kenny AM, Boxer R, Walsh S, Hager WD, Raisz LG. Femoral bone mineral density in patients with heart failure. Osteoporos Int. 2006;17(9):1420–7.
Kono Y, Izawa H, Aoyagi Y, Yamada R, Ishiguro T, Yoshinaga M, Okumura S, Fujiwara W, Hayashi M, Otaka Y. Impact of heart failure severity on bone mineral density among older patients with heart failure. Heart Vessels. 2021;36(12):1856–60.
Lai S-W, Liao K-F, Lai H-C, Tsai P-Y, Lin C-L, Chen P-C, Sung F-C. Risk of major osteoporotic fracture after cardiovascular disease: a population-based cohort study in Taiwan. J Epidemiol. 2013;23(2):109–14.
van Diepen S, Majumdar SR, Bakal JA, McAlister FA, Ezekowitz JA. Heart failure is a risk factor for orthopedic fracture: a population-based analysis of 16,294 patients. Circulation. 2008;118(19):1946–52.
Smith GD, Ebrahim S. “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22.
Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–98.
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, VanderWeele TJ, Higgins JPT, Timpson NJ, Dimou N, et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021;326(16):1614–21.
Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–52.
Zheng HF, Forgetta V, Hsu YH, Estrada K, Rosello-Diez A, Leo PJ, Dahia CL, Park-Min KH, Tobias JH, Kooperberg C, et al. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526(7571):112–7.
Morris JA, Kemp JP, Youlten SE, Laurent L, Logan JG, Chai RC, Vulpescu NA, Forgetta V, Kleinman A, Mohanty ST, et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet. 2019;51(2):258–66.
Trimpou P, Bosaeus I, Bengtsson B-A, Landin-Wilhelmsen K. High correlation between quantitative ultrasound and DXA during 7 years of follow-up. Eur J Radiol. 2010;73(2):360–4.
Shah S, Henry A, Roselli C, Lin H, Sveinbjornsson G, Fatemifar G, Hedman AK, Wilk JB, Morley MP, Chaffin MD, et al. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun. 2020;11(1):163.
Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64.
Shim H, Chasman DI, Smith JD, Mora S, Ridker PM, Nickerson DA, Krauss RM, Stephens M. A multivariate genome-wide association analysis of 10 LDL subfractions, and their response to statin treatment, in 1868 Caucasians. PLoS ONE. 2015;10(4): e0120758.
Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature. 2015;526(7571):68–74.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89.
Greco MFD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926–40.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783–802.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7: e34408.
Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–8.
Xing W, Lv X, Gao W, Wang J, Yang Z, Wang S, Zhang J, Yan J. Bone mineral density in patients with chronic heart failure: a meta-analysis. Clin Interv Aging. 2018;13:343–53.
Ge G, Li J, Wang Q. Heart failure and fracture risk: a meta-analysis. Osteoporos Int. 2019;30(10):1903–9.
Carbone L, Buzková P, Fink HA, Lee JS, Chen Z, Ahmed A, Parashar S, Robbins JR. Hip fractures and heart failure: findings from the Cardiovascular Health Study. Eur Heart J. 2010;31(1):77–84.
Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–46.
Vuong JT, Jacob SA, Alexander KM, Singh A, Liao R, Desai AS, Dorbala S. Mortality from heart failure and dementia in the United States: CDC WONDER 1999–2016. J Card Fail. 2019;25(2):125–9.
Bohlken J, Jacob L, Schaum P, Rapp MA, Kostev K. Hip fracture risk in patients with dementia in German primary care practices. Dementia. 2017;16(7):853–64.
Hou M, Zhang Y, Chen AC, Liu T, Yang H, Zhu X, He F. The effects of dementia on the prognosis and mortality of hip fracture surgery: a systematic review and meta-analysis. Aging Clin Exp Res. 2021;33(12):3161–72.
Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Loop diuretics increase bone turnover and decrease BMD in osteopenic postmenopausal women: results from a randomized controlled study with bumetanide. J Bone Miner Res. 2006;21(1):163–70.
Terrovitis J, Zotos P, Kaldara E, Diakos N, Tseliou E, Vakrou S, Kapelios C, Chalazonitis A, Nanas S, Toumanidis S, et al. Bone mass loss in chronic heart failure is associated with secondary hyperparathyroidism and has prognostic significance. Eur J Heart Fail. 2012;14(3):326–32.
Acknowledgements
The authors sincerely thank the HERMES consortium, the GEFOS consortium, and Morris, J. A. et.al for providing summary statistics.
Funding
This research was supported by grants from the National Natural Science Foundation of China (82170331 and U21A20337), and Key Research and Development Plan of Zhejiang Province (2020C03017).
Author information
Authors and Affiliations
Contributions
Study conception and design: HC; data analyses: HC and RY; draft preparation: HC and RY; supervision of the study: XG; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. Ethical approval and informed consent for studies included in the analyses was provided in the original publications.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. STROBE-MR Checklist [20]. Table S2. Genome-wide significant SNPs associated with HF and their association with BMDs and fracture. Table S3. Evaluation of heterogeneity and directional pleiotropy using different methods.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, H., Ye, R. & Guo, X. Lack of causal association between heart failure and osteoporosis: a Mendelian randomization study. BMC Med Genomics 15, 232 (2022). https://doi.org/10.1186/s12920-022-01385-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-022-01385-8