Abstract
Background
Common marmosets (Callithrix jacchus) are widely used as primate experimental models in biomedical research. Duodenal dilation with chronic vomiting in captive common marmosets is a recently described life-threatening syndrome that is problematic for health control. However, the pathogenesis and cause of death are not fully understood.
Case presentation
We report two novel necropsy cases in which captive common marmosets were histopathologically diagnosed with gastric emphysema (GE) and pneumatosis intestinalis (PI). Marmoset duodenal dilation syndrome was confirmed in each case by clinical observation of chronic vomiting and by gross necropsy findings showing a dilated, gas-filled and fluid-filled descending duodenum that adhered to the ascending colon. A diagnosis of GE and PI was made on the basis of the bubble-like morphology of the gastric and intestinal mucosa, with histological examination revealing numerous vacuoles diffused throughout the lamina propria mucosae and submucosa. Immunostaining for prospero homeobox 1 and CD31 distinguished gas cysts from blood and lymph vessels. The presence of hepatic portal venous gas in case 1 and possible secondary bacteremia-related septic shock in case 2 were suggested to be acute life-threatening abdominal processes resulting from gastric emphysema and pneumatosis intestinalis.
Conclusions
In both cases, the gross and histopathological findings of gas cysts in the GI tract walls matched the features of human GE and PI. These findings contribute to clarifying the cause of death in captive marmosets that have died of gastrointestinal diseases.
Similar content being viewed by others
Background
Common marmosets (Callithrix jacchus) are widely used as primate experimental models in biomedical researches [11]. Chronic recurrent gastrointestinal (GI) diseases are common in laboratory marmoset colonies and are sometimes becoming a life-threatening problem in health control.
Histopathological findings on necropsy have shown inflammatory bowel disease in the forms as chronic lymphocytic enteritis (CLE) and colitis [3, 10]. Duodenal lesions, such as strictures near the major duodenal papilla [17] and proximal duodenal obstruction and dilation (“marmoset duodenal dilation syndrome (MDDS)” [12]), are also considered significant causes of morbidity and mortality in laboratory marmosets [3]. Although many autopsy cases have been reported, further observation and analysis are required to determine the etiology of this disease.
An MDDS is a diagnosis of captive marmosets exhibiting repetitive vomiting, chronic bloating, and exhaustion [12]. The gross morphological criterion of MDDS consists of a dilated duodenum that is filled with a mixture of gas and fluid and whose descending part has a maximum diameter > 12 mm. Along with severe duodenitis, duodenal ulceration and fibrosis, and peritonitis, MDDS can cause adhesions between the duodenum and large intestine. Histopathological examination revealed CLE, cholangitis, cholecystitis, and pancreatitis in the affected marmosets. Multiple factors are suspected, as the etiology of MDDSs involves bacterial infection, ulceration, and fibrosis leading to stricture, as well as dietary factors such as overeating or consuming highly fermentable food; however, the causes of death have not been fully determined [3, 12].
In humans, gastric emphysema (GE) and pneumatosis intestinalis (PI) occur in the GI tract wall through the accumulation of gas from outside the GI tract or the production of gas within the GI wall [1, 6, 20]. Gross findings of bubble-like structures and histopathological findings of gas cysts are typical features of GE and PI [2, 6, 20]. A common clinical sign of GE is frequent vomiting [1, 5, 8], while those of PI are diarrhea, bloody stools, abdominal pain, constipation, weight loss, and tenesmus [19]. In PI, mucosal disruption allows invasion of the flatus and translocation of bacteria into the lesion [6, 19, 20], followed by the formation of hepatic portal venous gas (HPVG) and secondary bacteremia, which are life-threatening, acute abdominal processes [13,14,15, 19].
Here, we report two cases in which captive marmosets developed sudden hypothermia and heavy vomiting with hematemesis after long-term chronic vomiting. Gross and histopathological examination confirmed the diagnosis of MDDS, with concurrent GE and PI. To our knowledge, this is the first report of simultaneous MDDS, GE, and PI in the common marmoset.
Case presentation
Case 1, male, 9 years and 5 months old, 291 g body weight (BW), was euthanized because of sudden severe hypothermia (34.1 °C), seizures, and moderate vomiting (approximately 4–6 ml) with a small amount of blood. It had been treated for 5 years and exhibited frequent vomiting that fluctuated between mild (< 3 ml) and heavy (> 7 ml). Case 1 showed changes in BW with vomiting and tested negative for infectious pathogens (Table 1). Case 2, female, 6 years and 11 months old, 353 g BW, died the day after sudden severe hypothermia (34.7 °C) and heavy vomiting with hematemesis. This marmoset had been treated for frequent, fluctuating, mild to moderate vomiting without persistent diarrhea or weight loss for 2 years, and the frequency of heavy vomiting without hematemesis increased 1 month before its death. Both animals were treated with drugs as needed (Table 1).
Serum biochemical tests (Table 2) and complete blood counts (CBCs; Table 3) were performed for both animals. The last serum biochemistry panel before euthanasia suggested chronic maldigestion (hypoalbuminemia) and acute renal injury (azotemia, markedly elevated creatinine, hyperglycemia, hypercalcemia, hyperphosphatemia, and low Na/K ratio) in case 1 and mild maldigestion (slight hypoalbuminemia, reduced blood urea nitrogen, and creatinine) in case 2 (Table 2). We did not perform any antemortem imaging.
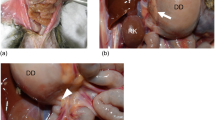
The necropsy of case 1 was performed soon after euthanasia. The stomach and proximal to the descending parts of the duodenum were markedly dilated (18.3 mm in diameter at the proximal duodenum; reference interval of our laboratory (n = 7): 7.0 mm ± 1.6 mm), as measured before opening the lumen, accompanying intraabdominal fibrous adhesion among the ascending colon, stomach, and descending part of the duodenum. The necropsy of case 2 was performed the morning after she died. The diameter of the region proximal to the descending part of the duodenum was 21.0 mm, with fibrous adhesions formed among the stomach, duodenum, and colon (Fig. 1a, b). A sanguineous exudate was retained in the lumen of the dilated stomach and duodenum. The findings of odd-shaped, edematous gastric mucosa with diffuse numerous bubble-like structures (Fig. 1b) matched the typical features of human GE and PI [2, 6, 20]. Thus, in addition to the major symptoms of vomiting and hypoalbuminemia, the gross necropsy findings in both cases were compatible with the diagnostic criteria of MDDS [3, 12].
Marmoset duodenal dilation syndrome, case 2. a Marked dilation of the stomach (S) and duodenum (D). The ascending colon (C) tightly adheres to the stomach (arrowhead) and duodenum (arrow). The peritoneum between the colon and stomach is thickened by fibrosis. The abdominal organs are slightly pale reddish in color, indicating gastritis, and the GI tract is bloated. b Inside of the dilated stomach. A bloody exudate is retained. Odd-shaped, edematous gastric mucosa with diffuse numerous bubble-like structures (gas cysts characteristic of gastric emphysema)
After gross examination, the tissues of both animals were immediately fixed in 10% neutral buffered formalin, and then paraffin-embedded 5-μm-thick staining sections were prepared at a commercial laboratory for histopathological examination. In case 1, numerous diffusely distributed vacuoles of various sizes were observed in the lamina progeria mucosae, submucosa, and subserosa of the stomach and duodenum (Fig. 2a,b, and d). Gastritis with ulcers reached the submucosa. Dilated and congested portal veins in Glisson’s capsules of the liver were also observed, which indicated HPVG (Fig. 2e).
Gastric emphysema and pneumatosis intestinalis, marmoset, HE. a, b Stomach, case 1. c Stomach, case 2. d Duodenum, case 1. The gastric and duodenal mucosa contain numerous empty spaces, which are gas cysts. e Liver, case 1. Abnormal dilation of the portal vein suggests hepatic portal venous gas. f Liver, case 2. There are numerous rods, neutrophil infiltration, and liver cell necrosis around the main trunk of the portal vein
In case 1, immunohistochemical staining for CD31 and prospero homeobox 1 (Prox1) was performed to distinguish the conditions from chronic lymphocytic enteritis, lymphangioma, and lymphangiectasis. The results showed that the walls of diffusely distributed vacuoles were mostly negative, and some vacuoles with positive endothelial cells were blood and lymph vessels (Fig. 3a, b). Thus, the vacuoles were gas cysts [6, 20] without an endothelium-lined cystic structure and were unlikely to have developed from lymph vessels.
Gas cysts identified by immunohistochemistry, case 1. Immunohistochemistry was performed with a mouse anti-human CD31 monoclonal antibody (clone JC70A) and a mouse anti-human prospero homeobox 1 (Prox1) monoclonal antibody (clone 1D4B1). The amino acid sequences of human CD31 and Prox1 almost completely match those of C. jacchus CD31 (90%) and Prox1 (100%), as indicated by Standard Protein Blast (NIH, Bethesda, MD, USA). a) Stomach. b) Duodenum. Gas cysts (asterisk) are observed in the mucosa, independent of the blood and lymph vessels (v and ly, respectively). Note that the nuclei of the lymphatic endothelial cells as well as some of the enteric epithelial cells and pancreatic acinar cells are positive, but vascular endothelial cells and gas cyst walls are negative. The universal immunoenzyme polymerase method is visualized with DAB (Histofine; Nichirei, Tokyo, Japan)
Case 1 showed marked congestion of the liver (with centrilobular congestion and liver cell necrosis) and bilateral kidneys. Mild chronic hepatitis with slight fibrosis, hemosiderin deposition in the liver, and acute and chronic cholecystitis, which are common histopathological findings in MDDS [12], were also observed. Except for cholecystitis, these findings indicated circulatory failure, consistent with acute renal failure, as suggested by the serum biochemistry results.
In case 2, numerous diffusely distributed vacuoles of various sizes were observed in the propria mucosae and submucosa of the stomach and duodenum and in the jejunum (Fig. 2c). In the liver, bacteremia with numerous rods, neutrophil infiltration, and liver cell necrosis around the main trunk of the portal vein were found (Fig. 2f). Circulatory failure due to septic shock was suspected from similar findings of liver and kidney congestion in case 1. The presence of neutrophilic infiltration indicated an inflammatory response to septicemia.
Discussion and conclusions
Here, we present the first report of GE and PI in marmosets. The gross and histopathological findings in both cases matched those of human GE and PI [2, 6, 20]. Numerous vacuoles were histopathologically observed in the stomach and duodenum, and immunohistochemistry confirmed gas cysts. Chronic vomiting had been observed for several years before sudden death. The stomachs and the duodenal segments proximal to the descending region were markedly dilated, accompanied by intra-abdominal fibrous adhesions. Taken together, these findings consistently supported the diagnosis of GE and PI in animals affected by MDDS.
In case 1, gas cysts were distributed in the stomach and duodenum, with gastric ulceration and dilated and congested portal veins. In case 2, the gas cysts reached the jejunum without gastric ulcers, but additional bacteremia was observed. HPVG can occur by high-pressure gas in the GI tract or alteration of the GI mucosa, allowing the gas and intestinal bacteria to enter the portal system through the mesenteric veins [12-14, 18]. Increased intraluminal pressure in MDDSs might lead to emphysema through gas migration into the GI mucosa and submucosa via ulcers and inflow to the portal vein. With these conditions, the animals could have fallen into shock, leading to different outcomes in case 1 (euthanasia) and in case 2 (unexpected death) depending on the site of gas inflow to the portal vein. Therefore, GE and PI in common marmosets constitute fatal conditions in MDDS.
We attempted to identify the etiology of GE in marmosets by considering its etiology in humans, with the assumption that GE and PI are the primary lesions in MDDS. Human GE is subclassified into traumatic (mechanical or nonmechanical), obstructive (secondary to malignancy, stricture, volvulus, pyloric stenosis, or duodenal stenosis), and pulmonary (primary) types depending on the etiology [1, 8]. The nonmechanical mucosal traumatic type was the most likely in our cases because its causes included ischemia of the GI tract and gastric dilation in eating disorders [5, 8]. Case 1 showed bloating and vomiting after binge-eating pelleted food and presented with cholecystitis, which is associated with diet [4] and was supposed to be the primary lesion in MDDS [12]. Alterations in the gut microbiome are associated with duodenal stricture [16, 17]. Small intestinal bacterial overgrowth, involving an abnormal increase in the overall bacterial population in the small intestine, could cause gas accumulation in the GI tract. Among the factors influencing the gut microbiome [16], diet was a likely etiology of MDDS in these cases, and GE may have occurred secondary to gastric mucosal damage, similar to what has been observed in human patients [5, 8, 9].
Fatal Clostridium (C.) perfringens infections have been reported in marmosets [18, 21]. C. perfringens type A toxin was demonstrated in cases of gas gangrene (clostridial myonecrosis) [21] and acute gastric dilation [18], with histopathological findings of not only bacterial enteritis but also subcutaneous, muscle, liver, and lung lesions. Although we did not observe C. perfringens infections, these findings did not apply in case 2. Thus, the GE and PI observed in the present cases were unlikely to have occurred as a primary infection [21] or secondary overgrowth [18] of C. perfringens.
In conclusion, we report the first cases of simultaneous MDDS, GE, and PI in two captive marmosets. Gross and histopathological findings of gas cysts in the GI tract walls matched the features of human GE and PI. In marmosets with MDDS caused by GE and PI, HPVG and/or secondary bacteremia could lead to hypothermia and shock, causing congestion of the liver and kidney and resulting in death. However, additional cases are needed to determine the prevalence and pathogenesis of GE and PI in MDDSs.
Availability of data and materials
All the data generated or analyzed during this study are included in this article.
Change history
12 June 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12917-024-04117-5
Abbreviations
- BW:
-
Body weight
- CLE:
-
Chronic lymphocytic enteritis
- GE:
-
Gastric emphysema
- GI:
-
Gastrointestinal
- HPVG:
-
Hepatic portal venous gas
- MDDS:
-
Marmoset duodenal dilation syndrome
- PI:
-
Pneumatosis intestinalis
References
Agha FP. Gastric emphysema: an etiologic classification. Australas Radiol. 1984;28:346–52. https://doi.org/10.1111/j.1440-1673.1984.tb02363.x.
Cordum NR, Dixon A, Campbell DR. Gastroduodenal pneumatosis: endoscopic and histological findings. Am J Gastroenterol. 1997;92:692–5.
Fitz C, Goodroe A, Wierenga L, Mejia A, Simmons H. Clinical Management of Gastrointestinal Disease in the Common Marmoset (Callithrix jacchus). ILAR J. 2020;61:199–217. https://doi.org/10.1093/ilar/ilab012.
Freston JW, Bouchier IA. Experimental cholelithiasis Gut. 1968;9:2–4. https://doi.org/10.1136/gut.9.1.2.
Inayat F, Zafar F, Zaman MA, Hussain Q. Gastric emphysema secondary to severe vomiting: a comparative review of 14 cases. BMJ Case Rep. 2018;bcr2018226594. https://doi.org/10.1136/bcr-2018-226594.
Koss LG. Abdominal gas cysts (pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53:523–49.
Kramer R, Burns M. Normal Clinical and Biological Parameters of the Common Marmoset (Callithrix jacchus). Part II: Clinical Pathology In: Marini R, Wachtman L, Tardif S et al., eds. The Common Marmoset in Captivity and Biomedical Research. Academic Press; 2019. p. 96–100.
Lee S, Rutledge JN. Gastric emphysema. Am J Gastroenterol. 1984;79:899–904.
Low VH, Thompson RI. Gastric emphysema due to necrosis from massive gastric distention. Clin Imaging. 1995;19:34–6. https://doi.org/10.1016/0899-7071(94)00011-z.
Ludlage E, Mansfield K. Clinical care and diseases of the common marmoset (Callithrix jacchus). Comp Med. 2003;53:369–82.
Mansfield K. Marmoset models commonly used in biomedical research. Comp Med. 2003;53:383–92.
Mineshige T, Inoue T, Yasuda M, Yurimoto T, Kawai K, Sasaki E. Novel gastrointestinal disease in common marmosets characterised by duodenal dilation: a clinical and pathological study. Sci Rep. 2020;10:3793. https://doi.org/10.1038/s41598-020-60398-4.
Morris MS, Gee AC, Cho SD, et al. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195:679–82. https://doi.org/10.1016/j.amjsurg.2008.01.011. PMID: 18424288.
Nault I, Lauzon C. Gas in the portomesenteric vessels from nonocclusive ischemic bowel disease. CMAJ. 2007;176:321–3. https://doi.org/10.1503/cmaj.060807.
Peloponissios N, Halkic N, Pugnale M, et al. Hepatic portal gas in adults: review of the literature and presentation of a consecutive series of 11 cases. Arch Surg. 2003;138:1367–70. https://doi.org/10.1001/archsurg.138.12.1367.
Sheh A. The Gastrointestinal Microbiota of the Common Marmoset (Callithrix jacchus). ILAR J. 2020;61:188–98. https://doi.org/10.1093/ilar/ilaa025.
Sheh A, Artim SC, Burns MA, et al. Alterations in common marmoset gut microbiome associated with duodenal strictures. Sci Rep. 2022;12:5277. https://doi.org/10.1038/s41598-022-09268-9.
Stein FJ, Lewis DH, Stott GG, Sis RF. Acute gastric dilatation in common marmosets (Callithrix jacchus). Lab Anim Sci. 1981;31:522–3.
St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138:68–75. https://doi.org/10.1001/archsurg.138.1.68.
Turnure PR IV. Gas Cysts of the Intestine: Pneumatosis Cystoides Intestinorum Hominis. Ann Surg. 1913;57:811–39. https://doi.org/10.1097/00000658-191306000-00005.
Yasuda M, Inoue T, Ueno M, Morita H, Hayashimoto N, Kawai K, Itoh T. A case of nontraumatic gas gangrene in a common marmoset (Callithrix jacchus). J Vet Med Sci. 2016;77:1673–6. https://doi.org/10.1292/jvms.15-0210.
Acknowledgements
We thank Takafumi Ogawa (Kyodobyori, Inc.) and Masahiro Yamaguchi (Kyodobyori, Inc.) for their technical assistance with the histopathological and immunohistochemical examinations.
Funding
Grants of Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) JP18dm0207001 at RIKEN from Japan Agency for Medical Research and Development (to AI), Grant-in-Aid for Scientific Research on Innovative Areas JP19H05736 “Integrative Human Historical Science of ‘Out of Eurasia’” at RIKEN (to AI). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
YS, SK, and YY prepared the manuscript. YY contributed to patient care, clinical examinations, and treatments. YS and SK provided clinical and pathological advice, respectively, based on medical and veterinary statements. AI organized the experimental research in the marmoset colony, kept the marmoset colony in his laboratory, coordinated the writing of the manuscript, and supervised the project. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal care and procedures involved in this case report were in accordance with the Guidelines for Conducting Animal Experiments of RIKEN and approved by the Animal Experiment Committees at RIKEN Center for Biosystems Dynamics Research (A2018-08).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kawarai, S., Sakai, Y., Iriki, A. et al. Gastric emphysema and pneumatosis intestinalis in two common marmosets with duodenal dilation syndrome. BMC Vet Res 20, 223 (2024). https://doi.org/10.1186/s12917-024-04087-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-04087-8