Abstract
Infectious bovine rhinotracheitis (IBR) is a major animal health hazard in many countries throughout the world, caused by bovine herpesvirus-1 (BoHV-1). The study’s goal was to evaluate the prevalence of BoHV-1 seropositivity among dromedary camels in three governorates in northern Egypt, as well as to identify risk variables related with BoHV-1 seropositivity. A total of 321 blood samples were collected randomly from dromedary camels living in the selected governorates and examined for presence of BoHV-1 antibody using ELISA test. The overall seroprevalence of BoHV-1 among examined camels was 5.92% (95%CI: 3.82–9.06). Univariable analysis confirmed that the significant association (P < 0.05) between sex, history of abortion, contact with small ruminants and herd size and BoHV-1 seropositivity. Using multiple logistic regression analysis, the following risk factors were identified to be related with the presence of BoHV-1 infection: sex (OR = 2.54, 95%CI: 0.63–10.22), history of abortion (OR = 4.16, 95%CI: 1.30–13.27), contact with small ruminants (OR = 5.61, 95%CI: 1.67–18.80) and large herd size (OR = 10.52, 95%CI: 2.46–44.91). This study estimated the disease’s seroprevalence in Egyptian dromedary camels, implying that camels could act as a BoHV-1 reservoir for transmission to other species.
Similar content being viewed by others
Introduction
Infectious bovine rhinotracheitis (IBR) is one of the most economically important diseases of ruminants with a global distribution [1]. Bovine herpes virus-1 (BoHV-1) is the causal agent of IBR, is a member of the family Herpesviridae and belongs to the genus Varicellovirus, subfamily Alphaherpesvirinae [2]. BoHV-1 has three subtypes (BoHV-1.1, BoHV-1.2a, and BoHV-1.2b) that can cause a variety of disease forms, including respiratory infections (nasal discharges, respiratory manifestations and conjunctivitis), reproductive disorders in both males and females (balanoposthitis, vulvovaginitis and abortions), fever, loss of appetite, decreased milk yield and neonatal mortalities [3,4,5]. BoHV-1 infection can cause immunosuppression, which raises the risk of subsequent bacterial infections accompanying with respiratory infections [6, 7]. The virus transmitted mostly through inhalation or vaginal contact and can survive for a long period within populations because of its ability to become latent, reactivate, and quickly transmit among animals maintained in intense production units [8, 9].
Several studies in Egypt have reported the presence of BoHV-1 in cattle and buffaloes of different ages [10], but there are few records of the virus’s exposure in camels. Camels in Iran, Tunisia and Egypt have shown serological evidence of BoHV-1 exposure [11,12,13]. The virus was isolated from dromedary camels’ lung in Egypt and Sudan [14, 15], and was detected in llamas’ lungs in North America [16].
Egypt is a country with a low camel breeding population. However, large numbers of camels are imported each year from Sudan and Somalia for meat consumption [17]. Previous studies were provided that Sudanese camels are naturally exposed to BoHV-1, and the infection was confirmed using ELISA and FAT [15].
Therefore, this study investigates the epidemiology of BoHV-1 seropositivity in dromedary camels in some Egyptian governorates with large camel populations, as well as the risk factors related with BoHV-1 infection.
Materials and methods
Ethical statement
The Benha University ethical committee for animal studies approved all operations involving the handling and collection of blood samples (approval No: BUFVTM02–03-22). The owners of the camels gave their informed agreement and permission to collect the samples. The ethical committee at Benha University’s Faculty of Veterinary Medicine approved all procedures involving laboratory animals, which were carried out in compliance with current standards and regulations. The ARRIVE guidelines were followed when doing this research.
Study area
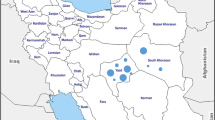
This study was conducted in three governorates in Northern Egypt (Cairo, Kafr ElSheikh, and Qalyubia). These governorates are geographically located at 30°02′N 31°13′E, 31.3°N 30.93°E and 30.41°N 31.21°E (Fig. 1). The climate is arid, desert climate, with summers that are lengthy, hot, humid, and dry, and winters that are cold, dry, and clearer. The temperature ranging from 25 to 40 °C in summer and 10–25 °C in winter.
Sample size and samples collection
The minimum sample size was calculated using a random sampling approach using the Thrusfield formula [18], assuming a 2.8% of prevalence as previously reported by Eisa [12], a confidence level of 95, and 5% precision.
Where n is the sample size, Z is standard normal distribution values (Z= 1.96), P is the predicted prevalence, and d2 is the absolute precision.
A total of 321 blood samples were collected randomly from camels living in three governorates at Northern Egypt. A total of 10 mL of blood was obtained through jugular venipuncture. Blood samples were stored at room temperature for 3 h to allow clotting before being centrifuged at 3.000 rpm for 15 minutes. The sera samples were separated and kept at − 20 °C until serological analysis.
Animals
The sera were collected from free-ranging camels raising with sheep and goats, and these animals were used mainly for meat and milk production and breeding. Additionally, all camels included in this study were dromedary camels. An epidemiological questionnaire was used to analyse the potential risk factors for BoHV-1 in this study, which was administered to all the selected camel owners. The questionnaire was created to identify the most essential variables that could be related to BoHV-1 infection: 1) camels data such as sex (male, female), age (1–3, 4–9, 10–15 and > 15 years), and number of calving; 2) herd data like location, herd size (0–70, 71–140 and > 140), contact with small ruminant and history of abortion.
Serological analysis
Serological investigation of antibodies against BoHV-1 in sera of sampled camels was performed using a competitive ELISA (ID Screen® IBR gB competition multi-species kits, IDVet, France) according to the manufacturer’s guidelines and protocols. The findings were represented as optical density (OD) and the absorbance was measured at 450 nm with an AllSheng ELISA microplate reader (AllSheng instrument Inc., Zhejiang, China). The proportion of inhibition of sample/negative control (S/N) was calculated to ascertain the result. If the S/N percent was equal to or less than 50%, the sample was declared positive for BHV-1gB.
Statistical analysis
The data was analysed statistically using SPSS version 24 software (SPSS Inc., Chicago, IL, USA). Pearson’s Chi-square test was used to determine the association between BoHV-1 seropositivity and different risk factors (locality, sex, age, history of abortion, contact with small ruminants and herd size). All variables with P-value less than 0.25 in the univariate analysis were fitted for multivariate logistic regression models [19, 20]. Variables with P-value less than 0.05 estimated significant and included in the final model. The Hosmer and Lemeshow goodness-of-fit test was used to evaluate the model fit.
Results
The percentage of camels with anti-BoHV-1 serum antibodies represented in Table 1. Out of 321 examined camels, 19 animals were seropositive, resulting in a prevalence of 5.92% (95%CI: 3.82–9.06). The prevalence rate of BoHV-1 in studied area showed non-disparity and the disease was mostly prevalent in Cairo (6.59%) and Kafr ElSheikh (6.84%) governorates. Univariate analysis was used to identify potential risk variables for BoHV-1 infection, Table 1. There was no correlation between seroprevalence of BoHV-1 in camels and age or calving number (P > 0.05). Moreover, the seroprevalence of BoHV1 increased significantly in females (9.68%) with history of abortion (13.48%), mainly in case of contact with small ruminants (9.80%), Table 1.
In the final multivariable analysis, the factors associated with seropositivity for BoHV-1 infection shown in Table 2. Females were 2.54 times more likely than males to have BoHV-1 antibody. Also, the results proved that history of abortion (OR = 4.16, 95%CI: 1.30–13.27), contact with small ruminants (OR = 5.61, 95%CI: 1.67–18.80) and large herd size > 140 (OR = 10.52, 95%CI: 2.46–44.91) were associated with BoHV-1 seropositivity, Table 2.
Discussion
BoHV-1 is a globally distributed disease with significant geographical variation in incidence. Several researches have been conducted using serological surveys to detect risk variables for BoHV-1 seropositivity in ruminants [21,22,23,24,25] but few studies have been performed in camels especially in Egypt [12]. The present work identified the seroprevalence of BoHV-1 in camels in some Egyptian governorates and assessed the associated risk factors.
The detected seroprevalence for BoHV-1 antibodies in camels in this study was found 5.92%, which was lower than studies in other parts of Sudan of 76.9% [15] and Saudi Arabia of 13% [26]. The seroprevalence of this study lie in range of previous study from Tunisia of 5.8% [11] and Algeria of 3.7% [27] but higher than previous rate reported in Iran of 0% [13].
Interestingly, there is significant disparities in Egypt regarding BoHV-1 seroprevalence rates in camels. Two studies found greater rates of seroprevalence than our reported rate 14.2% [28] and 18.2% [29], while a third one found a lower rate of seropositivity 2.8% in farm samples and 2.1% in samples from abattoir [12].
The disparities in antibody seroprevalence observed in different locations and countries could be attributed to some factors such as sample size, analyze test, herd size differences, breeding practices, production systems, disease-control strategies, and age of camels [30,31,32,33,34,35].
The present findings revealed that the seroprevalence rate of BoHV-1 is significant higher in females especially with one to two calving. Similar results have been reported previously by Benaissa et al. [27] and Tadeg et al. [36]. This might be explained due to stress factors related to female as pregnancy, lactation or due to frequent exposure to infection from male during breeding [23, 37,38,39].
The findings are consistent with prior research, which found that the seroprevalence of BoHV-1 was strongly associated with abortion [36], they found that cow with history of abortion was at a risk to get BoHV-1 infection than non-aborted animals. Therefore, those results confirmed that the reproductive problem increase the risk of BoHV-1 infection in camels [40]. Although a positive ELISA test reveals that an animal was exposed to the BoHV-1 virus during its life, it does not prove that the virus was the cause of the abortion because it is not a direct diagnostic approach [41]. For such reasons, direct diagnostic procedures such as antigen detection ELISA or PCR would be necessary [42].
Moreover, in the line with previous findings of Benaissa, Youngs, Mimoune, Faye, Mimouni and Kaidi [27], the seroprevalence of BoHV-1 was more frequent among camels of median age group (4–9 years). This could be explained by repeated virus exposure over time, particularly in old animals or due to presence of persistent infected animals among studied animals [43,44,45,46,47,48]. In contrast, age of the animal has been identified as a risk factor for seropositivity of BoHV-1 in cattle [49, 50].
The studied camels had the chance to come into contact with small ruminants that were BoHV-1-positive due to the shared grazing area, which enhanced the risk of infection [51, 52]. Similar conclusion was reached by Benaissa et al. [27].
Importantly, a larger herd was substantially associated with a high prevalence of BoHV1 infection, which is consistent with earlier research of Solis-Calderon et al. [53]. They found that a larger herd, particularly one with a high population density, is linked to a higher prevalence of IBR.
Conclusion
In Egypt, the BoHV-1 virus is circulating among camels, infecting a large number of animals. Contact with other small ruminants, sex, a history of abortion, and herd size are all risk factors for IBR infection in camels. Age and number of calving had not significant role in prevalence of the disease. More molecular and epidemiological investigations of BoHV-1 in camels are needed to assess the virus’s epidemiological and clinical implications in Egypt, estimate the true prevalence, and characterize the virus’s circulation.
Availability of data and materials
This article contains all of the data that was created or analyzed throughout the investigation.
References
Graham DA. Bovine herpes virus-1 (BoHV-1) in cattle–a review with emphasis on reproductive impacts and the emergence of infection in Ireland and the United Kingdom. Ir Vet J. 2013;66:1–12.
Muylkens B, Thiry J, Kirten P, Schynts F, Thiry E. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Vet Res. 2007;38:181–209.
Raaperi K, Orro T, Viltrop A. Epidemiology and control of bovine herpesvirus 1 infection in Europe. Vet J. 2014;201:249–56.
Nandi S, Kumar M, Yadav V, Chander V. Serological evidences of bovine Herpesvirus-1 infection in bovines of organized farms in India. Transbound Emerg Dis. 2011;58:105–9.
Dias J, Alfieri A, Ferreira-Neto J, Gonçalves V, Muller E. Seroprevalence and risk factors of bovine herpesvirus 1 infection in cattle herds in the state of Paraná, Brazil. Transbound Emerg Dis. 2013;60:39–47.
Jones C, Chowdhury S. A review of the biology of bovine herpesvirus type 1 (BHV-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines. Anim Health Res Rev. 2007;8:187–205.
Reisberg K, Selim AM, Gaede W. Simultaneous detection of Chlamydia spp., Coxiella burnetii, and Neospora caninum in abortion material of ruminants by multiplex real-time polymerase chain reaction. J Vet Diagn Investig. 2013;25:614–9.
İnce ÖB, Şevik M. Risk assessment and seroprevalence of bovine herpesvirus type 1 infection in dairy herds in the inner Aegean region of Turkey. Comp Immunol Microbiol Infect Dis. 2022;80:101741.
Adeli E, Pourmahdi Borujeni M, Haji Hajikolaei MR, Seifi Abad Shapouri M. Bovine Herpesvirus-1 in Khouzestan province in Iran: seroprevalence and risk factors. Iran J Rumin Health Res. 2017;2:47–56.
Hekal SHA, Al-Gaabary MH, El-Sayed MM, Sobhy HM, Fayed AAA. Seroprevalence of some infectious transboundry diseases in cattle imported from Sudan to Egypt. J Adv Vet Anim Res. 2019;6:92.
Burgemeister R, Leyk W, Goessler R. Investigations on the occurrence of parasites and of bacterial and virus infections in Southern Tunisian dromedaries, Deutsche Tieraerztliche Wochenschrift (Germany, FR); 1975.
Eisa M. Serological survey against some viral diseases in camels in Sharkia governorate, Egypt. In: Proceedings of the Third Annual Meeting for Animal Production Under Arid Conditions; 1998.
Raoofi A, Hemmatzadeh F, Ghanaei AM. Serological survey in camels (Camelus dromedarius) to detect antibodies against bovine herpesvirus type-1 and Mycobacterium avium paratuberculosis in Iran. J Camel Pract Res. 2012;19:65–8.
Nawal M, Gabry G, Hussien M, Omayma A. Occurrence of parainfluenza TYPE-3 (pi-3) and bovine herpes virus type-I (BHV-I) viruses (mixed infection) among camels. Egypt J Agric Res. 2003;81:781–92.
Intisar K, Ali Y, Khalafalla A, Mahasin ER, Amin A. Natural exposure of dromedary camels in Sudan to infectious bovine rhinotracheitis virus (bovine herpes virus-1). Acta Trop. 2009;111:243–6.
Williams J, Evermann J, Beede R, Scott E, Dilbeck P, Whetstone C, et al. Association of bovine herpesvirus type 1 in a llama with bronchopneumonia. J Vet Diagn Investig. 1991;3:258–60.
Klemmer J, Njeru J, Emam A, El-Sayed A, Moawad AA, Henning K, et al. Q fever in Egypt: epidemiological survey of Coxiella burnetii specific antibodies in cattle, buffaloes, sheep, goats and camels. Plos One. 2018;13:e0192188.
Thrusfield M. Veterinary epidemiology. Wiley; 2018.
Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models; 2006.
Selim AM, Elhaig MM, Moawed SA, El-Nahas E. Modeling the potential risk factors of bovine viral diarrhea prevalence in Egypt using univariable and multivariable logistic regression analyses. Vet World. 2018;11:259.
Lucchese L, Benkirane A, Hakimi I, El Idrissi A, Natale A. Seroprevalence study of the main causes of abortion in dairy cattle in Morocco. Vet Ital. 2016;52:3–19.
Nikbakht G, Tabatabaei S, Lotfollahzadeh S, Nayeri Fasaei B, Bahonar A, Khormali M. Seroprevalence of bovine viral diarrhoea virus, bovine herpesvirus 1 and bovine leukaemia virus in Iranian cattle and associations among studied agents. J Appl Anim Res. 2015;43:22–5.
Selim A, Abdelhady A. The first detection of anti-West Nile virus antibody in domestic ruminants in Egypt. Trop Anim Health Prod. 2020;52:3147–51.
Selim A, Manaa E, Khater H. Seroprevalence and risk factors for lumpy skin disease in cattle in northern Egypt. Trop Anim Health Prod. 2021;53:1–8.
Selim A, Marawan MA, Ali A-F, Manaa E, AbouelGhaut HA. Seroprevalence of bovine leukemia virus in cattle, buffalo, and camel in Egypt. Trop Anim Health Prod. 2020;52:1207–10.
Al-Afaleq A, Abu-Elzein E, Hegazy A, Al-Naeem A. Surveillance of camels (Camelus dromedarius) to detect antibodies against viral diseases in Saudi Arabia. J Camel Pract Res. 2007;14:91–6.
Benaissa MH, Youngs CR, Mimoune N, Faye B, Mimouni FZ, Kaidi R. First serological evidence of BHV-1 virus in Algerian dromedary camels: Seroprevalence and associated risk factors. Comp Immunol Microbiol Infect Dis. 2021;76:101638.
Elbayoumy MK, Allam AM, Albehwar AM, Elsayed EL. Investigation of the immune status of camels (Camelus dromedarius) against some viral diseases. Alex J Vet Sci. 2013;39:12–7.
Darwish SF, Hassanien TK, Salem HA. Patho-molecular studies on the occurrence of bovine herpes virus type 1 in the genitalia of she camels. Alexandria J Vet. 2015;46:130–7.
Ackermann M, Engels M. Pro and contra IBR-eradication. Vet Microbiol. 2006;113:293–302.
Selim A, Attia K, Ramadan E, Hafez YM, Salman A. Seroprevalence and molecular characterization of Brucella species in naturally infected cattle and sheep. Prev Vet Med. 2019;171:104756.
Selim A, Megahed AA, Kandeel S, Abdelhady A. Risk factor analysis of bovine leukemia virus infection in dairy cattle in Egypt. Comp Immunol Microbiol Infect Dis. 2020;72:101517.
Selim A, Abdelhady A. Neosporosis among Egyptian camels and its associated risk factors. Trop Anim Health Prod. 2020;52:3381–5.
Selim A, Alafari HA, Attia K, AlKahtani MD, Albohairy FM, Elsohaby I. Prevalence and animal level risk factors associated with Trypanosoma evansi infection in dromedary camels. Sci Rep. 2022;12:1–8.
Selim A, Ali A-F. Seroprevalence and risk factors for C. burentii infection in camels in Egypt. Comp Immunol Microbiol Infect Dis. 2020;68:101402.
Tadeg WM, Lemma A, Yilma T, Asgedom H, Reda AA. Seroprevalence of infectious bovine rhinotracheitis and brucellosis and their effect on reproductive performance of dairy cattle. J Vet Med Anim Health. 2021;13:106–13.
Elhaig MM, Selim A, Mandour AS, Schulz C, Hoffmann B. Prevalence and molecular characterization of peste des petits ruminants virus from Ismailia and Suez, Northeastern Egypt, 2014–2016. Small Rumin Res. 2018;169:94–8.
Selim A, Ali A-F, Ramadan E. Prevalence and molecular epidemiology of Johne’s disease in Egyptian cattle. Acta Trop. 2019;195:1–5.
Selim A, Attia KA, Alsubki RA, Kimiko I, Sayed-Ahmed MZ. Cross-sectional survey on Mycobacterium avium Subsp. paratuberculosis in dromedary camels: Seroprevalence and risk factors. Acta Trop. 2022;226:106261.
Krishnamoorthy P, Patil S, Shome R, Rahman H. Seroepidemiology of infectious bovine rhinotracheitis and brucellosis in organized dairy farms in southern India; 2015.
Alvarez J, Perez A, Mardones F, Pérez-Sancho M, García-Seco T, Pagés E, et al. Epidemiological factors associated with the exposure of cattle to Coxiella burnetii in the Madrid region of Spain. Vet J. 2012;194:102–7.
Muskens J, Van Engelen E, Van Maanen C, Bartels C, Lam T. Prevalence of Coxiella burnetii infection in Dutch dairy herds based on testing bulk tank milk and individual samples by PCR and ELISA. Vet Rec. 2011;168:79.
Segura-Correa J, Zapata-Campos C, Jasso-Obregón J, Martinez-Burnes J, López-Zavala R. Seroprevalence and risk factors associated with bovine herpesvirus 1 and bovine viral diarrhea virus in North-Eastern Mexico. Open Vet J. 2016;6:143–9.
Selim A, Radwan A. Seroprevalence and molecular characterization of West Nile virus in Egypt. Comp Immunol Microbiol Infect Dis. 2020;71:101473.
Selim A, Radwan A, Arnaout F, Khater H. The recent update of the situation of West Nile fever among equids in Egypt after three decades of missing information. Pak Vet J. 2020;40.
Selim A, Manaa EA, Alanazi AD, Alyousif MS. Seroprevalence, risk factors and molecular identification of bovine leukemia virus in Egyptian cattle. Animals. 2021;11:319.
Selim A, El-Haig M, Galila ES, Geade W. Direct detection of Mycobacterium avium subsp. Paratuberculosis in bovine milk by multiplex Real-time PCR. Anim Sci Papers Rep. 2013;31:291–302.
Selim AM, Elhaig MM, Gaede W. Development of multiplex real-time PCR assay for the detection of Brucella spp., Leptospira spp. and Campylobacter foetus. Vet Ital. 2014;50:75.
Guarino H, Nunez A, Repiso M, Gil A, Dargatz D. Prevalence of serum antibodies to bovine herpesvirus-1 and bovine viral diarrhea virus in beef cattle in Uruguay. Prev Vet Med. 2008;85:34–40.
Woodbine KA, Medley GF, Moore SJ, Ramirez-Villaescusa AM, Mason S, Green LE. A four year longitudinal sero-epidemiological study of bovine herpesvirus type-1 (BHV-1) in adult cattle in 107 unvaccinated herds in south West England. BMC Vet Res. 2009;5:1–12.
Yeşilbağ K, Güngör B. Antibody prevalence against respiratory viruses in sheep and goats in North-Western Turkey. Trop Anim Health Prod. 2009;41:421–5.
Gür S, Erol N, Yapıcı O, Kale M, Tan MT, Turan T, et al. The role of goats as reservoir hosts for bovine herpes virus 1 under field conditions. Trop Anim Health Prod. 2019;51:753–8.
Solis-Calderon J, Segura-Correa V, Segura-Correa J, Alvarado-Islas A. Seroprevalence of and risk factors for infectious bovine rhinotracheitis in beef cattle herds of Yucatan, Mexico. Prev Vet Med. 2003;57:199–208.
Acknowledgements
The authors extend their appreciation to Researchers Supporting Project number (RSP-2021/369), King Saud University, Riyadh, Saudi Arabia.
Funding
This research was funded by King Saud University, Riyadh, Saudi Arabia, grant number RSP-2021/369.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing-original draft preparation, A.S. and S.S., R.A., F.A. and K.A.; writing-review and editing, A.S. R.A., F.A., I. K and S.S.; project administration, A.S., K.A., F.A., R.A. and S.S.; funding acquisition, A.S., K.A., F.A. and S.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Benha University ethical committee for animal studies approved all operations involving the handling and collection of blood samples (approval No: BUFVTM02–03-22). The informed consent was obtained from owners of the camels to collect the samples. The ethical committee at Benha University’s Faculty of Veterinary Medicine approved all procedures involving laboratory animals, which were carried out in compliance with current standards and regulations. The ARRIVE guidelines were followed when doing this research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Selim, A., Shoulah, S., Alsubki, R.A. et al. Sero-survey of bovine herpes virus-1 in dromedary camels and associated risk factors. BMC Vet Res 18, 362 (2022). https://doi.org/10.1186/s12917-022-03448-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-022-03448-5