Abstract
Background
Porcine teschovirus (PTV) circulates among wild and domesticated pig populations without causing clinical disease, however neuroinvasive strains have caused high morbidity and mortality in the past. In recent years, several reports appeared with viral agents as a cause for neurologic signs in weanling and growing pigs among which PTV and new strains of PTV were described.
Case presentation
On two unrelated pig farms in the Netherlands the weanling pig population showed a staggering gate, which developed progressively to paresis or paralysis of the hind legs with a morbidity up to 5%. After necropsy we diagnosed a non-suppurative encephalomyelitis on both farms, which was most consistent with a viral infection. PTV was detected within the central nervous system by qPCR. From both farms PTV full-length genomes were sequenced, which clustered closely with PTV-3 (98%) or PTV-11 (85%). Other common swine viruses were excluded by qPCR and sequencing of the virus.
Conclusion
Our results show that new neuroinvasive PTV strains still emerge in pigs in the Netherlands. Further research is needed to investigate the impact of PTV and other viral agents causing encephalomyelitis within wild and domestic pig populations supported by the awareness of veterinarians.
Similar content being viewed by others
Background
The family Picornaviridae (order Picornavirales) comprises 110 named positive-strand RNA viruses, grouped into 47 genera [1]. In addition to enteroviruses, sapeloviruses and teschoviruses, many picornaviruses have been detected in pigs: Foot-and-mouth disease viruses, encephalomyocarditis viruses, porcine cosaviruses, kobuviruses, pasiviruses, senecaviruses and tottoriviruses. The genus Teschovirus has been frequently associated with neurologic disease in pigs [2,3,4,5]. Porcine teschovirus (PTV) currently comprises 13 known serotypes [6,7,8], which is likely to expand with the continuous discovery of novel genotypes [9, 10].
PTV is globally endemic in pig herds and is commonly isolated from fecal samples of clinically healthy wild and domestic pigs [11, 12], indicating that these viruses replicate in the intestinal tract without causing disease. However, when PTV invades the central nervous system (CNS) it can cause severe neurologic signs [13]. The first outbreak of severe CNS disease associated with PTV serotype 1 (PTV-1) occurred in 1929 in the Czech Republic in a town named Teschen [13, 14]. In 1957, a PTV-1 strain of lower pathogenicity caused an outbreak near the Talfan hill in Wales [15, 16]. Since that time, severe PTV-1-associated CNS disease is referred to as Teschen disease and the milder clinical form as Talfan disease. The most recent severe outbreak of PTV-1 with high mortality occurred in the Republic of Haiti in 2009 [17]. However, this virulent manifestation is now considered rare and only milder forms of neurologic disease associated with PTV-1 or other PTV serotypes have been reported in growing pigs in Canada, Spain and Brazil during the last years [2, 5, 8]. Although co-infections were not detected in these cases, immunosuppressive pathogens, such as porcine circovirus type 2 (PCV-2) and/or porcine reproductive and respiratory syndrome virus (PRRSV) could predispose animals to develop neurologic PTV disease [3, 18]. It is therefore important to screen for immunosuppressive pathogens when PTV is suspected to be associated with neurologic signs.
Case presentation
In 2014 and 2015, weanling piglets between the age of 6–12 weeks from two commercial pig farms showed periodically neurological signs. On both farms, the affected weanling piglets had a staggering gait, which developed progressively in 1 or 2 days from paresis to complete paralysis of the hind limbs. Paralyzed animals died within a few days or were euthanized. On farm A, a farrow-to-finish farm with more than 4000 animals (Topigs20), these neurologic signs occurred over a period of more than 5 years, during which the morbidity of the weanling piglets varied from 0 to 5%. On this farm no other significant health issues were noticed. On farm B, a fattening farm with 2000 animals (Topigs 40 x Pietrain), similar neurologic signs were noticed in pigs obtained repeatedly from one specific farrow farm. No further information is available for farm B. Both farms belong to separate companies and are located in two different parts of the Netherlands (> 100 km distance).
Clinical differential diagnoses for these neurologic signs include infectious (viral and bacterial) and non-infectious causes, such as tail-biting, sodium chloride intoxication and poisoning. Tail-biting was excluded based on clinical examination, and water nipples and water quality were checked regularly to prevent water deprivation, causing sodium chloride intoxication. No medication was used nor any change in feed was made, which makes poisoning unlikely. Only on farm A, the animals with comparable neurologic signs were tested repeatedly negative for Streptococcus suis and Escheria coli, which were considered the most likeley differential diagnosis. On the other hand, as ascending paresis/paralysis was the most typical clinical sign in the affected piglets bacterial agents causing septicaemia, like Streptococcus suis, Haemphilus parasuis or edema disease-associated Escheria coli, were considered unlikely. Also possible viral causes, like Suid herpesvirus type 1 (SuHV-1) and classical swine fever (CSF), were less likely based on clinical signs and/or the fact that clinical signs were observed over a long period of time.
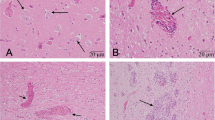
Piglets were submitted for pathological examination to identify the underlying cause of the neurologic signs. In total, six weanling piglets with clear clinical signs, aged between 7 and 10 weeks, from the two different farms (four animals from farm A and two animals from farm B) were necropsied. None of these piglets showed macroscopic changes. Tissue samples from lung, heart, kidney, liver, gastro-intestinal tract, trigeminal ganglion (only farm A) and CNS (spinal cord, cerebellum and cerebrum) were formalin-fixed and paraffin-embedded and tissue sections were stained with hematoxylin and eosin (H&E). In all animals histological changes were confined to the CNS and trigeminal ganglia. The trigeminal ganglion of the animals (farm A) showed infiltrates of mainly lymphocytes and macrophages around the neurons (Fig. 1a). The spinal cord, cerebrum and cerebellum showed perivascular infiltrates up-to 7 layers composed primarily of macrophages, lymphocytes and fewer plasma cells (mononuclear infiltrate Fig. 1b, c and d) and occasional eosinophils. These infiltrates extended into the neuropil where gliosis with glia nodules, neuronal degeneration and necrosis were encountered. The CNS lesions were most prominent in the gray matter of the spinal cord, where the lesions were bilateral and asymmetric. The cerebrum and cerebellum showed less severe changes (Additional file 1: Table S1). The meninges were mildly infiltrated with mainly macrophages and lymphocytes, which was more prominent around the brain compared to the spinal cord (Fig. 1d). Although there was variation in severity between the individual animals, there was no significant difference in morphology or severity of the CNS histopathology in the pigs of both farms. The observed histological changes were highly suggestive of a viral infection in the CNS. Possible viral causes of this non-suppurative or lymphohystiocytic encephalomyelitis in Western-European pigs without any other significant pathology could include: SuHV-1 (also known as Aujeszky’s disease or pseudorabies), PTV, PCV-2 and PRRSV [19, 20], and more recently described porcine sapelovirus (PSV) [21,22,23] and porcine astrovirus (PoAstV) [24, 25]. No additional research was performed to exclude bacterial pathogens, as a bacterial cause was highly unlikely based absence of gross inflammatory changes and the morphology the histopathological findings.
Sections of the central nervous system and trigeminal ganglion. Tissue sections from one animal from farm A stained with hematoxylin and eosin (H&E). a Trigeminal ganglion with infiltration of a large number of macrophages and lymphocytes (asterisk) (Bar = 100 μm); (b) Proximal spinal cord (gray matter) with perivascular infiltrates (perivascular cuffing; arrow) of mainly macrophages, lymphocytes and a lesser number of plasma cells, extending into the neuropil (Bar = 200 μm); (c) Cerebrum (gray matter) with perivascular cuffing, gliosis and neuronal degeneration and necrosis (arrow) (Bar = 100 μm); (d) Cerebellum with infiltration of a large number of lymphocytes and macrophages into the meninges (asterisk), and perivascular cuffing in the parenchyma (arrow) (Bar = 200 μm)
To identify a potential viral cause, brain and spinal cord tissues were investigated further. First an immunoperoxidase monolayer assay (IPMA) was performed on a monolayer of porcine kidney epithelial (PK15) cells incubated with 10% spinal cord or brain suspensions using serotype-specific hyperimmune sera. As PTV-1 is a notifiable disease in the Netherlands, the IPMA was performed with an antiserum specific for this serotype. As a negative control, a serum specific for PTV-2 was included. The IPMA was negative for PTV-1 and PTV-2, although a clear cytopathogenic effect (CPE) was observed in the cell cultures, suggesting the presence of a cytopathogenic agent.
Consecutively, we performed a real-time qPCR for PTV, PCV-2, PRRSV and suHV-1with hybridization probes specific for the amplification products. DNA or RNA was isolated from brain and spinal cord tissues by column chromatography. The primers and probe for PTV were specific for the 5′ untranslated region (5‘UTR), which is conserved among different serotypes of PTV [26], and were used with slight modifications in the primers and probes. The other viral qPCRs were used as described by van Rijn et al., 2004 [27] and Wellenberg et al., 2004 [28]. Primer and probe sequences are provided as Additional file 1: Table S2. Positive controls derived from cell-grown stocks of the different viral agents were included. All tested brain and/or spinal cord samples from farm A and B were positive for PTV (Cq values between 25.3–34.0 for the different CNS samples (Additional file 1: Table S3); Cq value of positive control for PTV is 29.0, low dilution control, and 35.5, high dilution control), whereas none of the other viral agents (PRRSV, PCV2, suHV-1) were detected.
Supernatants of cell lysates from the cultures in which CPE was detected were used to determine full genome sequences of the PTV isolates. Near full-length sequences of the three isolates were generated using the Illumina sequencing platform. A phylogenetic tree of the VP1 gene was reconstructed (Fig. 2) using the maximum likelihood method with 1000 bootstrap replicates and substitution model TIM2 + F + I + G4 in IQ-TREE v1.6.8 [29]. To determine similarity with known PTV serotypes, 40 reference PTV sequences of serotypes 1–13 were obtained from GenBank and included in the analyses. The phylogenetic tree was annotated in FigTree v1.4.3 http://tree.bio.ed.ac.uk/software/figtree/. From farm A, the PTV_WBVR_197_v01 and 199 strains from two different animals (identical in nucleotide sequence) clustered closely with the PTV-3 serotype with a high support value (98%) and PTV_strain_GD_v06 from farm B clustered closely with serotype 11 with a support value of 85%. The genome sequences are available on the European Nucleotide Archive (ENA) (PTV_197 as ERS3526779; PTV_199 as ERS3526780 and PTV_GDv06 as ERS3526781).
The maximum likelihood (ML) phylogeny of Porcine teschovirus (PTV) VP1. The gene of the three isolates (red and blue) and 40 references sequences with bootstrap values. The 40 reference sequences are labeled by name, GenBank accession number and serotype. The three PTV strains of interest are colored blue (PTV_strain_GD_v06) and red (PTV_WBVR_197_v01 and 199)
The alignment of the sequence data against the National Center for Biotechnology Information (NCBI) viral database followed by a manual inspection with the Integrative Genomics Viewer version 2.5.2 excluded the presence of PSV and PoAstV in the sequence data of both farms.
Discussion and conclusion
On two farms in the Netherlands with neurologic signs in weanling piglets we identified PTV as causative agent. This diagnosis was based on the location (prominent lesions in spinal cord) and the morphology of CNS histopathology and concurrent positive virus isolation and identification. Additional differential viral infections were excluded by absence of histopathological lesions in lung and lymph nodes, qPCR (PRRSV, PCV and suHV-1) and virus sequencing (PSV and PoAstV). Based on phylogenetic analyses we concluded that our isolated PTV strains are closely related to serotype 3 (strains WBVR_197_v01 and 199), first described in the USA (Ohio) [30] and serotype 11 (strain_GD_v06), first described in Europe (Dresden, Germany) [6]. Of note, PTV-3 [31] and PTV-11 [32] were previously shown to be neuropathogenic after experimental inoculation, manifesting with comparable clinical signs and histopathological changes in the CNS. Similar as described in this case report, the histopathological changes were most prominent in the spinal cord and less severe in cerebellum and cerebrum. Based on distribution, severity or morphology of the CNS histopathology we were unable to discriminate between the two newly identified PTV strains.
The Picornaviridae family contains small RNA viruses and their pathogenicity is associated with their ability to undergo mutation and genetic recombination, causing a high diversity over a short period of time [33]. Recombination of PTV strains have been reported [34], which may contribute significantly to the evolution of PTV. It is not known whether our isolated strains have evolved towards higher virulence or that their pathogenic potential was not recognized until today.
In several countries worldwide, such as Spain and Brazil, an increased prevalence of PTV-associated neurological disease is noted [2, 8]. Recent reports have identified other viral causes of encephalomyelitis in pigs of similar age. Eleven-week-old North American piglets infected with PSV presented comparable neurological signs as we have observed in the present work, and were diagnosed with lymphoplasmacellular and necrotizing polioencephalomyelitis [21, 22]. In 2017, PSV was detected during several porcine epidemic diarrhea outbreaks in Germany, noting the possible presence in the European commercially held swine population [35]. In Hungary, PoAstV type 3 was found in piglets with posterior paraplegia, encephalomyelitis and high mortality rates, although the authors were cautious in making a direct association between the virus and the central nervous lesions [24]. To the author’s knowledge, no research on PSV and PoAstV infections has been performed in swine herds in the Netherlands and in our report these viral causes were highly unlikely based on sequencing analyses.
Despite the fact that the encephalomyelitis was suggestive for a viral infection and that PTV was the only identified pathogen in the affected pigs, we have to recognize that not all Koch’s postulates were met in this case report, because this were all diagnostic cases. Investigations of future encephalomyelitis outbreaks would be facilitated greatly by including healthy age-matched control pigs. To our knowledge, no new cases of PTV have been diagnosed in the Netherlands since 2015. As only PTV-1 is a notifiable virus in the Netherlands, different strains of PTV could remain underreported or underdiagnosed. However, despite the low mortality on the farms investigated in the present work, it is very important that veterinarians are aware of this disease as the viruses may have the potential to evolve into more pathogenic strains. The objective of this report is to describe the diagnostic process for neuropathogenic PTV, where histopathology and additional molecular diagnostics are essential, and raise awareness for PTV and other viral agents, such as PSV and PoAstV causing encephalomyelitis in wild and domestic pig populations. Further research on pathology and epidemiology would facilicate assessment of the impact of PTV on global pig health and the swine industry.
In summary, on two different farms in the Netherlands, two new PTV strains were identified within the CNS of weanling piglets after earlier histopathological diagnosis of a lymphohistiocytic encephalomyelitis. Further research is needed to investigate the incidence and relevance of PTV and other viral agents, such as PSV and PoAstV causing encephalomyelitis within wild and domestic pig populations supported by the awareness of veterinarians and research institutes.
Availability of data and materials
The viral sequences are deposited in ENA (PTV_197 as ERS3526779; PTV_199 as ERS3526780 and PTV_GDv06 as ERS3526781). Phylogeny data, including alignments, are deposited in the TreeBASE repository http://purl.org/phylo/treebase/phylows/study/TB2:S25306. Other datasets used in the current study are available from the corresponding author on reasonable request.
Abbreviations
- CNS:
-
Central nervous system
- CSF:
-
Classical swine fever
- ENA:
-
European Nucleotide Archive
- IPMA:
-
Immunoperoxidase monolayer assay
- NCBI:
-
National Center for Biotechnology information
- PCV-2:
-
Porcine circovirus type 2
- PoAstV:
-
Porcine astrovirus
- PRRSV:
-
Porcine reproductive and respiratory virus
- PSV:
-
Porcine sapelovirus
- PTV:
-
Porcine teschovirus
- qPCR:
-
Quantitative polymerase chain reaction
- SuHV-1:
-
Suid herpesvirus type 1
References
Zell R, Delwart E, Gorbalenya AE, Hovi T, King AMQ, Knowles NJ, Lindberg AM, Pallansch MA, Palmenberg AC, Reuter G, et al. ICTV virus taxonomy profile: picornaviridae. J Gen Virol. 2017;98(10):2421–2.
Possatti F, Headley SA, Leme RA, Dall Agnol AM, Zotti E, de Oliveira TES, Alfieri AF, Alfieri AA. Viruses associated with congenital tremor and high lethality in piglets. Transbound Emerg Dis. 2018;65(2):331–7.
Chiu SC, Hu SC, Chang CC, Chang CY, Huang CC, Pang VF, Wang FI. The role of porcine teschovirus in causing diseases in endemically infected pigs. Vet Microbiol. 2012;161(1–2):88–95.
Bangari DS, Pogranichniy RM, Gillespie T, Stevenson GW. Genotyping of porcine teschovirus from nervous tissue of pigs with and without polioencephalomyelitis in Indiana. J Vet Diagn Investig. 2010;22(4):594–7.
Salles MW, Scholes SF, Dauber M, Strebelow G, Wojnarowicz C, Hassard L, Acton AC, Bollinger TK. Porcine teschovirus polioencephalomyelitis in western Canada. J Vet Diagn Investig. 2011;23(2):367–73.
Zell R, Dauber M, Krumbholz A, Henke A, Birch-Hirschfeld E, Stelzner A, Prager D, Wurm R. Porcine teschoviruses comprise at least eleven distinct serotypes: molecular and evolutionary aspects. J Virol. 2001;75(4):1620–31.
Cano-Gomez C, Jimenez-Clavero MA. Complete coding genome sequence of a putative novel teschovirus serotype 12 strain. Genome Announc. 2016;4(2):1-2.
Carnero J, Prieto C, Polledo L, Martinez-Lobo FJ. Detection of teschovirus type 13 from two swine herds exhibiting nervous clinical signs in growing pigs. Transbound Emerg Dis. 2018;65(2):e489–93.
Yang T, Li R, Yao Q, Zhou X, Liao H, Ge M, Yu X. Prevalence of porcine teschovirus genotypes in Hunan, China: identification of novel viral species and genotypes. J Gen Virol. 2018;99(9):1261–7.
Oba M, Naoi Y, Ito M, Masuda T, Katayama Y, Sakaguchi S, Omatsu T, Furuya T, Yamasato H, Sunaga F, et al. Metagenomic identification and sequence analysis of a teschovirus A-related virus in porcine feces in Japan, 2014-2016. Infect Genet Evol. 2018;66:210–6.
Chiu SC, Yang CL, Chen YM, Hu SC, Chiu KC, Lin YC, Chang CY, Wang FI. Multiple models of porcine teschovirus pathogenesis in endemically infected pigs. Vet Microbiol. 2014;168(1):69–77.
Prodelalova J. The survey of porcine teschoviruses, sapeloviruses and enteroviruses B infecting domestic pigs and wild boars in the Czech Republic between 2005 and 2011. Infect Genet Evol. 2012;12(7):1447–51.
Manuelidis EE, Sprinz H, Horstmann DM. Pathology of teschen disease; virus encephalomyelitis of swine. Am J Pathol. 1954;30(3):567–97.
Trefny L. Massive illness of swine in Teschen area. ZverolekObz. 1930;23:235–6.
Harding J. A transmissible polioencephalomyelitis of pigs (Talfan disease). Vet Rec. 1957;69:824–32.
Doherty M, Todd D, McFerran N, Hoey EM. Sequence analysis of a porcine enterovirus serotype 1 isolate: relationships with other picornaviruses. J Gen Virol. 1999;80(Pt 8):1929–41.
Deng MY, Millien M, Jacques-Simon R, Flanagan JK, Bracht AJ, Carrillo C, Barrette RW, Fabian A, Mohamed F, Moran K, et al. Diagnosis of porcine teschovirus encephalomyelitis in the Republic of Haiti. J Vet Diagn Investig. 2012;24(4):671–8.
Vlasakova M, Leskova V, Sliz I, Jackova A, Vilcek S. The presence of six potentially pathogenic viruses in pigs suffering from post-weaning multisystemic wasting syndrome. BMC Vet Res. 2014;10:221.
Bukovsky C, Schmoll F, Revilla-Fernandez S, Weissenbock H. Studies on the aetiology of non-suppurative encephalitis in pigs. Vet Rec. 2007;161(16):552–8.
Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32(6):648–60.
Arruda PH, Arruda BL, Schwartz KJ, Vannucci F, Resende T, Rovira A, Sundberg P, Nietfeld J, Hause BM. Detection of a novel sapelovirus in central nervous tissue of pigs with polioencephalomyelitis in the USA. Transbound Emerg Dis. 2017;64(2):311–5.
Schock A, Gurrala R, Fuller H, Foyle L, Dauber M, Martelli F, Scholes S, Roberts L, Steinbach F, Dastjerdi A. Investigation into an outbreak of encephalomyelitis caused by a neuroinvasive porcine sapelovirus in the United Kingdom. Vet Microbiol. 2014;172(3–4):381–9.
Kumari S, Ray PK, Singh R, Desingu PA, Varshney R, Saikumar G. Pathological and molecular investigation of porcine sapelovirus infection in naturally affected Indian pigs. Microb Pathog. 2019;127:320–5.
Boros A, Albert M, Pankovics P, Biro H, Pesavento PA, Phan TG, Delwart E, Reuter G. Outbreaks of neuroinvasive astrovirus associated with encephalomyelitis, weakness, and paralysis among weaned pigs, Hungary. Emerg Infect Dis. 2017;23(12):1982–93.
Matias, Ferreyra FS, Bradner LK, Burrough ER, Cooper VL, Derscheid RJ, Gauger PC, Harmon KM, Madson D, Pineyro PE, Schwartz KJ, et al. Polioencephalomyelitis in domestic swine associated with porcine astrovirus type 3. Vet Pathol. 2020;57(1):82-89.
Jimenez-Clavero MA, Fernandez C, Ortiz JA, Pro J, Carbonell G, Tarazona JV, Roblas N, Ley V. Teschoviruses as indicators of porcine fecal contamination of surface water. Appl Environ Microbiol. 2003;69(10):6311–5.
van Rijn PA, Wellenberg GJ, Hakze-van der Honing R, Jacobs L, Moonen PL, Feitsma H. Detection of economically important viruses in boar semen by quantitative RealTime PCR technology. J Virol Methods. 2004;120(2):151–60.
Wellenberg GJ, Stockhofe-Zurwieden N, Boersma WJ, De Jong MF, Elbers AR. The presence of co-infections in pigs with clinical signs of PMWS in the Netherlands: a case-control study. Res Vet Sci. 2004;77(2):177–84.
Minh BQ, Nguyen MA, von Haeseler A. Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol. 2013;30(5):1188–95.
Kasza L. Swine polioencephalomyelitis viruses isolated from the brains and intestines of pigs. Am J Vet Res. 1965;26:131–7.
Holman JE, Koestner A, Kasza L. Histopathogenesis of porcine polioencephalomyelitis in the germ free pig. Pathol Vet. 1966;3(6):633–51.
Matias Ferreyra F, Arruda B, Stevenson G, Schwartz K, Madson D, Yoon KJ, Zhang J, Pineyro P, Chen Q, Arruda P. Development of polioencephalomyelitis in cesarean-derived colostrum-deprived pigs following experimental inoculation with either teschovirus a serotype 2 or serotype 11. Viruses. 2017;9(7):179.
Lukashev AN. Recombination among picornaviruses. Rev Med Virol. 2010;20(5):327–37.
Qiu Z, Wang Z, Zhang B, Zhang J, Cui S. The prevalence of porcine teschovirus in the pig population in northeast of China. J Virol Methods. 2013;193(1):209–14.
Hanke D, Pohlmann A, Sauter-Louis C, Hoper D, Stadler J, Ritzmann M, Steinrigl A, Schwarz BA, Akimkin V, Fux R, et al. Porcine epidemic diarrhea in europe: in-detail analyses of disease dynamics and molecular epidemiology. Viruses. 2017;9(7):177.
Acknowledgements
The authors thank Bas Kolpa for his assistance in analysing the clinical cases and Mike Brouwer and Dre Kampfraath for making the data available in ENA and TreeBase.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
WK analysed the clinical cases. SV, KP and NC performed the necropsies and the pathological examinations (national certified and ECVP boarded veterinary pathologists). FH and CH performed the sequencing lab work and analysed the sequencing data; JB and SV did the qPCR lab work and analysed the data; JK, SV and NC analysed the overall data, discussed the results and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case report was approved and performed by guidelines of author’s institutions (Wageningen University & Research, Utrecht University and GD Animal Health) and the animal owners from farm A and B provided written consent to participate in this case report.
Consent for publication
The animal owners from farm A and B provided written consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
Table S1. Overview histopathology central nervous system for animals farm A and B. Table S2. Primer and probe overview for qPCR. Table S3. Overview animals and analysis.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Vreman, S., Caliskan, N., Harders, F. et al. Two novel porcine teschovirus strains as the causative agents of encephalomyelitis in the Netherlands. BMC Vet Res 16, 51 (2020). https://doi.org/10.1186/s12917-020-2275-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-020-2275-0