Abstract
Background
High protein diets shift the faecal microbiota into a more unfavourable composition in obese humans. In lean dogs, higher protein consumption is accompanied with increased production of putrefactive fermentation products, whereas obese dogs have a different gut microbiota compared to lean dogs. Still, the impact of high dietary protein on gut microbiota in obese dogs remains unclear. The aim of this study was to investigate faecal microbial changes in lean and obese dogs in response to two different levels of dietary protein.
Six healthy lean and six obese Beagles were fed a high protein diet (HP) and a low protein diet (LP) for 28 days each in a crossover design. Denaturing gradient gel electrophoresis and quantitative PCR were performed on faecal samples for microbial profiling. Plasma acylcarnitine and fermentation metabolites were measured.
Results
Dogs fed HP had higher concentrations of protein fermentation metabolites including faecal ammonia, isovalerate, isobutyrate, phenol, indole, serum indoxyl sulphate and plasma 3-OH isovalerylcarnitine compared to dogs fed LP, whereas no changes in faecal concentrations of acetate and butyrate were observed. The abundances of clostridial clusters IV and XIVa, covering the majority of butyrate-producing bacteria, and of the butyrate kinase gene, one of the terminal genes of the butyrate synthesis pathway were higher in dogs on HP compared to LP. Significant interactions between diet and body condition were found for the abundance of Firmicutes, Lactobacillus and clostridial cluster I. The similarity coefficient of faecal microbiota between the two diets was smaller in obese dogs than in lean dogs.
Conclusions
High protein diet increased the abundance and activity of butyrate-producing bacteria in Beagles independent of the body condition. In addition, increasing dietary protein content had a greater overall impact on faecal microbiota in obese compared to lean dogs.
Similar content being viewed by others
Background
Obesity is defined as an accumulation of excessive amounts of adipose tissue in the body, and it is the most common nutritional disorder in companion animals [1]. The prevalence of obesity has been reported to range from 8 to 34% in dogs [2, 3]. In the last decade, the link between obesity and gut microbiota has been established in humans and rodents [4,5,6], however, compared to humans, the relationship between gut microbiota and obesity is less well understood in dogs and needs to be investigated [7, 8].
Gut microbiota are mainly influenced by undigested dietary carbohydrates and protein. In dogs, there is increasing interest in feeding high protein diets, e.g. raw meat-based diets and weight management diets [9, 10]. In obese humans, high protein diets result in decreased butyrate concentration and numbers of butyrate-producing bacteria. Additionally, several studies carried out in lean dogs reported negative effects of colonic protein fermentation including increased faecal pH and elevated production of putrefactive substances [11]. Furthermore, a high protein diet has shown to promote the growth of Clostridium perfringens and to reduce the abundance of clostridial cluster XIVa in dogs [12]. However, the impact of high protein diets on faecal microbiota in obese dogs has not been studied.
The interactions between gut microbiota and host metabolism are of great importance thus receiving increased attention more recently [13]. Short-chain fatty acids (SCFA) are major fermentation products that are rapidly absorbed and utilized by the host. The metabolism of SCFA requires activation with coenzyme (CoA) [14]. Intracellular CoA bound acylgroups are then transported from the cytoplasm to the mitochondria by means of carnitine groups. Therefore, acetylcarnitine, propionylcarnitine, and butyrylcarnitines are measures of the respective SCFA-CoA by which SCFA influence cellular metabolism [14, 15]. In addition, the protein fermentation product indole can be metabolized to indoxyl sulphate by the liver [16], and the latter has been associated with chronic kidney disease in dogs [17]. Therefore, the assessment of these metabolites might be a useful approach to evaluate host metabolism of the gut fermentation end-products [18, 19].
Butyrate-producing bacteria supply energy to the gut epithelium, regulate host cell responses, and therefore, are considered to exert health-promoting effects on the colon [20]. The reduction of butyrate-producing bacteria has been associated with colon cancer and inflammatory bowel disease [21, 22]. The biosynthesis of butyrate can occur via the butyrate kinase (BK) pathway or via the butyryl CoA: acetate CoA transferase (BCoAT) pathway [23]. Although clostridial clusters IV and XIVa consist the majority of butyrate-producing bacteria from human colon [24], they still habour a diverse collection of non-butyrate producers. Therefore, assessing terminal genes of butyrate synthesis pathways could provide valuable information specifically target the activity of the butyrate-producing bacterial community.
The aim of the present study was (1) to investigate the impact of dietary protein on faecal microbial profile and functionality especially focusing on butyrate-producing microbiota and the concomitant fermentation and host metabolic profile in dogs; and (2) to compare the response of lean and obese dogs to two different levels of dietary protein. We hypothesize that high protein diet alters the profile and functionality of faecal microbiota and thus induces associated changes to host metabolism. The diversity and composition of faecal microbiota were measured through DGGE and quantitative PCR. The functionality of faecal microbiota was assessed by quantifying faecal fermentation metabolites and terminal genes expression for butyrate synthesis. The effect on the host metabolism was evaluated through the concentrations of plasma acylcarnitines and serum indoxyl sulphate.
Methods
Animals and diets
This study was approved by the Ethical Committee of the Faculty of Veterinary Medicine, Ghent University, Belgium (EC 2011/056).
Twelve healthy Beagles (Marshall Farms and Domaine des Souches) with a mean age of 6.0 years (range 3.8–8.3 year) were included in this study. Six Beagles (one spayed and three intact females; two intact males) were lean with a body condition score (BCS) of 4–5/9 and six Beagles (three intact females, one castrated and two intact males) were obese with a BCS of 8–9/9 [25]. Obesity was induced approximately one year prior to the present study by feeding the dogs a high fat commercial diet as described by Van de Velde et al., [26]. Prior to the study, dogs were deemed healthy, apart from obesity in six dogs, based on physical exams, complete blood counts, and serum biochemistry.
Two isocaloric experimental diets (Table 1), a high protein diet (HP) which consisted of 50.0 g crude protein (CP), 12.2 g ether extract (EE) and 32.2 g nitrogen free extract (NFE) on 100 g dry matter (DM) basis and a low protein diet (LP) which consisted of 17.8 g CP, 13.6 g EE and 62.3 g NFE on 100 g DM basis were formulated with the same ingredients (NV Versele-Laga). Both diets met the Minimal Requirement for adult dogs according to the National Research Council (NRC) [27]. The initial amount of food offered was calculated based on individual maintenance energy requirements according to population history and adjusted to maintain a stable body weight throughout the study. Dogs were fed twice daily and had free access to water.
Animal experimental procedures
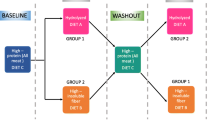
The study was designed as a crossover with two 4-weeks periods. The first 3 weeks were an adaptation period and samples were taken in the fourth week. In the first period, three lean and three obese dogs were randomly selected and assigned to LP first and the other three lean and obese dogs first received HP. In the second period, diets were switched. Each dog was therefore assigned to one of four groups (group 1: lean dogs received LP first; group 2 lean dogs received HP first; group 3 obese dogs received LP first; group 4 obese dogs received HP first).
Sampling procedure
Leftover food was collected and weighed after each feeding. Body weight and BCS were measured weekly. After overnight fasting, blood samples were drawn from the jugular vein on day 27 of each period. Heparinized plasma and serum were obtained by centrifugation at 1620 g for 15 min at 4 °C and stored at −20 °C until assayed. On day 27 of each period, fresh faecal samples (± 10 g) were collected within 10 min after spontaneous voiding. An aliquot of ±2 g was put into a sterile plastic tube, frozen immediately on dry ice and stored at −80 °C for microbial analyses, and the rest was stored at −20 °C for chemical analyses.
Chemical analyses
Dry matter of diets and faecal samples was analyzed by drying to a constant weight at 103 °C (ISO 1442, 1997), and crude ash was determined by combustion at 550 °C (ISO 936, 1998). Dietary crude protein was calculated from Kjeldahl nitrogen (6.25 × N, ISO 5983–1, 2005), ether extract was analyzed by the Soxhlet method (ISO 1443, 1973) and crude fibre was determined by acid-alkali digestion (ISO 5498, 1981). Nitrogen-free extract was calculated by subtracting crude ash, crude protein, crude fat, and crude fibre of the DM content. Total dietary fibre (TDF) and insoluble dietary fibre was measured with a Total Dietary Fibre Assay Kit (Sigma–Aldrich Co.), using procedures based on a combination of enzymatic and gravimetric methods [28]. Soluble dietary fibre was calculated by subtracting insoluble dietary fibre for the TDF [28].
Faecal pH was measured with a portable pH meter (Hanna Instruments). Faecal ammonia was analysed by steam distillation and titration [29]. Faecal SCFA concentrations were determined via gas chromatography after extraction with diethyl ether [30]. Faecal phenol, indole and p-cresol concentrations were measured as described by Depauw et al. [31].
Serum cobalamin and folate concentrations were measured using commercially available ARCHITECT B12 and ARCHITECT Folate assays, respectively, on ARCHITECT i System (Abbott Diagnostics). Plasma acylcarnitine profile was determined according to Zytkovicz et al. [32]. Serum indoxyl sulphate concentrations were measured according to Depauw et al. [31].
Microbial analyses
Total bacterial DNA extractions from 500 mg faeces were performed according to Boon et al. [33]. Isolated DNA was subsequently used as a template to amplify the 16S rDNA for all members of the Bacteria with forward primer P338F-GC and the reverse primer P518r, and a GC-clamp of 40 bp was incorporated into the forward primer. DGGE based on the protocol of Muyer et al. [34] was performed on the Bio-Rad D gene system (Bio-Rad). The PCR products (10 μL of mixture from 20 μL PCR product and 5 μL loading dye) of the second round were loaded. The obtained DGGE patterns were normalized and analyzed using BioNumerics 2.0 (Applied Maths) [35]. The number of bands in the DGGE profile was used to calculate the richness in the present study. A matrix of similarities for the densiometric curves of the band patterns was calculated based on the Pearson product-moment correlation coefficient, and dendrograms were created by using Ward linkage [36].
The quantification of DNA by qPCR was performed with a C1000 Thermal Cycler (Bio-Rad). The amplification and detection were carried out in 96-well plates using SensiMixTM SYBR No-ROX Kit (Bioline Reagents Ltd). Each reaction was done in triplicate in 12 μL total reaction mixture using 2 μL of 50 ng of the DNA sample except for BK where 2 μL of undiluted DNA was used. All qPCR results were expressed as gene copies per g of fresh faeces. The primer sets used in this study are listed in Table 2. A melting curve analysis was done after amplification to confirm specificity of the reaction. Quantification was done by using standard curves made from known concentrations of plasmid DNA containing the respective amplicon for each set of primers.
Statistical analyses
Statistical analyses were performed with RStudio (The R Foundation for Statistical Computing, version 3.1.0) using the gamm4 package for R (version 0.2–2).
To test the effect of both dietary protein levels on composition and functionality of microbiota in lean and obese dogs and their effect on host metabolism, a general additive mixed model was used: Y = μ + dog + diet + BC + group + D × BC + ε, where μ is the overall mean, Dog a random effect; diet refers to HP or LP; BC is the body condition of the dogs (lean vs. obese), group is the order by which dogs received the diets (groups 1–4) and refers to the carry-over effect, D × BC is the interaction between diet and body condition and refers to the direction or size of the effect of dietary protein on lean and obese animals; and ε is the error term.
Faecal concentration of valerate and p-cresol were only detected in three and one samples, respectively, and hence these parameters were not taken into consideration when performing the statistical analysis.
For the D × BC, the nparcomp package of R was used (version 2.0) for post-hoc test by creating dummy variables and an own contrast matrix.
Statistical significance was accepted at P < 0.05.
Results
Food, energy and protein intake
Two obese dogs (one from group 3 and one from group 4) were excluded from the study due to injuries not associated with the present study. Significant D × BC interactions were observed on BW and BCS, and post hoc analysis could only detect the differences between lean and obese dogs (Table 3). No difference was observed on food and energy intake between diets and BC (Table 3). Protein intake was significantly higher in HP compared to LP (P < 0.001).
Faecal metabolites
Faecal concentrations of ammonia (P < 0.001), isovalerate (P < 0.001), isobutyrate (P < 0.001), phenol (P = 0.004) and indole (P = 0.002) were higher in dogs on HP compared to LP (Table 3). In addition, faecal isovalerate (P = 0.010) and isobutyrate (P = 0.009) concentrations were higher in obese than lean dogs. A significant D × BC interaction was observed on faecal propionate (P = 0.017), however, a carry-over effect was observed for faecal propionate (P = 0.049, data not shown). No differences in faecal concentrations of acetate, butyrate, and total SCFA were observed between diets or BC (Table 3).
Blood parameters
Serum indoxyl sulphate concentration was higher in dogs fed HP than LP (P = 0.027) (Table 4). Within the plasma acylcarnitine profile, only 3-OH isovalerylcarnitine (3OH-C5) was higher (P = 0.012) for dogs fed HP compared to LP (Table 4).
Microbial ecology and populations
Neither the type of diets nor the individual played an important role in the clustering of the microbial community (Fig. 1). Although seven out of ten dogs had greater richness when fed HP compared to LP irrespective of BC, no significant difference was observed between diets and between lean and obese dogs. The average value of faecal microbial richness was 17.0 in this study (Fig. 2). The order of the diet (effects of group) did not affect the similarity coefficient of faecal microbiota (Fig. 3a). However, faecal microbiota of obese dogs had a lower similarity value compared to lean dogs (52% vs. 76%) when the two diets were switched (P = 0.050) (Fig. 3b).
Dice cluster analysis of the DGGE gel profile of lean (L, n = 6) and obese (O, n = 4) dogs fed low protein diet (LP) and high protein diet (HP) in a crossover design. DGGE: denaturing gradient gel electrophoresis; LP: low protein diet; HP: high protein diet; L: lean dog; O: obese dog (Additional file 1)
Higher faecal abundances of clostridial cluster IV (P = 0.025), XIVa (P = 0.001) and of the BK gene (P = 0.019) were observed when dogs were fed HP compared to LP (Table 5). In addition, a significant D × BC interaction was observed for Firmicutes (P = 0.007), Lactobacillus (P = 0.017) and clostridial cluster I (P = 0.022). Compared to LP, HP increased the abundance of Firmicutes in lean dogs (P < 0.05), while no changes were observed in obese dogs. Clostridial cluster I was more abundant in lean dogs when fed HP than LP (P < 0.05), whereas post-hoc test did not reveal any differences between the four groups for Lactobacillus. No effects of diet or BC were found for faecal concentrations of total bacteria, Bacteroidetes, Enterobacteriaceae and gene express of BCoAT.
Discussion
Understanding how dietary components alter the composition and activity of gut microbiota direct nutritional interventions and disease prevention strategies. The present study has demonstrated an impact of dietary protein content on both composition and activity of faecal microbiota in dogs. In particular, the major butyrate producing bacterial groups, Clostridial cluster IV and XIVa, and BK, the terminal gene of butyrate production were increased in dogs fed HP. Interestingly the present study has also shown a different response to dietary protein content in lean and obese dogs, which to the author’s knowledge has not yet been reported. In previous studies, human obesity has been associated with a number of changes in faecal microbial groups, such as consistently reported changes in two predominant phyla Firmicutes and Bacteroidetes, which have been suggested to be important for energy harvest [5, 6]; and an increase in the family Enterobacteriaceae, an important producer of inflammatory lipopolysaccaride [37]. In addition, decreases in butyrate producing bacteria have been reported in obese humans with reduced carbohydrate intake [24]. Therefore, these bacterial groups were selected and measured in the present study. Surprisingly, in contrast to humans, increases in the abundance of major butyrate-producing bacterial groups, clostridial clusters IV and XIVa were observed in this study. However, clostridial clusters IV and XIVa still harbour non-butyrate-producing bacteria and some butyrate-producing strains within these clusters (eg. Coprococcus catus and Roseburia inulinivorans) can switch from butyrate to propionate production [38, 39]. When measuring the terminal genes of the two butyrate production pathways, the BK gene was increased whereas BCoAT remained unchanged in dogs fed HP. This is in contrast to humans where the BCoAT pathway has been found to be the dominant pathway for butyrate formation [23]. Possible reasons for dogs utilizing a different butyrate synthesis pathway compared to humans are explained below.
The accumulation of butyrate-producing bacteria is often associated with intake of fibre. In this study, TDF in HP was two-fold higher compared to LP. Generally, TDF is comprised of plant fibre which in this study was provided by brewer’s rice, rice meal, and beet pulp (Table 1), with TDF ranging from 1.6–16.4% [40, 41], 2.4–4.6% [27], and 60–80% [42], respectively. However, TDF does not clearly differ from HP to LP in terms of plant fibre content. Therefore, the higher TDF content in HP vs. LP could be of animal origin [31, 43], where the content of pork greaves was clearly higher in HP vs LP. TDF in the pork greaves was thus analyzed and observed to be 7.0% on DM basis.
Although TDF was higher, soluble fibre was much lower in HP compared to LP. In contrast to insoluble fiber which cannot be fermented, soluble fibre is readily fermentable [44]. Thus, it is unlikely that the dietary fibre contributed to the increase in butyrate producing bacteria. Importantly, butyrate can be formed by fermentation of amino acids, such as glutamate and leucine [45]. Thus, in the present study, amino acids might be the important substrates for the growth of butyrate producing bacteria. This is supported by increased BK gene expression because BK pathway might be associated more with protein-rich environment, whereas BCoAT pathway is depending on a consistent supply of acetate that is derived from carbohydrates [46]. Recent studies have found that the BK gene was more abundant in carnivorous animals and BCoAT gene was enriched in omnivores and herbivores [46].
As dogs may use a different pathway for butyrate production, it raises the question whether dogs possess the same predominant butyrate producers as humans (clostridial clusters IV and XIVa). The butyrate kinase gene pathway is linked to C. perfringens dominated in many Carnivores (e.g. ferret, tiger, African lion) and non-carnivorous Carnivora (e.g. red panda and giant panda) [46]. In this study C. perfringens was not measured, but it is the major component of clostridial cluster I. The clostridial cluster I was increased when dogs were fed HP, however, it still needs to be confirmed if increased clostridial cluster I abundance is due to increases in C. perfringens numbers. Nevertheless, other canine studies have also shown that high protein diets promoted the growth of C. perfringens [12]. Thus, closteridial cluster I might also be important for butyrate production in dogs. Further studies are warranted to investigate the diversity, metabolism and microbial ecology of butyrate-producing bacteria from the dog gut.
In contrast to our results, another study reported high dietary protein decreased the abundance of clostridial cluster XIVa in dogs [47]. This contradiction might be due to the different quality/components in the greaves meal (e.g. bone and cartilage), and/or different level of greaves meal included in the diet, 80.0% in that study vs. 53.4% in our study, in particular, diarrhea has been observed in that study, which possibly indicates gut dysbiosis in those dogs fed that diet. Furthermore, faecal butyrate concentrations and plasma C4 concentrations did not differ between the two diets, this could be due to the fact that faecal concentrations may not necessarily reflect SCFA production in the proximal colon because more than 95% of SCFA are absorbed rapidly [39]. Nevertheless, a recent study observed an increased butyrate concentration in a high minced beef diet compared to commercial dry food, whereas protein content was 46.2% versus 27.1% on dry matter basis [48]. Moreover, the technique used to measure acylcarnitines could not separate butyrylcarnitine and isobutyrylcarnitine. Therefore, the accurate estimation of in vivo butyrate production is hardly feasible in dogs.
Although several studies have shown that high protein diets can alter faecal microbiota in obese human subjects [24], whether the diet independent of the obese phenotype is responsible for the changes remains largely unknown. One study reported an increase in Enterobacteriales in the faecal microbiota of obese but not of lean rats [49]. In the present study, the similarity coefficient of faecal microbiota between the two diets was lower in obese dogs than in lean dogs (Fig. 2b), suggesting faecal microbiota in lean dogs might be more resilient to dietary protein changes than in obese dogs. This is in accordance with another study that found dietary protein and carbohydrate ratios have more significant impacts on gut microbial compositions in obese dogs than in lean dogs [50]. In addition, within the quantified bacterial groups, a high protein diet promoted bacterial growth, i.e. Firmicutes, Lactobacillus and clostridial cluster I in lean dogs whereas no changes were observed obese dogs (Table 5). Therefore, dietary modulation on faecal microbiota was also affected by body condition in dogs.
As expected, colonic protein fermentation was increased in dogs fed HP as indicated by higher faecal concentrations of putrefactive compounds (ammonia, indole and phenol) and branched-chain fatty acids (isovalerate and isobutyrate) compared to dogs consuming LP. These results are in agreement with previous studies in dogs fed high protein diets [11, 51]. In addition, higher serum concentrations of indoxyl sulphate and 3-OH isovalerylcarnitine in dogs fed HP were observed, supporting colonic production and absorption of protein fermentation metabolites [45]. To date, no toxicity or tolerance tests have been performed on the effects of protein fermentation metabolites on canine gut health, and the only studies available concerning the link between protein fermentation and canine gut health have evaluated the effects of prebiotics, probiotics and synbiotics [52, 53]. Studies have found Novosphingobium sp. and Haliangium ochraceum, which are capable of breaking down aromatic putrefactive substances were increased in cats fed a high protein diet [54]. Therefore, whether the increased protein fermentation metabolites are harmful for dogs needs to be further investigated, especially the beneficial effects of indole that have been proposed by in vitro studies, such as the increase in the expression of anti-inflammatory genes and strengthening of epithelial cell barrier properties [55].
Conclusions
In our study, high protein diet promoted the growth of butyrate-producing bacteria which includes clostridial clusters I, IV and XIVa in dogs independent of body condition, and importantly this butyrate synthesis is suggested to relate to the BK pathway as compared to BCoAT pathway in humans. Thus, the different metabolic pathway used for butyrate production provides valuable information for modulation of gut microbiota and their fermentation metabolites in dogs. In addition, HP induced several bacterial changes (including Firmicutes, Lactobacillus and clostridial cluster I) that are body condition dependent. Further, faecal microbiota in obese dogs seemed to be less stable compared to that of lean dogs. Together, the results of the present study suggest that diet composition per se had an important effect on the faecal microbiota, however, body condition also affected microbiota composition in dogs. This should be taken into consideration in future nutritional interventions and disease prevention.
Abbreviations
- 3OH-C4:
-
3-OH butyrylcarnitine
- 3OH-C5:
-
3-OH isovalerylcarnitine
- BC:
-
Body condition
- BCoAT:
-
Butyryl CoA: acetate CoA transferase
- BCS:
-
Body condition score
- BK:
-
Butyrate kinase
- BW:
-
Body weight
- C0:
-
Free carnitine
- C2:
-
Acetylcarnitine
- C3:
-
Propionylcarnitine
- C3DC:
-
Malonylcarnitine
- C4:
-
Butyryl- and isobutyrylcarnitine
- C5:
-
Isovalerylcarnitine
- CoA:
-
Co-enzyme A
- CP:
-
Crude protein
- D × BC:
-
Interaction between diet and body condition
- DGGE:
-
Denaturing gradient gel-electrophoresis
- DM:
-
Dry matter
- EE:
-
Ether extract
- EI:
-
Energy intake
- FI:
-
Food intake
- HP:
-
High protein diet
- LP:
-
Low protein diet
- NFE:
-
Nitrogen free extract
- PI:
-
Protein intake
- SCFA:
-
Short-chain fatty acids
- TDF:
-
Total dietary fibre
References
German AJ. The growing problem of obesity in dogs and cats. J Nutr. 2006;136:1940S–6S.
Diez M, Picavet P, Ricci R, Dequenne M, Renard M, Bongartz A, Farnir F. Health screening to identify opportunities to improve preventive medicine in cats and dogs. J Small Anim Pract. 2015;56:463–9.
McGreevy PD, Thomson PC, Pride C, Fawcett A, Grassi T, Jones B. Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Vet Rec. 2005;156:695–702.
Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, Gómez-Delgado F, Pérez-Martínez P, Delgado-Lista J, et al. Intestinal microbiota is influenced by gender and body mass index. PLoS One. 2016;11:e0154090.
Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18:190–5.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Park HJ, Lee SE, Kim HB, Isaacson RE, Seo KW, Song KH. Association of Obesity with serum leptin, adiponectin, and serotonin and gut microflora in beagle dogs. J Vet Intern Med. 2015;29:43–50.
Handl S, German AJ, Holden SL, Dowd SE, Steiner JM, Heilmann RM, et al. Faecal microbiota in lean and obese dogs. FEMS Microbiol Ecol. 2013;84:332–43.
Diez M, Nguyen P, Jeusette I, Devois C, Istasse L, Biourge V. Weight loss in obese dogs: Evaluation of a high-protein, low-carbohydrate diet. J Nutr. 2002;132:1685s–7s.
Freeman LM, Chandler ML, Hamper BA, Weeth LP. Current knowledge about the risks and benefits of raw meat-based diets for dogs and cats. J Am Vet Med Assoc. 2013;243:1549–58.
Hesta M, Roosen W, Janssens GP, Millet S, De Wilde R. Prebiotics affect nutrient digestibility but not faecal ammonia in dogs fed increased dietary protein levels. Br J Nutr. 2003;90:1007–14.
Zentek J, Marquart B, Pietrzak T, Ballevre O, Rochat F. Dietary effects on bifidobacteria and Clostridium Perfringens in the canine intestinal tract. J Anim Physiol Anim Nutr (Berl). 2003;87:397–407.
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7.
Bremer J. Carnitine--metabolism and functions. Physiol Rev. 1983;63:1420–80.
den Besten G, Lange K, Havinga R, van Dijk TH, Gerding A, van Eunen K, et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol. 2013;305:G900–10.
Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–703.
Cheng FP, Hsieh MJ, Chou CC, Hsu WL, Lee YJ. Detection of indoxyl sulfate levels in dogs and cats suffering from naturally occurring kidney diseases. Vet J. 2015;205:399–403.
Verbrugghe A, Janssens GP, Meininger E, Daminet S, Piron K, Vanhaecke L, et al. Intestinal fermentation modulates postprandial acylcarnitine profile and nitrogen metabolism in a true carnivore: the domestic cat (Felis Catus). Br J Nutr. 2010;104:972–9.
Rochus K, Janssens GP, Van de Velde H, Verbrugghe A, Wuyts B, Vanhaecke L, Hesta M. Highly viscous guar gum shifts dietary amino acids from metabolic use to fermentation substrate in domestic cats. Br J Nutr. 2013;109:1022–30.
Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12:304–14.
Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111–20.
Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5.
Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, Flint HJ. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J Bacteriol. 2004;186:2099–106.
Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73:1073–8.
Laflamme D. Development and validation of a body condition score system for dogs. Canine Pract. 1997;22:10–5.
Van de Velde H, Janssens GP, Rochus K, Duchateau L, Scharek-Tedin L, Zentek J, et al. Proliferation capacity of T-lymphocytes is affected transiently after a long-term weight gain in beagle dogs. Vet Immunol Immunopathol. 2013;152:237–44.
National Research Council. Energy. In: Nutrient Requirements of Dogs and Cats. National Academies, Washington, D.C, USA, 2006. p. 28–48.
Prosky L, Asp NG, Schweizer TF, DeVries JW, Furda I. Determination of insoluble, soluble, and total dietary fiber in foods and food products: interlaboratory study. J Assoc of off Anal Chem. 1988;71:1017–23.
Bremner J, Keeney DR. Steam distillation methods for determination of ammonium, nitrate and nitrite. Anal Chim Acta. 1965;32:485–95.
Greenberg AE, Clesceri LS, Eaton AD. Aggregate organic constituents: organic and volatile acids. Standard Methods for the Examination of Water and Wastewater 1992. 53–54.
Depauw S, Hesta M, Whitehouse-Tedd K, Vanhaecke L, Verbrugghe A, Janssens GP. Animal fibre: the forgotten nutrient in strict carnivores? First insights in the cheetah. J Anim Physiol Anim Nutr (Berl). 2011;97:146–54.
Zytkovicz TH, Fitzgerald EF, Marsden D, Larson CA, Shih VE, Johnson DM, et al. Tandem mass spectrometric analysis for amino, organic, and fatty acid disorders in newborn dried blood spots: a two-year summary from the New England newborn screening program. Clin Chem. 2001;47:1945–55.
Boon N, Top EM, Verstraete W, Siciliano SD. Bioaugmentation as a tool to protect the structure and function of an activated-sludge microbial community against a 3-chloroaniline shock load. Appl Environ Microbiol. 2003;69:1511–20.
Muyzer G, Dewaal EC, Uitterlinden AG. Profiling of complex microbial-populations by denaturing gradient gel-electrophoresis analysis of polymerase chain reaction-amplified genes-coding for 16s ribosomal-Rna. Appl Environ Microbiol. 1993;59:695–700.
Boon N, Windt W, Verstraete W, Top EM. Evaluation of nested PCR-DGGE (denaturing gradient gel electrophoresis) with group-specific 16S rRNA primers for the analysis of bacterial communities from different wastewater treatment plants. FEMS Microbiol Ecol. 2002;39:101–12.
Ward JH Jr. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58:236–44.
Santacruz A, Marcos A, Wärnberg J, Martí A, Martin-Matillas M, Campoy C, et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring). 2009;17:1906–15.
Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8:1323–35.
Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–9.
de -Oliveira LD, Carciofi AC, Oliveira MC, Vasconcellos RS, Bazolli RS, Pereira GT, Prada F. Effects of six carbohydrate sources on diet digestibility and postprandial glucose and insulin responses in cats. J Anim Sci. 2008;86:2237–46.
Carciofi AC, Takakura FS. De-Oliveira LD, Teshima E, Jeremias JT, Brunetto MA, Prada F. Effects of six carbohydrate sources on dog diet digestibility and post-prandial glucose and insulin response. J Anim Physiol Anim Nutr (Berl). 2008;92:326–36.
Middelbos IS, Fastinger ND, Fahey GC Jr. Evaluation of fermentable oligosaccharides in diets fed to dogs in comparison to fiber standards. J Anim Sci. 2007;85:3033–44.
Codex Alimentarius. Report of the 30th session of the Codex Committee on Nutrition and Foods for Special Dietary Uses. 2008, ALINORM 09/32/26. Cape Town, South Africa.
McIntyre A, Gibson PR, Young GP. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut. 1993;34:386–91.
Michal G. Amino acids and derivatives. In: Michal G, editor. Biochemical pathways: an atlas of biochemistry and molecular biology. 1st ed. New York: Wiley; 1999. p. 47–8.
Vital M, Gao J, Rizzo M, Harrison T, Tiedje JM. Diet is a major factor governing the fecal butyrate-producing community structure across Mammalia, Aves and Reptilia. ISME J. 2015;9:832–43.
Hang I, Rinttila T, Zentek J, Kettunen A, Alaja S, Apajalahti J, et al. Effect of high contents of dietary animal-derived protein or carbohydrates on canine faecal microbiota. BMC Vet Res. 2012;8:90.
Herstad KMV, Gajardo K, Bakke AM, Moe L, Ludvigsen J, Rudi K, et al. A diet change from dry food to beef induces reversible changes on the faecal microbiota in healthy, adult client-owned dogs. BMC Vet Res. 2017;13:147.
de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–8.
Li Q, Lauber CL, Czarnecki-Maulden G, Pan Y, Hannah SS. Effects of the dietary protein and carbohydrate ratio on gut microbiomes in dogs of different body conditions. MBio. 2017;8:e01703–16.
Nery J, Goudez R, Biourge V, Tournier C, Leray V, Martin L, et al. Influence of dietary protein content and source on colonic fermentative activity in dogs differing in body size and digestive tolerance. J Anim Sci. 2012;90:2570–80.
Swanson KS, Dowd SE, Suchodolski JS, Middelbos IS, Vester BM, Barry KA, et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2011;5:639–49.
Flickinger EA, Schreijen EMWC, Patil AR, Hussein HS, Grieshop CM, Merchen NR, Fahey GC. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J Anim Sci. 2003;81:2008–18.
Lubbs DC, Vester BM, Fastinger ND, Swanson KS. Dietary protein concentration affects intestinal microbiota of adult cats: a study using DGGE and qPCR to evaluate differences in microbial populations in the feline gastrointestinal tract. J Anim Physiol Anim Nutr (Berl). 2009;93:113–21.
Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A. 2010;107:228–33.
Ovreas L, Forney L, Daae FL, Torsvik V. Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl Environ Microbiol. 1997;63:3367–73.
Lee DH, Zo YG, Kim SJ. Nonradioactive method to study genetic profiles of natural bacterial communities by PCR-single-strand-conformation polymorphism. Appl Environ Microbiol. 1996;62:3112–20.
Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol. 2008;47:367–73.
Bartosch S, Fite A, Macfarlane GT, McMurdo ME. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol. 2004;70:3575–81.
Abdulamir AS, Hafidh RR, Bakar FA. Molecular detection, quantification, and isolation of streptococcus gallolyticus bacteria colonizing colorectal tumors: inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol Cancer. 2010;9:249.
Song Y, Liu C, Finegold SM, Real-time PCR. Quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004;70:6459–65.
Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microb. 2004;70:7220–8.
Malinen E, Rinttila T, Kajander K, Matto J, Kassinen A, Krogius L, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–82.
Charrier C, Duncan GJ, Reid MD, Rucklidge GJ, Henderson D, Young P, et al. A novel class of CoA-transferase involved in short-chain fatty acid metabolism in butyrate-producing human colonic bacteria. Microbiology. 2006;152:179–85.
Vital M, Penton CR, Wang Q, Young VB, Antonopoulos DA, Sogin ML, et al. A gene-targeted approach to investigate the intestinal butyrate-producing bacterial community. Microbiome. 2013;1:8.
Thompson A. Ingredients: where pet food starts. Top Companion Anim Med. 2008;23:127–32.
Acknowledgments
The work of J. X. was supported by a grant attributed by the China Scholarship Council (CSC). M.M is a Postdoctoral Fellow of the Research Foundation-Flanders (FWO, Belgium). The authors wish to thank Dr. Birgitte Wuyts for the analysis of plasma acylcarnitine profile, Versele-Lage NV (Deinze, Belgium) for providing the diets used in this study, Herman De Rycke, Julie Vanden Bussche, Tim Lacoere, Siska Maertens, Laura Statius and Saartje Van Beirs for technical assistance.
Funding
The work of J. X. was supported by a grant attributed by the China Scholarship Council (CSC). The funding body did not play any role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiment: JX, AV, MH. Performed the experiment: JX, VE, LV, MC. Contributed to/carried out analyses: JX, TVW, MM, VE, FVE, LV, MH. Performed statistical analysis: JX, ML, AC. Drafted and amended the manuscript: JX, AV, ML, AC, DJXL, TVW, VE, FVE, MC, MH. All authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethical Committee of the Faculty of Veterinary Medicine, Ghent University, Belgium (EC 2011/056).
Consent for publication
Not applicable
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
The DGGE gel of the present study. Description of data: the original DGGE gel of the present study that includes the markers, blanks, and 20 faecal samples. (PNG 1049 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xu, J., Verbrugghe, A., Lourenço, M. et al. The response of canine faecal microbiota to increased dietary protein is influenced by body condition. BMC Vet Res 13, 374 (2017). https://doi.org/10.1186/s12917-017-1276-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-017-1276-0