Abstract
Background
The objective of the present study was to optimize and validate an automated method to assess the total antioxidant capacity (TAC) in serum of dogs using the cupric reducing antioxidant capacity (CUPRAC) methodology (TACc) with bathocuproinedisulfonic acid disodium salt as chelating agent, evaluating also possible variations due to the use of two different automated analyzers. The method is based on the reduction of Cu2+ into Cu1+ by the action of the non-enzymatic antioxidants that are present in the sample.
Results
Imprecision was low in both apparatus utilized, and the results were linear across serial Trolox and canine serum samples dilutions. Lipids did not interfere with the assay; however, hemolysis increased the TACc concentrations. When TACc concentrations were determined in ten healthy (control) dogs and in twelve dogs with inflammatory bowel disease (IBD), dogs with IBD had lower TACc concentrations when compared with the healthy dogs.
Conclusions
The method validated in this paper is precise, simple, and fast and can be easily adapted to automated analyzers.
Similar content being viewed by others
Background
Oxidative stress is characterized by the inability of endogenous antioxidants to counteract the oxidative damage on biological targets [1, 2]. Antioxidant response can be monitored by analysis of individual biomarkers such as α-tocopherol (vitamin E), carotenoids, glutathione peroxidase, selenium, among others, and/or by assays that measure the total antioxidant capacity (TAC). TAC can be measured by direct methods (e.g., Trolox equivalent antioxidant capacity) [3] that are based on the ability of inhibiting the oxidation of a chemical substance, or by indirect methods based on the determination of the reductive properties of the sample such as the cupric reducing antioxidant capacity (CUPRAC) and the ferric reducing ability of plasma (FRAP) [4, 6, 16]. TAC assays provide a global measurement of the antioxidant capacity of the body, including in some cases possible in vivo interaction between different antioxidants [5, 6].
In humans, a decrease of TAC values has been reported in several conditions, including metabolic syndrome, prediabetes or surgery [7–10]. In dogs, decreased TAC values have been reported after surgery and when anaesthetized with isoflurane [11, 12]. Furthermore, increases in this analyte have been described in demodicosis, parvoviral enteritis, and lymphoma [13–15].
CUPRAC assay evaluates the capacity of the sample in reducing the Cu2+ to Cu1+ in the presence of a chelating agent. Neocuproine, bathocuproine and bathocuproinedisulfonic acid disodium salt are different chelating agents used for this purpose. This method has been applied to human serum, food, and plant extracts [16–19].
To the authors’ knowledge, no CUPRAC assay has been validated for TAC measurements in canine serum samples. The objective of the present study was to optimize and validate an automated method to assess the TAC in serum of dogs using the CUPRAC methodology (TACc) and the bathocuproinedisulfonic acid disodium salt as the chelating agent. The evaluation was performed using two different automated analyzers; therefore possible variations of the assay due to the use of different equipment were also evaluated.
Methods
Chemicals
Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), potassium chloride (KCl), sodium chloride (NaCl), potassium phosphate (KH2PO4), bathocuproinedisulfonic acid disodium salt, and copper(II) sulphate (CuSO4) were obtained from Sigma-Aldrich. Di-sodium hydrogen phosphate anhydrous (Na2HPO4) was obtained from Panreac.
Apparatus
The analyses were performed in the Cobas Mira Plus Biochemical Auto Analyzer (ABX Diagnostic) and in the Olympus AU400 Automatic Chemistry Analyzer (Olympus Europe GmbH).
Principle of the assay
The CUPRAC assay is based on the reduction of Cu2+ into Cu1+ by the action of the non-enzymatic antioxidants presented in the sample. The oxidant complex, consisted of Cu2+-bathocuproinedisulfonic acid (Cu2+-BCS) reacts with the antioxidants of the sample and is reduced to a Cu1+-bathocuproinedisulfonic acid (Cu1+-BCS), a stable complex which has a maximum absorbance at 490 nm [19]. The antioxidant capacity of the sample is assumed to be equal to the extent of the complex Cu1+-BCS formation [19]. The assay used for CUPRAC in the present study was based on the method described by Campos et al. [19] with some modifications.
Measurement procedure for the Cobas Mira Plus biochemical auto analyzer
In brief, a 5 μL volume of sample was pipetted. Then, 195 μL of the reagent 1 were added and a first read at 500 nm was taken. Subsequently, 50 μL of the reagent 2 were added to the reaction and incubated at 37 °C during 200 seconds. After incubation, a second read at 500 nm was taken and the difference between the first and the second read was used to calculate the antioxidant capacity of the sample. Distilled water was used for blanks.
Measurement procedure for the Olympus AU400 automatic chemistry analyzer
An amount of 195 μL of reagent 1 and 5 μL of sample were pipetted. A first read at 480 nm was taken before the addition of the second reagent. Then, 50 μL of reagent 2 were added to the mixture and incubated at 37 °C during 280 seconds. A second read at 480 nm was taken and the difference between the first and the second read was used for calculation of the antioxidant capacity of the sample. Distilled water was used instead of sample or standard for blanks.
Preparation of standards
Trolox solution at a concentration of 2.0, 1.0, 0.5, 0.25, 0.125, 0.0625 and 0.0 mmol/L were used. The results obtained for test samples were compared with a standard curve obtained with Trolox and were expressed as millimoles of Trolox equivalents per liter.
Optimization of reagents concentrations
To adjust the assay for measurements in canine serum, different concentrations of reagent 1 and reagent 2 were tested with the standards at different concentrations and also with different samples in the Cobas Mira Plus biochemical analyzer.
Reagent 1 was prepared at 0.1, 0.25, 1.0 and 1.6 mmol/L of bathocuproinedisulfonic acid disodium salt in 10 mmol/L phosphate buffer (pH 7.4) while reagent 2 was prepared at 0.1, 0.5, and 0.8 mmol/L of CuSO4 in ultrapure water.
The optimal concentrations were selected based on the production of a higher signal with lower background and a lower intra-assay imprecision calculated after analysis of one sample five times in one assay run.
Analytical validation of the assay
For the analytical validation of the CUPRAC assay, imprecision, accuracy, and sensitivity were evaluated following previously reported protocols [19–22].
Imprecision
Imprecision was expressed as coefficient of variation (CV) and was calculated as inter- and intra-assay variations. The CV was calculated as the standard deviation (SD) divided by the mean value (Xmean) of analyzed replicates x 100 % in the formula CV = (SD x 100 %)/ Xmean. To determine inter-assay variation, four serum samples were used. Inter-assay CV was determined by analyzing the same samples in separate runs performed on five different days. Five aliquots of each serum sample were stored in plastic vials at −20 °C until analysis. On the day of analysis, the samples were brought to room temperature prior to TACc measurement. The intra-assay CV was calculated after analysis of four samples five times in one assay run. Intra-assay CV tests were performed for all the different combination of reagents tested, although in the results only appear the values for the final concentration selected for the assay.
Accuracy
The accuracy was evaluated through assessment of linearity and spiking recovery. The linearity was evaluated by linearity under dilution, then duplicate determinations of TACc were made of a canine serum diluted at 1/2, 1/4, 1/8, 1/16 and 1/32 using ultrapure water. Dilution of a Trolox solution (2.0, 1.0, 0.5, 0.25, 0.125, and 0.0625 mmol/L) was also analyzed. For the spiking recovery, two canine serum samples with a known TACc concentration were mixed in different percentages (12.5, 25, 50, 75 and 87.5 %). The percentages of the measured TACc concentrations to the expected TACc concentrations were then calculated.
Sensitivity
The detection limit was calculated on the basis of data from 20 replicate TACc determinations of ultrapure water as mean value plus 3 SDs. The lower limit of quantification (LLOQ) was calculated based on the lowest TACc concentration that could be measured within a CV less than 15 % [20].
Effects of hemolysis and lipemia
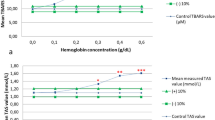
In order to examine the effect of hemolysis and lipemia, serum samples from three dogs were mixed with various concentrations of hemoglobin and lipids solution, respectively, and TACc was measured [23]. To study the effects of hemolysis, fresh hemolysate was prepared by the addition of distilled water to packed, washed canine red blood cells from one dog, followed by centrifugation to remove cell debris. The hemoglobin concentration was adjusted to 80, 40, 20, 10, 5, and 0.0 g/L. Ten μL of each concentration were added to three 90 μL samples of canine serum to produce test samples with final hemoglobin concentration of 8, 4, 2, 1, 0.5, and 0.0 g/L, respectively. The 0.0 g/L concentration was reached by adding 10 μL of distilled water to 90 μL of the serum sample. Prepared samples were used to determine the TACc concentrations.
To investigate the effects of lipemia, a commercial fat emulsion (Lipofundina 20 %; Braun Medical S.S.) with triglycerides concentration of 200 g/L was serially diluted to 50, 25, 12.5, 6.25,3.125 and 0.0 g/L. Ten μL of each dilution were added to 90 μL of the serum samples and were used to determine the TACc concentration. The final concentrations of triglycerides in the samples were 5, 2.5, 1.25, 0.625, 0.3125, and 0.0 g/L (10 μL of distilled water were added to 90 μL of the serum samples).
The TACc measurements to evaluate the effect of hemolysis and lipemia in the assay were made in the Olympus AU400.
Clinical validation
TACc levels were determined in healthy (control) dogs and dogs with inflammatory bowel disease (IBD). The control samples were from ten (seven males and three females) clinically healthy dogs of several different breeds aged between 3 and 8 years old, that were presented for routine checkups and had normal physical examination. Twelve dogs with IBD were included in this study. They were four female and eight male dogs aged between 3 and 8 years old also of different several breeds. A diagnosis of IBD was made on the basis of clinical signs (vomiting, diarrhea, and weight loss) of at least 3 weeks’ duration, and detection of lymphoplasmacytic inflammation during histologic examination of duodenal biopsy samples following the criteria of Ohta et al. [24]. Exclusion of other causes of chronic gastrointestinal tract signs including urinalysis, abdominal ultrasound, fecal exam, complete blood count and serum biochemistry were made [25, 26].
Blood samples of the healthy and diseased dogs were collected from each via jugular or lateral saphenous venipuncture into tubes without anticoagulant. Samples were centrifuged at 3,500 x g for 5 min at 20 °C. The serum samples were stored in plastic vials at - 20 °C until analysis.
Statistical analysis
Arithmetic means, medians, intra- and inter-assay CVs were calculated by use of routine descriptive statistical procedures and computer software (Excel 2013, Microsoft; GraphPad Statistics Guide). Linearity under dilution was investigated by linear regression. To compare the TACc results from both analyzers a Spearman correlation coefficient was used. The influence of hemolysis or lipemia on TACc concentration was investigated by use of 1-way ANOVA and Dunnett posttests. Interferograms were prepared to show the differences in TACc concentrations when hemoglobin or lipids were added. Kolmogorov-Smirnov’s test was performed to assess normality of data. Comparison of the TACc concentrations between healthy dogs and dogs with IBD were made by use of Student’s t test once a parametric distribution was given. For all tests, P < 0.05 was considered as statistically significant.
Results
Analytical validation
Results of the optimization of the reagents appear in Figs. 1 and 2. When concentrations of 0.1 mmol/L of reagent number 1 was tested with Trolox, the method lost its linearity at high Trolox concentrations. The other concentrations (0.25, 1.0 and 1.6 mmol/L) showed a similar reaction kinetic, but the background of the reaction increased with increased concentrations. When different concentrations of reagent number 2 were tested with Trolox, the concentration of 0.1 mmol/L did not produce a reaction. Concentrations of 0.5 and 0.8 mmol/L showed a similar reaction kinetic, but when 0.8 mmol/L was used, the background was higher.
Optimization of both reagent concentrations on a Cobas Mira Plus biochemical analyzer. a, Effect of varying concentrations of reagent 1 (▼, 0.1; ○, 0.25; ●, 1.0; □, 1.6 mmol/L) on the calibration curves obtained at a fixed concentration of CuSO4 at 0.5 mmol/L. b, Effect of varying concentrations of reagent 2 (▼, 0.1; ●, 0.5; □, 0.8 mmol/L) on the calibration curves obtained at a fixed concentration of bathocuproinedisulfonic acid disodium salt at 0.25 mmol/L
Absorbance obtained of a reagent blank (H2O instead of sample) (▲) and of two different canine serum samples (●, ■) studied with different reagent 1 (A, 0.25; B, 1.0; C, 1.6 mmol/L) and reagent 2 concentrations (D, 0.5; E, 0.8 mmol/L). When the reagent 1 was tested, the reagent 2 was fixed at 0.5 mmol/L and when the reagent 2 was tested, the reagent 1 was fixed at 0.25 mmol/L
When canine serum samples were tested, higher values were obtained when the concentrations of reagents 1 and 2 were increased (Fig. 2). However, the background was also higher. Also, when the reagent 2 was set at 0.8 mmol/L, the imprecision of the assay increased (data not shown). Therefore final concentrations of 0.25 mmol/L bathocuproinedisulfonic acid disodium salt (reagent 1) and 0.5 mmol/L CuSO4 (reagent 2) were established as optimal for the assay.
The mean, SD, and the intra- and inter-assay CVs obtained with the CUPRAC assay in the two apparatus are shown in Table 1. Intra-assay CVs were lower than 2 % in all cases. The inter-assay CVs were lower than 7 % and 9 % for the Cobas Mira Plus and Olympus AU400, respectively.
The method showed a high linearity with the Trolox and with canine serum sample in both apparatus (Fig. 3). The results of spiking recovery obtained in the Cobas Mira Plus were between 100 % and 103 % (Table 2), and in the Olympus AU400 were between 97 % and 101 % (Table 3).
a and b, Regression lines showing the TACc concentrations in the Cobas Mira Plus with the Trolox solution and canine serum sample, respectively. c and d, Regression lines showing the linearity of TACc concentrations in the Olympus AU400 with the Trolox solution and canine serum sample, respectively. Coefficients of determination (R2) are shown
The assay detection limit in the Cobas Mira Plus and Olympus AU400 were 0.017 mmol/L (mean ± SD, 0.001 ± 0.005) and 0.003 mmol/L (mean ± SD, 0.0001 ± 0.001), respectively. The lower limit of quantification could not be determined because the CVs were less than 15 % in all cases.
Data of the TACc obtained with the Cobas Mira Plus were significantly correlated with the data obtained in the Olympus AU400 (r = 0.973, P < 0.001).
Effects of hemolysis and lipemia
Lipids did not interfere with TACc concentrations (Fig. 4a). The addition of hemoglobin significantly increased the TACc concentrations, the increase being proportional with the hemoglobin concentration of the sample (Fig. 4b).
Clinical validation
The TACc results for serum samples in both apparatus are shown in Table 4. The TACc concentrations in dogs with IBD were significantly lower than those in healthy dogs when measured with both apparatus.
Discussion
In this study, an assay for evaluation of TAC by the CUPRAC method was described and validated for the first time in serum of dogs. This method is automated and uses bathocuproinedisulfonic acid disodium salt. The preparation of the reagents and measurement steps are simpler and faster compared to what has reported in other CUPRAC assays [16], making easier its application on routine analysis. Although in this paper the description of the assay is for automated analyzers, the method can be easily adapted to other formats such as 96 microplate well.
The optimal reagent concentrations determined in this work were different from those described in humans. Campos et al. [19] reported that the final established concentrations were 0.2 and 0.1 mmol/L for reagent number 1 and 2, respectively. Another study reported that the optimal concentrations for the reagent number 1 and 2 were 0.7 and 0.128 mmol/L, respectively [27]. In our study, it was found that higher concentrations of both reagents, especially reagent 2, were needed for the development of an optimal reaction (lower background and higher difference in absorbance between healthy and diseased individuals, and lower imprecision) in dog serum.
The validation results showed that the method has a good precision since all the intra- and inter-assay CVs in both apparatus were lower than 9 %. The method showed a good linearity with dog samples and with serial dilutions of Trolox. Campos et al. [19] also reported a linearity of this assay with Trolox dilutions. The assay showed recovery rates of around 100 % when canine serum samples were mixed at different proportions, indicating that the assay was accurate when measuring the TACc in canine serum samples.
The validation was performed in two different automated analyzers, the Cobas Mira Plus and Olympus AU400 in order to evaluate their agreement [28] and the repeatability of method in different equipment [29]. In this study, a high correlation between the TACc results obtained using the Cobas Mira Plus and Olympus AU400 was achieved revealing that this assay can be performed in both apparatus. Regarding the analytical validation results, the two automated equipment also gave similar results with the exception of the detection limit that was higher in the Cobas Mira Plus compared with the Olympus AU400. However, both detection limits were lower than the values usually observed in routine analyses and do not compromise the analytical sensitivity of the assay.
The presence of lipemia and hemolysis can interfere with the results of various analytes leading to an erroneous interpretation if the effect is unknown [23]. In the case of the CUPRAC assay, the lipemia did not interfere with the assay, which is an advantage to use it in clinical setting. However, hemolysis resulted in higher TACc concentrations. Therefore, results of this assay should be interpreted with caution when hemolytic samples are used.
Dogs with IBD had lower TACc concentrations than healthy dogs. The IBD is a progressive gastrointestinal tract disorder of unknown cause [25, 30]. During active episodes of IBD, the uncontrolled overproduction of reactive oxygen species could easily overwhelm the antioxidants, which are protective mechanisms, resulting in oxidative damage to cells and tissue. It was suggested that, this event may play a role in the pathogenesis of the disease [31–33]. In humans, the serum TAC, evaluated by the crocin bleaching method, was significantly reduced in IBD patients compared to healthy controls [32]. This is in line with the results of the present study, where our assay was able to demonstrate diminished concentrations of total antioxidants likely in response to oxidative stress in dogs with IBD.
Conclusions
In conclusion, the automated CUPRAC assay validated in this study can measure the TAC in serum of dogs in a simple, fast and reliable manner and could be adapted to two common automated analyzers. It is expected that this assay could contribute to a wider use of TACc measurements and therefore the evaluation of the antioxidant status in canine medicine.
References
López-Alarcón C, Denicola A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal Chim Acta. 2013;763:1–10.
Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–3.
Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci. 1993;84:407–12.
Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6.
Rice-Evans CA, Diplock AT, Symons MC. Techniques in free radical research. Laboratory techniques in biochemistry and molecular biology. London: Elsevier; 1991.
Bartosz G. Non-enzymatic antioxidant capacity assays: limitations of use in biomedicine. Free Radic Res. 2010;44:711–20.
Glantzounis GK, Tselepis AD, Tambaki AP, Trikalinos TA, Manataki AD, Galaris DA, Tsimoyiannis EC, Kappas AM. Laparoscopic surgery-induced changes in oxidative stress markers in human plasma. Surg Endosc. 2001;15:1315–9.
Zulfikaroglu B, Koc M, Soran A, Isman FK, Cinel I. Evaluation of oxidative stress in laparoscopic cholecystectomy. Surg Today. 2002;32:869–74.
Hopps E, Canino B, Montana M, Lo Presti R, Averna MR, Caimi G. Behavior of the total antioxidant status in a group of subjects with metabolic syndrome. Diabetes Metab Syndr. 2014;8:166–9.
Mohieldein AH, Hasan M, Al-Harbi KK, Alodailah SS, Azahrani RM, Al-Mushawwah SA. Dyslipidemia and reduced total antioxidant status in young adult Saudis with prediabetes. Diabetes Metab Syndr. 2015;9:287–91.
Lee JY. Evaluation of the total oxidant and antioxidant status of the plasma of dogs anaesthetised with isoflurane. Vet Rec. 2013;173:96.
Lee JY, Kim MC. Comparison of oxidative stress status in dogs undergoing laparoscopic and open ovariectomy. J Vet Med Sci. 2014;76:273–6.
Martínez-Subiela S, Bernal LJ, Tvarijonaviciute A, Garcia-Martinez JD, Tecles F, Cerón JJ. Canine demodicosis: the relationship between response to treatment of generalised disease and markers for inflammation and oxidative status. Vet Dermatol. 2014;25:72–e24.
Kocaturk M, Tvarijonaviciute A, Martinez-Subiela S, Tecles F, Eralp O, Yilmaz Z, Ceron JJ. Inflammatory and oxidative biomarkers of disease severity in dogs with parvoviral enteritis. J Small Anim Pract. 2015;56:119–24.
Winter JL, Barber LG, Freeman L, Griessmayr PC, Milbury PE, Blumberg JB. Antioxidant status and biomarkers of oxidative stress in dogs with lymphoma. J Vet Intern Med. 2009;23:311–6.
Apak R, Güçlü K, Özyürek M, Karademi˙ r SEN, Altun M. Total antioxidant capacity assay of human serum using copper (II)-neocuproine as chromogenic oxidant: the CUPRAC method. Free Radic Res. 2005;39:949–61.
Güçlü K, Sözgen K, Tütem E, Özyürek M, Apak R. Spectrophotometric determination of ascorbic acid using copper (II)–neocuproine reagent in beverages and pharmaceuticals. Talanta. 2005;65:1226–32.
Özyürek M, Güçlü K, Bektaşoğlu B, Apak R. Spectrophotometric determination of ascorbic acid by the modified CUPRAC method with extractive separation of flavonoids–La (III) complexes. Anal Chim Acta. 2007;588:88–95.
Campos C, Guzmán R, López-Fernández E, Casado Á. Evaluation of the copper (II) reduction assay using bathocuproinedisulfonic acid disodium salt for the total antioxidant capacity assessment: The CUPRAC–BCS assay. Anal Biochem. 2009;392:37–44.
Tiwari G, Tiwari R. Bioanalytical method validation: An updated review. Pharm Methods. 2010;1:25–38.
Tvarijonaviciute A, Tecles F, Caldin M, Tasca S, Cerón J.-Validation of spectrophotometric assays for serum paraoxonase type-1 measurement in dogs. Am J Vet Res. 2012;73:34–41.
Kjelgaard-Hansen M, Jacobsen S. Assay validation and diagnostic applications of major acute-phase protein testing in companion animals. Clin Lab Med. 2011;31:51–70.
Martínez-Subiela S, Cerón JJ. Effects of hemolysis, lipemia, hyperbilirrubinemia, and anticoagulants in canine C-reactive protein, serum amyloid A, and ceruloplasmin assays. Can Vet J. 2005;46:625–9.
Ohta H, Sunden Y, Yokoyama N, Osuga T, Lim SL, Tamura Y, Morishita K, Nakamura K, Yamasaki M, Takiguchi M. Expression of apical junction complex proteins in duodenal mucosa of dogs with inflammatory bowel disease. Am J Vet Res. 2014;75:746–51.
Washabau RJ, Day MJ, Willard MD, Hall EJ, Jergens AE, Mansell J, Minami T, Bilzer TW. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med. 2010;24:10–26.
Rossi G, Cerquetella M, Antonelli E, Pengo G, Magi GE, Villanacci V, Rostami-Nejad M, Spaterna A, Bassotti G. The importance of histologic parameters of lacteal involvement in cases of canine lymphoplasmacytic enteritis. Gastroenterol Hepatol Bed Bench. 2015;8:33–41.
Gosmaro F, Bagnati M, Berto S, Bellomo G, Prenesti E. Measurement of total antioxidant capacity of human plasma: setting and validation of the CUPRAC–BCS method on routine apparatus ADVIA 2400. Talanta. 2013;115:526–32.
Sutton A, Dawson H, Hoff B, Grift E, Shoukri M. Analyte comparisons between 2 clinical chemistry analyzers. Can Vet J. 1999;40:255.
Baral R, Morton J, Dhand NK, Krockenberger MB, Govendir M. Repeatability of results from three in-house biochemistry analyzers and a commercial laboratory analyzer used in small animal practice. Comp Clin Path. 2015;24:755–765.
Jackson M, Jewell D. Metabolomic and clinical disturbances in canine inflammatory bowel disease. FASEB J. 2015;29:265–7.
Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, Grisham MB. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease 1,2. Free Radic Biol Med. 2002;33:311–22.
Koutroubakis I, Malliaraki N, Dimoulios PD, Karmiris K, Castanas E, Kouroumalis EA. Decreased total and corrected antioxidant capacity in patients with inflammatory bowel disease. Dig Dis Sci. 2004;49:1433–7.
Minamoto Y, Otoni CC, Steelman SM, Büyükleblebici O, Steiner JM, Jergens AE, Suchodolski JS. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes. 2015;6:33–47.
Acknowledgements
Not applicable.
Funding
The National Council for Scientific and Technological Development (CNPq) of Brazil funded the PhD scholarship of CPR. The Ministerio de Economia y Competitividad of Spain funded AT through a postdoctoral grant (Juan de la Cierva). This study was also funded by the Seneca Foundation of Murcia Region (19894/GERM/15).
Availability of data and materials
The authors prefer not to show the raw data.
Authors’ contributions
CPR and JJC designed and led the project, and wrote the manuscript; SMS, JHR and AT contributed to the interpretation of the data and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the University of Murcia (Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Rubio, C.P., Tvarijonaviciute, A., Martinez-Subiela, S. et al. Validation of an automated assay for the measurement of cupric reducing antioxidant capacity in serum of dogs. BMC Vet Res 12, 137 (2016). https://doi.org/10.1186/s12917-016-0760-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-016-0760-2