Abstract
Background
Patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer and primary resistance to trastuzumab have a poor clinical outcome and lack good evidence to inform clinical decision. This study investigated the efficacy and safety of pyrotinib plus capecitabine in this population.
Methods
This phase 2 trial was conducted at 16 sites in China. Patients received oral pyrotinib 400 mg once daily and capecitabine 1000 mg/m2 twice a day on days 1–14 of each 21-day cycle until disease progression or intolerable toxicity. The primary endpoint was investigator-assessed progression-free survival (PFS).
Results
Between June 2019 and September 2021, 100 patients were enrolled with a median age of 51 years (range, 24–69). All patients had been treated with trastuzumab and 21 (21.0%) patients had prior use of pertuzumab. As of August 31, 2022, the median follow-up duration was 20.1 months (range, 1.3–38.2). The median PFS was 11.8 months (95% confidence interval [CI], 8.4–15.1), which crossed the pre-specified efficacy boundary of 8.0 months. The objective response rate was 70.0% (70/100), with a median duration of response of 13.8 months (95% CI, 10.2–19.3). The disease control rate was 87.0% (87/100). The median overall survival was not reached. The most common grade ≥ 3 treatment-emergent adverse event was diarrhea (24 [24.0%]). No treatment-related deaths occurred.
Conclusions
Pyrotinib plus capecitabine can be considered to be a treatment option in HER2-positive advanced breast cancer patients who have shown primary resistance to trastuzumab. Even in the era of modern anti-HER2 treatments, this clinical setting warrants more investigations to meet unmet needs.
Trial registration
ClinicalTrials.gov, NCT04001621. Retrospectively registered on June 28, 2019.
Similar content being viewed by others
Background
Human epidermal growth factor receptor 2 (HER2)-positive breast cancer is an aggressive disease subtype, which accounts for 15–20% of breast cancers [1]. Trastuzumab is the first approved targeted biological, which significantly changes the natural course of this disease. In exposure to trastuzumab, 14–31% of early breast cancer and nearly all of advanced breast cancer can develop primary or secondary resistance to trastuzumab [2,3,4,5,6,7,8]. Those primary-resistant patients have either early relapse in the early setting or early progression in the advanced setting, representing approximately 30–50% of total recurrence [2,3,4,5,6] and approximately 1–25% of total progression [7, 8]. Patients with primary trastuzumab resistance are likely to be more aggressive and might derive no benefit from rechallenge of trastuzumab [9], while almost all recruited patients in the early-stopped phase 3 GBG26/BIG 3–05 trial were secondarily resistant to trastuzumab and could really benefit from it [10]. Therefore, there are unmet needs for patients with primary trastuzumab resistance.
Definition of primary trastuzumab resistance is derived from and serves for clinical trials, while its value of guiding clinical practice is not clarified. The cutoff to differentiate primary resistance from secondary resistance is not completely consistent in different clinical trials [11,12,13,14,15,16,17,18]. Previous clinical trials which only enrolled primarily trastuzumab-resistant, HER2-positive advanced breast cancer added the mammalian target of rapamycin (mTOR) inhibitor [11,12,13], tyrosine kinase inhibitor (TKI) [14], phosphatidylinositol 3-kinase (PI3K) inhibitor [15,16,17], or programmed cell death-1 inhibitor [18] to anti-HER2 therapy with or without chemotherapy, but all showed disappointing clinical benefits. Two phase 3 trials showed that patients with primary trastuzumab resistance had a median progression-free survival (PFS) of only 5.5 months with afatinib plus vinorelbine and 7.0 months with everolimus plus trastuzumab and vinorelbine, respectively [13, 14]. These PFS results were significantly shorter than others reported in patients with HER2-positive advanced breast cancer in a similar second-line setting, suggesting the aggressiveness of primary trastuzumab resistance and needing more investigations. Currently, the standard second-line treatment for HER2-positive advanced breast cancer has shifted from trastuzumab emtansine (T-DM1) to trastuzumab deruxtecan (T-DXd). However, the enrolled patients in the phase 3 trials of these antibody–drug conjugates were mixed population and patients with primary trastuzumab resistance were under-represented [19, 20]. It remains to be elucidated whether the primarily trastuzumab-resistant patients can derive the same benefit from novel anti-HER2 agents as others [19, 20].
Primary resistance mostly stands for intrinsic resistance and HER2 independency, while secondary resistance reflects enquired loss of sensitivity or presence of dominant resistant subclones [21]. Possible mechanisms of primary trastuzumab resistance include impaired binding to the extracellular domain of HER2, such as MUC4 or MUC1 expression [22, 23]; high expression of HER2 carboxy-terminal fragment p95HER2 [24]; HER2△16 lacking exon 16 [25]; activation of alternative signaling pathways, such as PI3K/protein kinase B (AKT)/mTOR and mitogen-activated protein kinase (MAPK) pathways [26, 27]; overexpression of insulin-like growth factor-1 receptor [28]; and epidermal growth factor receptor (EGFR) or HER3 amplification [29, 30]. Some newly investigated mechanisms include induction of immune suppression, vascular mimicry, breast cancer stem cells, and metabolic escape [31]. It is indicated that P95HER2, a truncated form of HER2 that lacks the extracellular domain to bind trastuzumab, is responsive to lapatinib [32, 33]. Pan-HER TKIs can also hinder tumor development through targeting other receptors (such as EGFR) that increase with trastuzumab exposure [34]. Thus, primary trastuzumab resistance might be overcome by pan-HER TKIs [32,33,34,35].
Pyrotinib is an irreversible pan-HER TKI that targets EGFR, HER2, and HER4. The phase 3 PHOEBE study confirmed the superiority of pyrotinib plus capecitabine over lapatinib plus capecitabine in patients with trastuzumab-taxane-treated, HER2-positive metastatic breast cancer [36]. Here we designed this phase 2 PICTURE study to investigate the efficacy and safety of pyrotinib plus capecitabine in patients with HER2-positive advanced breast cancer and primary trastuzumab resistance.
Methods
Study design and participants
This was an investigator-initiated, single-arm, phase 2 trial conducted at 16 sites in China. Patients were included if they were females aged 18–70 years; had pathologically confirmed HER2-positive (score 3 + by immunohistochemistry, or 2 + with positive results of fluorescence in-situ hybridization) locally advanced or metastatic breast cancer; had primary resistance to trastuzumab; had an Eastern Cooperative Oncology Group performance status of 0 or 1; had known hormone receptor status; had an expected survival of ≥ 3 months; had at least one measurable lesion according to the Response Evaluation Criteria In Solid Tumors (RECIST), version 1.1 [37]; and had adequate bone marrow (neutrophil count ≥ 1.5 × 109/L; platelet count ≥ 100 × 109/L; hemoglobin ≥ 90 g/L), hepatic (total bilirubin ≤ 1.5 × upper limit of normal [ULN]; alanine aminotransferase and aspartate aminotransferase ≤ 3 × ULN [≤ 5 × ULN for patients with liver metastases]), renal (creatinine ≤ 1.5 × ULN; creatinine clearance rate ≥ 50 mL/min), and cardiac (left ventricular ejection fraction ≥ 50%; Fridericia’s corrected QT interval < 480 ms) function. Based on the definitions used in previous clinical trials [14], primary trastuzumab resistance was defined as progression during (neo)adjuvant trastuzumab (subgroup A) or within 12 months of completing (neo)adjuvant trastuzumab (subgroup B; treatment must have been for ≥ 9 weeks), or progression within 6 months after initiation of first-line trastuzumab for advanced disease (subgroup C; treatment must have been for ≥ 6 weeks). A washout period of 4 weeks was required after last trastuzumab-based therapy. Patients with brain metastases that had been treated with local treatment or patients with stable brain metastases could be enrolled if they did not require dexamethasone or mannitol. The key exclusion criteria were meningeal and/or spinal cord metastases; other malignancies within 5 years, except for cured cervical cancer in situ, skin basal cell carcinoma, and skin squamous cell carcinoma; previous use of anti-HER2 TKI or antibody–drug conjugate with proven efficacy; active hepatitis B or C; history of transplantation; uncontrolled hypertension or diabetes mellitus; or lactating or pregnant women.
The study was conducted in accordance with the Declarations of Helsinki and Good Clinical Practice and was approved by the ethics committee of each participating center. Written informed consent was obtained from each patient. The study was registered with ClinicalTrials.gov, NCT04001621.
Procedures
Patients received oral pyrotinib 400 mg once daily and oral capecitabine 1000 mg/m2 twice a day on days 1–14 of each 21-day cycle. Treatment was continued until disease progression, intolerable toxicity, or withdrawal of consent. Dose reductions, interruptions, and discontinuations of study drugs were allowed according to AEs. The dose of pyrotinib could be reduced stepwise from 400 to 320 mg to 240 mg. The dose of capecitabine was permitted to be reduced stepwise by 25%. Dose escalation was not permitted upon resolution of toxicity. The cumulative interruption time of pyrotinib should be no more than 14 days in each cycle; otherwise, patients would be withdrawn from the study.
Imaging examinations were performed every 6 weeks for the first 20 treatment cycles, and every 12 weeks thereafter. Radiological response was assessed by investigators according to RECIST 1.1. For patients who discontinued the study treatment before disease progression or death, subsequent imaging examinations were performed every 12 weeks until the initiation of other anti-cancer therapies, disease progression, or death. OS was followed every 12 weeks until loss to follow-up, death, or completion of the study. Adverse events (AEs) during the study treatment and until 28 days after the last dose of study drug were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Endpoints
The primary endpoint was PFS, defined as the time from the initiation of study treatment to the first disease progression per RECIST 1.1 or any-cause death, whichever came first.
Secondary endpoints included objective response rate (ORR; defined as the proportion of patients with the best response of complete response [CR] or partial response [PR] per RECIST 1.1), duration of response (defined as the time from the first CR or PR to disease progression per RECIST 1.1 in patients with confirmed objective response), disease control rate (DCR; defined as the proportion of patients with the best response of CR, PR, or stable disease per RECIST 1.1), overall survival (OS; defined as the time from the initiation of study treatment to any-cause death), and safety.
Statistical analysis
Trastuzumab plus vinorelbine was the backbone treatment for trastuzumab-pretreated patients in 2019 in China; thus, the median PFS (5.78 months) of the control group in BOLERO-3 was considered as the historical control in our study [13]. The study treatment was expected to increase the median PFS to 8.0 months. Assuming that the survival time was in accordance with exponential distribution, 64 disease progression or death events were required to test the difference between the study treatment and historical control with a significance level of 5% and a power of 80%. The planned enrollment period was 32 months and the planned follow-up period was 24 months. Considering a dropout rate of 5%, 96 patients were required.
Efficacy and safety were analyzed in all patients with at least one dose of study drug. Continuous variables were expressed as median (range), and categorical variables were expressed as frequency (percentage). The 95% confidence intervals (CIs) of ORR and DCR were calculated using the Clopper-Pearson method. Comparison of ORR was performed between subgroups using the chi-square test. Median PFS and OS were estimated using the Kaplan–Meier method, and their 95% CIs were calculated using the Brookmeyer-Crowley method. Comparison of PFS was performed between subgroups using the Cox proportional hazard regression model. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Two-sided P < 0.05 was considered statistically significant.
Results
Patient characteristics and treatment
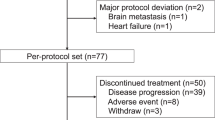
Between June 20, 2019, and September 19, 2021, a total of 108 patients were screened for eligibility, and 100 patients were enrolled and included in the efficacy and safety analyses (Fig. 1). Baseline characteristics are shown in Table 1. Of 100 patients, 65 (65.0%) had hormone receptor-negative disease, 94 (94.0%) had metastatic disease, 66 (66.0%) had visceral metastases, and 21 (21.0%) had prior use of pertuzumab. By the data cutoff date on August 31, 2022, the median follow-up duration was 20.1 months (range, 1.3–38.2). Median duration of the study treatment was 9.3 months (range, 0.2–38.2). Twenty-six (26.0%) patients were still on treatment.
Efficacy
By the data cutoff date, 66 (66.0%) of 100 patients had disease progression or died. The median PFS was 11.8 months (95% CI, 8.4–15.1; Fig. 2A). Twenty-five deaths occurred, and the median OS was not reached (95% CI, 29.0–not reached). The 1-year OS rate was 86.6%.
Kaplan–Meier curves for progression-free survival. A Total population. B Subgroup by the subcategory of primary trastuzumab resistance. Subgroup A included patients who had progression during adjuvant trastuzumab; subgroup B included patients who had progression within 12 months of completing adjuvant trastuzumab; subgroup C included patients who had progression within 6 months after the initiation of first-line trastuzumab for advanced disease. C Subgroup by hormone receptor status
Further analyses showed that the median PFS was 8.2 months (95% CI, 3.0–20.7) in subgroup A (progression during adjuvant trastuzumab; n = 21), 17.8 months (95% CI, 13.8–not reached) in subgroup B (progression within 12 months of completing adjuvant trastuzumab; n = 49), and 5.6 months (95% CI, 4.1–6.9) in subgroup C (progression within 6 months after initiation of first-line trastuzumab for advanced disease; n = 30; Fig. 2B). No significant difference in median PFS was observed in subgroup by hormone receptor status (hormone receptor-positive: 9.7 months [95% CI, 6.4–18.4]; hormone receptor-negative: 12.3 months [95% CI, 8.2–17.8]; hazard ratio, 0.926 [95% CI, 0.559–1.533]; P = 0.765; Fig. 2C).
Seven of 100 patients achieved confirmed CR and 63 achieved confirmed PR, with a confirmed ORR of 70.0% (95% CI, 60.0–78.8). Median duration of response was 13.8 months (95% CI, 10.2–19.3). Seven patients with CR were all from subgroup B. The ORR was significantly higher in subgroup B than in subgroups A and C (Additional file 1: Table S1), showing a similar trend with PFS. The DCR was 87.0% (95% CI, 78.8–92.9). Waterfall plot for best percent change in target lesions from baseline among individual patients is shown in Fig. 3.
Waterfall plot for best percent change in target lesions from baseline among individual patients (n = 96). Four of 100 patients discontinued treatment before the first post-baseline imaging examination; their responses could not be assessed and were not shown in this figure. One of 96 patients had a stable disease and withdrew from the study due to personal reason without confirmation of response; the final response was deemed as not evaluable
Safety
Treatment-emergent AEs (TEAEs) are summarized in Table 2. All patients had TEAEs, and 56 (56.0%) reported grade ≥ 3 TEAEs. The most common grade ≥ 3 TEAEs included diarrhea (24 [24.0%]), palmar-plantar erythrodysesthesia syndrome (nine [9.0%]), decreased neutrophil count (eight [8.0%]), hypokalemia (six [6.0%]), and anorexia (five [5.0%]). No grade 4 diarrhea occurred, while grade 3 diarrhea mostly (17/24, 70.8%) occurred in the first treatment cycle (Additional file 1: Fig. S1).
Twelve (12.0%) of 100 patients had dose reductions of pyrotinib due to AEs, and 37 (37.0%) had dose reductions of capecitabine. Four (4.0%) patients discontinued capecitabine due to increased blood bilirubin, anemia, dyspepsia, and palmar-plantar erythrodysesthesia syndrome, respectively. No AEs leading to discontinuation of pyrotinib occurred. No treatment-related deaths occurred.
Discussion
To our knowledge, this phase 2 trial was the first positive multicenter study for patients with HER2-positive advanced breast cancer and primary trastuzumab resistance, in contrast to previous failed trials in this clinical setting. The median PFS of 11.8 months met the primary endpoint, significantly longer than the pre-trial hypothesis of 8.0 months in the trial protocol. The combination of pyrotinib and capecitabine was also demonstrated well-tolerated and had no new safety signals in this study.
Patients with primary trastuzumab resistance are under-represented in two pivotal phase 3 trials (EMILIA and DESTINY-Breast 03). Even for those who received real second-line treatment in these two trials, they were either primary- or secondary-resistant to trastuzumab. The EMILIA study confirmed the role of T-DM1 in patients with HER2-positive advanced breast cancer previously treated with trastuzumab and a taxane when compared with lapatinib plus capecitabine (PFS: 9.6 vs. 6.4 months; hazard ratio, 0.65; P < 0.001; OS: 30.9 months vs. 25.1 months; hazard ratio, 0.68; P < 0.001) [19]. T-DXd defeated T-DM1 based on the amazing results from the phase 3 DESTINY-Breast 03 study (PFS: 28.8 months vs. 6.8 months; hazard ratio, 0.33; P < 0.0001) [38]. Both of these two studies included a subset of primary trastuzumab-resistant patients, mostly in the context of advanced disease, but the specific number of these patients and corresponding efficacy of T-DXd and T-DM1 are not available. While the whole world rejoices in the emergence of T-DXd, the hard-to-treat, primarily trastuzumab-resistant population still needs attention.
Among previous clinical trials focusing on trastuzumab-resistant patients, the definition of primary trastuzumab resistance is not unified, leading to hard indirect comparisons across studies (Additional file 1: Table S2) [11,12,13,14,15,16,17,18]. This definition in our study was generally consistent with that in the phase 3 LUX-Breast 1 study [14]. We have to acknowledge that the definition used in this trial is still arbitrary; a thorough research on baseline re-biopsy sample might be more informative. In LUX-Breast 1, the ORR and median PFS were 46.1% and 5.5 months with afatinib plus vinorelbine [14], respectively. Compared with these results, pyrotinib plus capecitabine in our study had a higher ORR (70.0%) and doubled the median PFS (11.8 months), demonstrating its high potency. Randomized controlled trials are warranted to validate the role of pyrotinib plus capecitabine in patients with primarily trastuzumab-resistant, HER2-positive advanced breast cancer and under the unified definition of primary trastuzumab resistance.
Patients who had progression within 12 months of completing adjuvant trastuzumab (subgroup B in our study) are generally included in the trastuzumab-resistant population [12,13,14, 16]. Pyrotinib plus capecitabine resulted in a long median PFS in this subpopulation (17.8 months), which may be not inferior to reported in the first-line trials for advanced disease [39,40,41,42,43]. On the other hand, patients with rapid progression on trastuzumab during adjuvant therapy (subgroup A: 8.2 months) or for advanced disease (subgroup C: 5.6 months) only achieved modest PFS benefit with pyrotinib plus capecitabine, suggesting that different mechanisms of drug resistance might be involved. Of course, caution should be taken to interpret the data due to the limited subgroup sample size in our study, and confirmation of real differences in subpopulations might need separate investigations in statistically powered trials.
The safety profile of pyrotinib plus capecitabine was consistent with results from previous clinical trials [36, 44,45,46]. As expected, the most common grade ≥ 3 AE was diarrhea, which mainly occurred in the first treatment cycle and could be managed with dose reductions of pyrotinib and antidiarrheal agents. No diarrhea or other AEs resulted in discontinuation of pyrotinib.
This study has some limitations. First, there might be potential bias due to the single-arm design without control group. In fact, it is very hard to conduct randomized controlled trials since such patients were rare due to advance in early diagnosis and HER2-targeted therapy, and it took us 27 months to enroll 100 patients at 16 sites. In the future, direct comparison between our study treatment and modern treatment option, especially T-DXd, is urgently needed. Second, only Chinese patients were enrolled and the effect of pyrotinib plus capecitabine in other populations needs to be established. Third, the efficacy was assessed by investigators rather than independent review committee. Fourth, due to the small sample size, multivariable analyses were not performed to analyze the independent factors influencing the treatment response and survival. Finally, OS data are not mature yet, which will be reported in the future.
Conclusions
Pyrotinib plus capecitabine can be considered to be a treatment option in HER2-positive advanced breast cancer patients who have shown primary resistance to trastuzumab. Even in the era of modern anti-HER2 treatments, this clinical setting warrants more investigations to meet unmet needs.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AE:
-
Adverse event
- AKT:
-
Protein kinase B
- CI:
-
Confidence interval
- CR:
-
Complete response
- DCR:
-
Disease control rate
- EGFR:
-
Epidermal growth factor receptor
- HER2:
-
Human epidermal growth factor receptor 2
- MAPK:
-
Mitogen-activated protein kinase
- mTOR:
-
Mammalian target of rapamycin
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PI3K:
-
Phosphatidylinositol 3-kinase
- PR:
-
Partial response
- RECIST:
-
Response Evaluation Criteria In Solid Tumors
- T-DM1:
-
Trastuzumab emtansine
- T-DXd:
-
Trastuzumab deruxtecan
- TEAE:
-
Treatment-emergent adverse event
- TKI:
-
Tyrosine kinase inhibitor
- ULN:
-
Upper limit of normal
References
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12.
Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369(9555):29–36.
Perez EA, Romond EH, Suman VJ, Jeong J-H, Davidson NE, Geyer CE, Martino S, Mamounas EP, Kaufman PA, Wolmark N. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–73.
Perez EA, Romond EH, Suman VJ, Jeong J-H, Sledge G, Geyer CE, Martino S, Rastogi P, Gralow J, Swain SM, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–52.
Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G, Untch M, Smith I, Gianni L, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–205.
Piccart M, Procter M, Fumagalli D, de Azambuja E, Clark E, Ewer MS, Restuccia E, Jerusalem G, Dent S, Reaby L, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol. 2021;39(13):1448–57.
Perez EA, Barrios C, Eiermann W, Toi M, Im Y-H, Conte P, Martin M, Pienkowski T, Pivot X, Burris H, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35(2):141–8.
Swain SM, Miles D, Kim S-B, Im Y-H, Im S-A, Semiglazov V, Ciruelos E, Schneeweiss A, Loi S, Monturus E, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–30.
Montemurro F, Donadio M, Clavarezza M, Redana S, Jacomuzzi ME, Valabrega G, Danese S, Vietti-Ramus G, Durando A, Venturini M, et al. Outcome of patients with HER2-positive advanced breast cancer progressing during trastuzumab-based therapy. Oncologist. 2006;11(4):318–24.
von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann M, Bauer W, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27(12):1999–2006.
Morrow PK, Wulf GM, Ensor J, Booser DJ, Moore JA, Flores PR, Xiong Y, Zhang S, Krop IE, Winer EP, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29(23):3126–32.
Hurvitz SA, Dalenc F, Campone M, O’Regan RM, Tjan-Heijnen VC, Gligorov J, Llombart A, Jhangiani H, Mirshahidi HR, Tan-Chiu E, et al. A phase 2 study of everolimus combined with trastuzumab and paclitaxel in patients with HER2-overexpressing advanced breast cancer that progressed during prior trastuzumab and taxane therapy. Breast Cancer Res Treat. 2013;141(3):437–46.
André F, O’Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G, Masuda N, Wilks S, Arena F, Isaacs C, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15(6):580–91.
Harbeck N, Huang C-S, Hurvitz S, Yeh D-C, Shao Z, Im S-A, Jung KH, Shen K, Ro J, Jassem J, et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1): an open-label, randomised, phase 3 trial. Lancet Oncol. 2016;17(3):357–66.
Guerin M, Rezai K, Isambert N, Campone M, Autret A, Pakradouni J, Provansal M, Camerlo J, Sabatier R, Bertucci F, et al. PIKHER2: A phase IB study evaluating buparlisib in combination with lapatinib in trastuzumab-resistant HER2-positive advanced breast cancer. Eur J Cancer. 2017;86:28–36.
Pistilli B, Pluard T, Urruticoechea A, Farci D, Kong A, Bachelot T, Chan S, Han HS, Jerusalem G, Urban P, et al. Phase II study of buparlisib (BKM120) and trastuzumab in patients with HER2+ locally advanced or metastatic breast cancer resistant to trastuzumab-based therapy. Breast Cancer Res Treat. 2018;168(2):357–64.
Jain S, Shah AN, Santa-Maria CA, Siziopikou K, Rademaker A, Helenowski I, Cristofanilli M, Gradishar WJ. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res Treat. 2018;171(2):371–81.
Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, Campone M, Biganzoli L, Bonnefoi H, Jerusalem G, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019;20(3):371–82.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh D-Y, Diéras V, Guardino E, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91.
Cortés J, Kim S-B, Chung W-P, Im S-A, Park YH, Hegg R, Kim MH, Tseng L-M, Petry V, Chung C-F, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–54.
Wong H, Leung R, Kwong A, Chiu J, Liang R, Swanton C, Yau T. Integrating molecular mechanisms and clinical evidence in the management of trastuzumab resistant or refractory HER-2+ metastatic breast cancer. Oncologist. 2011;16(11):1535–46.
Fessler SP, Wotkowicz MT, Mahanta SK, Bamdad C. MUC1* is a determinant of trastuzumab (Herceptin) resistance in breast cancer cells. Breast Cancer Res Treat. 2009;118(1):113–24.
Nagy P, Friedländer E, Tanner M, Kapanen AI, Carraway KL, Isola J, Jovin TM. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65(2):473–82.
Sperinde J, Jin X, Banerjee J, Penuel E, Saha A, Diedrich G, Huang W, Leitzel K, Weidler J, Ali SM, et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin Cancer Res. 2010;16(16):4226–35.
Castiglioni F, Tagliabue E, Campiglio M, Pupa SM, Balsari A, Ménard S. Role of exon-16-deleted HER2 in breast carcinomas. Endocr Relat Cancer. 2006;13(1):221–32.
Dave B, Migliaccio I, Gutierrez MC, Wu M-F, Chamness GC, Wong H, Narasanna A, Chakrabarty A, Hilsenbeck SG, Huang J, et al. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol. 2011;29(2):166–73.
Loibl S, von Minckwitz G, Schneeweiss A, Paepke S, Lehmann A, Rezai M, Zahm DM, Sinn P, Khandan F, Eidtmann H, et al. PIK3CA mutations are associated with lower rates of pathologic complete response to anti-human epidermal growth factor receptor 2 (her2) therapy in primary HER2-overexpressing breast cancer. J Clin Oncol. 2014;32(29):3212–20.
Nahta R, Yuan LXH, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65(23):11118–28.
Krop IE, Flores L, Najita JS, Mayer IA, Hobday TJ, Falkson CI, Arteaga CL, Wolff AC, Dees EC, Rimawi MF, et al. The role of EGFR amplification in trastuzumab resistance: a correlative analysis of TBCRC003. J Clin Oncol. 2011;29(15_suppl):528.
Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68(14):5878–87.
Vivekanandhan S, Knutson KL. Resistance to trastuzumab. Cancers (Basel). 2022;14(20):5115.
Scaltriti M, Rojo F, Ocaña A, Anido J, Guzman M, Cortes J, Di Cosimo S, Matias-Guiu X, Ramon y Cajal S, Arribas J, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99(8):628–638.
Scaltriti M, Chandarlapaty S, Prudkin L, Aura C, Jimenez J, Angelini PD, Sánchez G, Guzman M, Parra JL, Ellis C, et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res. 2010;16(9):2688–95.
Schlam I, Tarantino P, Tolaney SM. Overcoming resistance to HER2-directed therapies in breast cancer. Cancers (Basel). 2022;14(16):3996.
Wu X, Yang H, Yu X, Qin J-J. Drug-resistant HER2-positive breast cancer: Molecular mechanisms and overcoming strategies. Front Pharmacol. 2022;13:1012552.
Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q, Tong Z, Li H, Zhang Q, Sun T, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(3):351–60.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
Hurvitz SA, Hegg R, Chung W-P, Im S-A, Jacot W, Ganju V, Chiu JWY, Xu B, Hamilton E, Madhusudan S, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105–17.
Xu B, Yan M, Ma F, Li W, Ouyang Q, Tong Z, Teng Y, Wang S, Wang Y, Geng C, et al. LBA19 Pyrotinib or placebo in combination with trastuzumab and docetaxel for HER2-positive metastatic breast cancer (PHILA): A randomized phase III trial. Ann Oncol. 2022;33(7_Supplement):S1387.
Wang X, Huang J. 239P Pyrotinib in combination with docetaxel as first-line treatment for HER2-positive metastatic breast cancer (PANDORA): a single-arm, multicenter phase II trial. Ann Oncol. 2022;33:S646–7.
Baselga J, Cortés J, Kim S-B, Im S-A, Hegg R, Im Y-H, Roman L, Pedrini JL, Pienkowski T, Knott A, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–19.
Xu B, Li W, Zhang Q, Shao Z, Li Q, Wang X, Li H, Sun T, Yin Y, Zheng H, et al. Pertuzumab, trastuzumab, and docetaxel for Chinese patients with previously untreated HER2-positive locally recurrent or metastatic breast cancer (PUFFIN): a phase III, randomized, double-blind, placebo-controlled study. Breast Cancer Res Treat. 2020;182(3):689–97.
Wardley AM, Pivot X, Morales-Vasquez F, Zetina LM, de Fátima Dias Gaui M, Reyes DO, Jassem J, Barton C, Button P, Hersberger V, et al. Randomized phase II trial of first-line trastuzumab plus docetaxel and capecitabine compared with trastuzumab plus docetaxel in HER2-positive metastatic breast cancer. J Clin Oncol. 2010; 28(6):976–983.
Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu Y, Li H, Yu S, Feng J, Wang S, et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study. J Clin Oncol. 2019;37(29):2610–9.
Yan M, Bian L, Hu X, Zhang Q, Ouyang Q, Feng J, Yin Y, Sun T, Tong Z, Wang X, et al. Pyrotinib plus capecitabine for human epidermal factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Transl Breast Cancer Res. 2020;1:13.
Yan M, Ouyang Q, Sun T, Niu L, Yang J, Li L, Song Y, Hao C, Chen Z, Orlandi A, et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 2022;23(3):353–61.
Acknowledgements
We thank all the patients who participated in this trial and their families. We thank Mian Wei (Medical Manager, Jiangsu Hengrui Pharmaceuticals Co., Ltd) for her input in data interpretation, Yitao Wang (Clinical Statistics Manager, Jiangsu Hengrui Pharmaceuticals Co., Ltd) for statistical support, and Fangzhou Xia (Medical Writer, Jiangsu Hengrui Pharmaceuticals Co., Ltd) for medical writing assistance.
Funding
This work was supported by Jiangsu Hengrui Pharmaceuticals Co., Ltd. The funder provided all the study drugs and participated in data interpretation, but had no role in study design, data collection, or drafting of the manuscript.
Author information
Authors and Affiliations
Contributions
XH conceived and designed the study. All authors contributed to the acquisition of data. JC, YT, HL, and XH contributed to the analysis and interpretation of data. JC and XH drafted the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content. XH contributed to the administrative support and study supervision. All authors are accountable for the accuracy and integrity of this work and approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declarations of Helsinki and Good Clinical Practice and was approved by the ethics committee of each participating center (approval number: 1903198–13, (2019) 2019–270, 2020YJZ11, 2019–029, 2020SR (No. 1), CS2019 (57), 2019KYER (No. 126), 2019155, (2019) ER (No. 22), (2020) RCR (No. 1), (S) ER (No. 2019051), LDYYLL2019-213, BF2019-145–01, SYL-201938–01, ER (R) 2019–235-01, R (2019) ER (No. 3)). All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Tumor response. Table S2. Indirect comparison across studies. Figure S1. The incidence of diarrhea in each treatment cycle.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cao, J., Teng, Y., Li, H. et al. Pyrotinib plus capecitabine for trastuzumab-resistant, HER2-positive advanced breast cancer (PICTURE): a single-arm, multicenter phase 2 trial. BMC Med 21, 300 (2023). https://doi.org/10.1186/s12916-023-02999-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-02999-0