Abstract
Background
Micronutrients play an essential role at every stage of the immune response, and deficiencies can therefore lead to increased susceptibility to infections. Previous observational studies and randomized controlled trials of micronutrients and infections are limited. We performed Mendelian randomization (MR) analyses to evaluate the effect of blood levels of eight micronutrients (copper, iron, selenium, zinc, beta-carotene, vitamin B12, vitamin C, and vitamin D) on the risk of three infections (gastrointestinal infections, pneumonia, and urinary tract infections).
Methods
Two-sample MR was conducted using publicly available summary statistics from independent cohorts of European ancestry. For the three infections, we used data from UK Biobank and FinnGen. Inverse variance-weighted MR analyses were performed, together with a range of sensitivity analyses. The threshold for statistical significance was set at P < 2.08E−03.
Results
We found a significant association between circulating levels of copper and risk of gastrointestinal infections, where a one standard deviation increase in blood levels of copper was associated with an odds ratio of gastrointestinal infections of 0.91 (95% confidence interval 0.87 to 0.97, P = 1.38E−03). This finding was robust in extensive sensitivity analyses. There was no clear association between the other micronutrients and the risk of infection.
Conclusions
Our results strongly support a role of copper in the susceptibility to gastrointestinal infections.
Similar content being viewed by others
Background
Gastrointestinal infections, pneumonia, and urinary tract infections are common causes of hospital admission and important causes of death [1]. Identifying modifiable risk factors for those infections is essential since the disease burden is projected to increase due to antibiotic resistance, an aging population, and emerging pathogens [2]. Multiple micronutrients have been established to have vital roles in the immune system and are important components for the proliferation and maturation of immune cells, cytokine release, and enzymes involved in immune cell activity for antioxidant host defense [3]. Deficiency can significantly impair host immunity, increasing susceptibility to infections [3].
Previous observational studies and randomized controlled trials have found that certain micronutrients reduce the risk of specific infections [3]. However, the results are conflicting, possibly due to factors such as high variability between studies and the use of different outcomes. It can be difficult to conduct randomized controlled trials due to logistical issues and costs, and not many adequately powered trials have evaluated the effect of micronutrients and infections. Also, it can be challenging to quantify the causal effects from traditional observational studies due to residual confounding and reverse causation [4].
Mendelian randomization (MR) provides an alternative method to determine evidence of causality. MR uses single-nucleotide polymorphisms (SNPs) identified by genome-wide association studies (GWASs) as genetic instruments to evaluate the effect of an exposure (e.g., blood levels of copper) on the risk of an outcome (e.g., gastrointestinal infection). GWASs have successfully identified several genetic variants involved in the metabolic pathway of several vitamins and minerals [5,6,7,8,9,10,11,12,13,14,15]. Importantly, since these genetic variants are allocated randomly at conception, MR studies are much less susceptible to reverse causation and confounding than traditional observational studies [4].
The aim of this study was to estimate the association between genetically predicted blood levels of micronutrients on the genetically predicted risk of infectious diseases. We identified eight micronutrients of interest that have previously been linked to the risk of infection and for which genetic instruments were available—copper, iron, selenium, zinc, beta-carotene, vitamin B12, vitamin C, and vitamin D—and evaluated the risk of the following three infections: gastrointestinal infections, pneumonia, and urinary tract infections.
Methods
Study design
This study is reported according to the STROBE-MR (Additional file 1: Table S1) [16]. A schematic summary of the study design is given in Fig. 1. Briefly, we conducted a two-sample MR study using data from publicly available summary statistics from fourteen GWASs: eight for the exposures and six for the outcomes. Both exposure and outcome cohorts were restricted to subjects of European ancestry to reduce bias from population stratification [17]. All data used in this work are publicly available from studies with relevant participant consent and ethical approval, and ethical approval from an institutional review board was therefore not necessary for the present study.
Data on the genetically predicted levels of circulating micronutrients
We searched for published GWASs evaluating individuals of European ancestry on the GWAS Catalog and PubMed (the last search was performed in May 2022). We did not find any GWAS conducted for vitamins B1, B2, B3, B5, B7, sulfur, iodine, chloride, and fluoride. The GWASs conducted for vitamin K, potassium, sodium, cobalt, chromium, and molybdenum were excluded because of no significant genome-wide results [8, 18, 19]. In total, fourteen micronutrients of potential interest were identified: calcium [5], copper [6], iron [7], magnesium [8], selenium [6], zinc [6], beta-carotene [9], folate [10], vitamin A [11], vitamin B6 [12], vitamin B12 [10], vitamin C [13], vitamin D [14], and vitamin E [15] (Additional file 2: Additional Text) [5,6,7,8,9,10,11,12,13,14,15, 20, 21]. For copper, we also identified a more recent and larger GWAS by Jäger et al. [20], but given that this study reported Z-scores and not beta-coefficients, we used the study by Evans et al. [6] in order to improve interpretability. However, the genetic instruments from the GWAS by Jäger et al. [20] were used in secondary analyses. Vitamin A and vitamin E were excluded because those GWASs were adjusted for body mass index (BMI) [22] which might introduce collider bias if the genetic instruments of the exposure of interest also have an effect on BMI [23].
For the main MR analysis, we included independent SNPs (r2 < 0.001 within 10,000-kb windows), strongly associated (P ≤ 5E−08) with the blood level of each micronutrient.
Data on the genetically predicted risk of infectious diseases
Based on the disease incidence and availability of published summary statistics, we evaluated the risk of the following three infections: gastrointestinal infections, pneumonia, and urinary tract infections. We used publicly available summary statistics from two independent cohorts of European ancestry: UK Biobank (UKBiobank HRC-imputed) [24] and FinnGen Release 6 [25, 26] (Table 1). The GWAS conducted using the UKBiobank HRC-imputed data was performed using SAIGE (a generalized mixed model association test that uses the saddlepoint approximation to account for case-control imbalance), adjusted for genetic relatedness, sex, birth year, and the first four principal components [24]. The GWASs conducted on the FinnGen dataset were analyzed using SAIGE and were adjusted for sex, age, first ten principal components, and genotyping batch [25].
In both the UK Biobank and FinnGen, cases and controls were defined based on International Classification of Diseases codes (10th revision) from hospital records (Additional file 2: Tables S2-S4) [27,28,29,30,31,32]. For each infectious disease, summary statistics were meta-analyzed using a random-effects model in METAL (version 2011-03-25) [33] (Additional file 3: Table S5). The meta-analyses included genomic control to account for residual population stratification [33]. Additionally, we conducted Cochran’s Q statistical test, included in METAL [33], to assess the heterogeneity between the two cohorts for the genetic instruments used for the outcomes.
Statistical power
The strength of each genetic instrument was estimated using the F statistic: F = R2(N − 2)/(1 − R2), where R2 equals the proportion of variance explained by the genetic instrument and N is the effective sample size of the GWAS for the SNP-micronutrient association [34]. The R2 value was calculated using the formula 2 × MAF(1 − MAF)beta2, where beta represents the effect estimate of the genetic variant in the exposure, measured in standard deviation (SD) units, and MAF represents the minor allele frequency [35] (Table 2). The effect allele frequency was not available for the genetic instruments used for copper, selenium, and zinc, published by Evans et al. [6]. However, for the main analysis, none of the copper or selenium-associated SNPs were palindromic (A/T or G/C alleles), so it was clear which allele was the effect allele. For zinc, we removed one genetic instrument, rs10931753, due to being palindromic. The effect allele frequencies from the meta-analysis of FinnGen and UK Biobank were used to estimate the F statistics and R2 for copper, selenium, and zinc. Although this may result in incorrect calculations of R2 (and thus F statistic and statistical power), our results aligned well with the reported R2 from Evans et al. [6], with an R2 of 5% for copper. We reported a lower R2 value for selenium (R2 of 2.4%), but we only used 1 SNP, while Evans et al. [6] reported a total R2 for 2 SNPs (R2 of 4%) and a lower R2 value for zinc (R2 of 4.25%), where we used 2 SNPs, while Evans et al. [6] reported a total R2 for 3 SNPs (R2 of 8%). Additionally, for the secondary analysis of copper, we observed an unlikely R2 which was explained by one extremely outlying SNP: rs12582659 (Additional file 2: Fig. S1). After removing this SNP, we found an R2 of 7.10%.
Power calculations were done using http://cnsgenomics.com/shiny/mRnd/ [36]. The statistical power was calculated to capture an odds ratio (OR) of 0.90 or 1.10 per SD change in the circulating micronutrient concentration, given the sample size used for the meta-analyses at a type 1 error of 5% (Additional file 2: Table S6). In addition to the main MR analyses, we performed secondary analyses using more liberal criteria for including genetic variants to enhance statistical power; r2 < 0.01 and P ≤ 5E−06. For our study, we only considered micronutrients with an R2 > 1% and/or statistical power > 50% for at least one of the infectious disease outcomes, thereby excluding calcium, magnesium, folate, and vitamin B6 (Table 2 and Additional file 2: Tables S6-S7). For the remaining micronutrients, we excluded SNPs with an F statistic < 10 [4] to reduce the risk of weak instrument bias [34]. None of the included genetic instruments was shared by any of the considered micronutrients (Additional file 2: Table S7).
MR analysis
We calculated the Wald ratio for each SNP, defined as the SNP-outcome association divided by the SNP-exposure association [37]. When multiple SNPs were available for a micronutrient, we summarized the effect calculated by the Wald ratio using an inverse-variance weighted (IVW) analysis [38]. All reported associations correspond to an OR for the outcome per SD increase in the genetically predicted circulating concentrations of the micronutrient. The MR analyses were performed separately for the outcome GWASs from UK Biobank, FinnGen, and meta-analysis of the two cohorts. If not otherwise specified, the meta-analysis was used as the outcome study. P < 0.05 was considered nominally significant, whereas the level for statistical significance corrected for multiple testing (8 exposures × 3 outcomes = 24 tests) was set at P = 0.05/24 = 2.08E−03.
Sensitivity analyses
For an instrumental variable to be valid, three key assumptions must be met: the instrument must be robustly associated with the exposure, it cannot affect a confounder of the exposure-outcome association, and it must only affect the outcome through the risk factor [4]. Horizontal pleiotropy—that the SNP has multiple effects—can violate those assumptions. MR-Egger, weighted median, simple mode, and weighted mode are some of the most common sensitivity analyses to account for horizontal pleiotropy [17]. These analyses were only conducted when the number of genetic instruments was ≥ 3. The MR-Egger method allows some SNPs to affect the outcome through a pathway other than through the exposure. If the intercept term differs from zero, this indicates that not all the included instruments are valid, and the standard estimates (i.e., IVW) may be biased [39]. The weighted median method provides a valid MR estimate when up to 50% of the included instruments are invalid. This method calculates the weighted median estimate by ordering the genetic variants according to the magnitude of their estimates [40]. The mode-based methods (simple mode and weighted mode) assume that the most common causal effect is consistent with the true causal effect, allowing some instruments to be invalid without biasing the estimated causal effect [41].
To evaluate if the differences in the individual effect sizes among the genetic instruments may be related to pleiotropic effects rather than chance, we conducted Cochran’s Q statistical test [42]. This test was only conducted when two or more variants were available, and a P < 0.05 was considered significant in the test for heterogeneity.
We further evaluated whether the genetic instruments were associated with other phenotypes using PhenoScanner V2 [43], available at http://www.phenoscanner.medschl.cam.ac.uk/ (accessed 30 October 2022). Additionally, we performed leave-one-out analyses for micronutrients containing > 2 SNPs. This was performed to examine the robustness of the IVW estimates and if any specific SNP drove the association (which could be due to pleiotropy) [4].
Multivariable MR was used to evaluate whether any of the identified phenotypes on PhenoScanner had introduced bias due to pleiotropy [17]. The genetic variants for the potentially pleiotropic phenotypes were collected from IEU OpenGWAS [44]. Based on the identified potentially pleiotropic pathways, we conducted one multivariable MR analysis of copper on the risk of gastrointestinal infections where we included erythrocyte count (GWAS identifier: ukb-d-30250_irnt) and hemoglobin concentration (GWAS identifier: ebi-a-GCST004615) in the analysis.
Secondary analyses using less stringent criteria for the selection of genetic instruments
In secondary analyses, we included variants at a more liberal threshold of r2 < 0.01 and P ≤ 5E−06. While this could increase statistical power, it also may increase the risk of violating the MR assumptions and introduce weak instrument bias. Therefore, we included MR-RAPS, an MR method for correcting for bias introduced by weak instruments, using robust adjusted profile scores [45]. For copper, we carried out the secondary analyses both using instruments from Evans et al. [6] (as in the main analysis) and using instruments from Jäger et al. [20].
Post hoc analyses
Finally, to validate the association between copper and gastrointestinal infections (see the “Results” section), we conducted the following two post hoc analyses: first, we retrieved the results from an additional European GWAS on gastrointestinal infections conducted by Nudel et al. [46] and conducted MR analyses on this independent cohort. Only one of the two copper SNPs, rs2769264, was available from this study, and no reliable proxy for the other copper SNP was available (defined by r2 > 0.9; using European ancestry in the LDproxy tool from National Cancer Institute LDlink [47]).
Second, to assess the possibility of reverse causation, we conducted MR analyses of the association between the genetically predicted risk of gastrointestinal infection on the genetically predicted blood levels of copper. We used the meta-analysis results on gastrointestinal infection as exposure in this analysis, including SNPs suggestively associated with gastrointestinal infection (r2 < 0.01 within 10,000-kb windows, P ≤ 5E−06). For the outcome, we retrieved the copper association summary-level statistics from Evans et al. [6].
Statistical analysis
All MR analyses were conducted using the TwoSampleMR package (version 0.5.6) [42] in R (version 4.0.3). METAL (version 2011-03-25) [33] was used to perform the meta-analyses of the outcomes.
Results
Main analyses
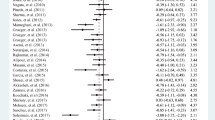
After correction for multiple testing, the only statistically significant micronutrient-infection association was that of genetically predicted blood levels of copper and risk of gastrointestinal infections (Fig. 2). One SD increase in genetically predicted blood levels of copper was associated with an OR of 0.91 (95% confidence interval [CI] 0.87 to 0.97, P = 1.38E−03), 0.89 (95% CI 0.80 to 0.98, P = 1.67E−02), and 0.93 (95% CI 0.87 to 0.99, P = 1.98E−02), in the meta-analysis, UK Biobank, and FinnGen, respectively (Fig. 2 and Additional file 2: Table S8). A nominally significant association was observed for both selenium and vitamin D on the risk of gastrointestinal infections, with an OR of 0.92 (95% CI 0.85 to 0.99, P = 2.39E−02) and OR of 1.11 (95% CI 1.02 to 1.21, P = 2.00E−02), respectively (Fig. 2 and Additional file 2: Table S8).
Mendelian randomization analyses of circulating levels of micronutrients on the risk of gastrointestinal infections, pneumonia, and urinary tract infections. Legend: Forest plot of inverse-variance weighted Mendelian randomization analyses. The x-axis represents the results expressed as per standard deviation increase in genetically proxied levels of the exposure. Abbreviations: Cu, copper; Fe, iron; Se, selenium; UTI, urinary tract infection; Zn, zinc
We observed little evidence that the circulating concentrations of iron, zinc, beta-carotene, vitamin B12, and vitamin C were associated with the risk of any of the evaluated infections (Fig. 2 and Additional file 2: Tables S8-S10).
Sensitivity and secondary analyses
The MR-Egger, weighted median, and mode-based sensitivity analyses supported the findings from the IVW analyses. As only two instruments were used for copper, MR-Egger regression, weighted median, simple mode, and weighted mode were not carried out in the main analysis. No heterogeneity was observed in the main MR analyses for copper and vitamin D on the risk of gastrointestinal infections (Cochran’s Q test P = 5.99E−01 and Cochran’s Q test P = 5.56E−01, respectively; Additional file 2: Table S8). Heterogeneity was observed for vitamin B12 and gastrointestinal infections (Cochran’s Q test P = 2.19E−04): iron (Cochran’s Q test P = 1.04E−02) and vitamin C (Cochran’s Q test P = 3.70E−02) for pneumonia and vitamin D (Cochran’s Q test P = 2.27E−02) for urinary tract infection. For the other micronutrients, no heterogeneity was observed (Additional file 2: Tables S8-S10).
For the secondary analyses, we generally observed comparable effects as in the main analyses (Additional file 2: Table S11). However, for copper, using 6 SNPs from Evans et al. [6] yielded an IVW estimate of OR 1.01 (95% CI 0.96 to 1.06, P = 7.45E−01). There was considerable heterogeneity in this estimate (Cochran’s Q test P = 4.68E−03), which was explained by one extremely outlying SNP: rs12582659 (Additional file 2: Fig. S1). Excluding this SNP from the analysis yielded comparable results to the main analysis (OR 0.97, 95% CI 0.90 to 1.05, P = 4.35E−01; Additional file 2: Table S12). However, we still found evidence of heterogeneity (Cochran’s Q test P = 1.47E−02), and the results should be interpreted with caution. Using genetic instruments from Jäger et al. [20] supported the main analysis (OR 0.95, 95% CI 0.91 to 1.00, P = 3.07E−02). In both secondary analyses using data from Evans et al. [6] and Jäger et al. [20], we observed the presence of potential pleiotropy, e.g., MR-Egger OR 1.01 (95% CI 0.94 to 1.09, P = 7.18E−01) and OR 1.07 (95% CI 0.93 to 1.23, P = 3.99E−01); it is unclear whether this pleiotropy reflects the presence of pleiotropy in the main analysis.
Using PhenoScanner, we found that several of the genetic instruments used for the micronutrients have previously been reported to be associated with numerous traits and diseases (Additional file 3: Tables S13-S20). Of note, rs1175550 used for copper was strongly associated with reticulocyte count and hemoglobin concentration. We therefore performed a multivariable MR analysis between copper, reticulocyte count, and hemoglobin concentration and the risk of gastrointestinal infection. We observed a similar effect as in the main analysis with an OR of 0.96 (95% CI 0.93 to 0.99, P = 1.64E−02). The genetic instruments for vitamin D were associated with several traits related to smoking, BMI, and alcohol. In the leave-one-out analysis, the observed association of vitamin D with gastrointestinal infection did not change meaningfully, indicating that no specific SNP drove the result nor that the observed association was due to pleiotropy (Additional file 2: Table S21).
Post hoc analyses
In the post hoc analysis of copper and gastrointestinal infections, we observed a similar effect as in the main analysis when using another GWAS on gastrointestinal infections [46]: OR 0.94 (95% CI 0.81 to 1.09, P = 3.82E−01). The CI was wide because only one of the two SNPs was available. Meta-analyzing the results from using Nudel et al. [46] with the main analysis yielded an OR of 0.92 (95% CI 0.87 to 0.97, P = 1.14E−03).
Finally, we conducted two-sample MR analyses using gastrointestinal infections as the exposure and blood levels of copper as the outcome. We found that gastrointestinal infections did not affect circulating copper levels (beta = − 0.35, 95% CI − 1.35 to 0.71, P = 5.40E−01) using two genetic instruments from Evans et al. [6], and no heterogeneity was observed (Cochran’s Q test P = 5.50E−01).
Discussion
In this MR study of eight micronutrients and the risk of three infectious diseases, we found genetically predicted blood levels of copper to be robustly associated with the genetically predicted risk of gastrointestinal infections. We did not find a clear association between the other micronutrients and infections.
Copper plays an essential role in innate and adaptive immunity: it regulates the function of T helper cells, B cells, neutrophils, natural killer cells, and macrophages; it accumulates at sites of inflammation, including the gastrointestinal and respiratory tract and in blood and urine, and is vital for interleukin 2 production and response [3, 48]. Blood levels of copper have not previously been robustly linked to the risk of gastrointestinal infections in humans. A small randomized controlled trial (RCT) found that supplementation with high doses of copper, zinc, and selenium significantly reduced the risk of infections among hospitalized patients with severe burns [49]. Another trial found that copper supplementation increased the interleukin 2 production by blood cells in healthy individuals with low to normal copper levels, which is crucial for T helper cell proliferation and natural killer cell cytotoxicity [50]. In addition, a previous study showed that cell cultures pretreated with added Cu boosted macrophage antibacterial activity and enhanced intracellular killing of Escherichia coli [51]. These results align with our finding that high levels of copper have a protective effect against infectious diseases and that higher blood levels of copper might lead to increased immune response.
Regarding vitamin D, a previous MR study found that lower plasma levels of this micronutrient were associated with an increased risk of pneumonia [52], which was not supported in our study and also not supported by a systematic review of trials of vitamin D supplementation [53]. The same MR study found no evidence of an association between vitamin D and the risk of urinary tract infections or gastroenteritis [52]. While we also found no association between vitamin D and urinary tract infections, we did observe a nominally significant positive association between vitamin D and gastrointestinal infection. However, this finding may be a chance finding due to multiple testing, and it did not pass our stringent threshold for statistical significance.
Interestingly, we found no associations between genetically predicted circulating iron, zinc, beta-carotene, vitamin B12, and vitamin C and the risk of gastrointestinal infections, pneumonia, or urinary tract infection. Systematic reviews of RCTs have found limited evidence of micronutrient supplementation on the risk of infections but have also underscored the paucity of studies [54,55,56,57]. Among those reviews, one reported no difference in the incidence of diarrhea and lower respiratory tract infection in infants with zinc supplementation [54]. Another review found uncertain and limited evidence for vitamin C supplementation in preventing pneumonia [55]. Two reviews found no clear evidence that emerged in favor of selenium supplementation for developing infections [56] and the incidence of new infections [57] among critically ill patients. This may indicate that several of these micronutrients are not important risk factors for the infections considered. Finally, high levels of serum iron have in previous MR studies been associated with skin and soft tissue infections and sepsis, but we did not find any evidence of an association for the infections that we considered [58, 59]. This discrepancy may be due to organ-specific effects of iron (e.g., iron levels were also associated with damages to skin-related structures) and that the infectious diseases are not comparable (e.g., sepsis is an inflammatory syndrome in response to severe infection) [59, 60].
Our study has several strengths and limitations. By applying an MR design, we reduced the risk of confounding, which often affects observational studies. Additionally, we considerably reduced random error and increased statistical power by combining summary data from multiple cohorts [35]. However, despite the large sample sizes, several of the genetic instruments used for exposures and the outcomes, to a varying degree, suffered from low statistical power and imperfect phenotype definitions, which may contribute to the null findings of the majority of associations explored. Larger GWASs on micronutrients and infections, with more precise phenotype definitions, would be beneficial. Also, summarized data does not allow for stratification by factors such as sex, age, diet, micronutrient supplement use, or co-morbidities. Due to the use of summary-level data, we could not identify individuals with a combination of two or more infections, which might lead to bias. The quality control, genotyping, and imputation were performed using different criteria and programs for the two cohorts. Additionally, different phenotype definitions were used in the two cohorts, which may introduce heterogeneity between the association estimates. However, we observed minimal heterogeneity between the two cohorts in the meta-analysis.
The genetic instruments used as exposure for each micronutrient have widely been used to evaluate the association with other complex diseases or phenotypes, which supports their use in this study [61,62,63]. Throughout, we tried to use data on our exposures and outcomes from separate GWASs to reduce the risk of confounding bias due to overlapping samples [64], but this was not possible for vitamin D (since the other published GWASs for vitamin D adjusted for BMI) [21, 65]. To reduce the risk of population stratification, we only evaluated participants of European ancestry. However, this affects our findings’ external validity to other ancestry groups. Our findings were supported by conducting a range of sensitivity analyses, including evaluating the presence of pleiotropy, and by evaluating two distinct biobanks for each outcome (i.e., UK Biobank and FinnGen). While only two instruments were available for the main MR analysis of copper, the more liberal threshold for SNP inclusion in the secondary analyses allowed for more genetic instruments to be included; these analyses were generally consistent with the main analysis. For copper and risk of gastrointestinal infections, we conducted an extended set of sensitivity analyses to evaluate the robustness of our findings, including following up our results in an additional GWAS of gastrointestinal infections, conducting multivariable MR to account for potentially pleiotropic pathways, and conducting bi-directional MR: These analyses all supported our main finding.
Conclusions
In conclusion, our findings support that copper may play a role in the susceptibility to gastrointestinal infections. More research is needed to evaluate whether this finding replicates in other settings and to learn more about the potential underlying mechanisms.
Availability of data and materials
Data described in the manuscript are provided within the article. Genetic instrumental variables and data sources are presented in the additional files. The UKBiobank HRC-imputed can be obtained via https://pheweb.org/UKB-SAIGE/. The summary-level data for FinnGen can be obtained via https://www.finngen.fi/en/access_results. We used the following web-based resources: Phenoscanner (http://www.phenoscanner.medschl.cam.ac.uk/), LDlink (https://analysistools.cancer.gov/LDlink/?tab=ldproxy), and IEU OpenGWAS (https://gwas.mrcieu.ac.uk/).
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- GWAS:
-
Genome-wide association study
- ICD:
-
International Classification of Diseases Code
- IVW:
-
Inverse-variance weighted
- MAF:
-
Minor allele frequency
- MR:
-
Mendelian randomization
- OR:
-
Odds ratio
- RCT:
-
Randomized controlled trial
- SD:
-
Standard deviation
- SNP:
-
Single-nucleotide polymorphism
References
Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210.
Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Medicin Chem. 2014;6:PMC. S14459.
Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236.
Davies NM, Holmes MV, Smith GD. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601.
O’Seaghdha CM, Wu H, Yang Q, Kapur K, Guessous I, Zuber AM, et al. Meta-analysis of genome-wide association studies identifies six new loci for serum calcium concentrations. PLoS Genet. 2013;9(9):e1003796.
Evans DM, Zhu G, Dy V, Heath AC, Madden PA, Kemp JP, et al. Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum Mol Genet. 2013;22(19):3998–4006.
Bell S, Rigas AS, Magnusson MK, Ferkingstad E, Allara E, Bjornsdottir G, et al. A genome-wide meta-analysis yields 46 new loci associating with biomarkers of iron homeostasis. Commun Biol. 2021;4(1):156.
Meyer TE, Verwoert GC, Hwang S-J, Glazer NL, Smith AV, Van Rooij FJ, et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six loci influencing serum magnesium levels. PLoS Genet. 2010;6(8):e1001045.
Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, et al. Common variation in the β-carotene 15, 15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84(2):123–33.
Grarup N, Sulem P, Sandholt CH, Thorleifsson G, Ahluwalia TS, Steinthorsdottir V, et al. Genetic architecture of vitamin B12 and folate levels uncovered applying deeply sequenced large datasets. PLoS Genet. 2013;9(6):e1003530.
Mondul AM, Yu K, Wheeler W, Zhang H, Weinstein SJ, Major JM, et al. Genome-wide association study of circulating retinol levels. Hum Mol Genet. 2011;20(23):4724–31.
Hazra A, Kraft P, Lazarus R, Chen C, Chanock SJ, Jacques P, et al. Genome-wide significant predictors of metabolites in the one-carbon metabolism pathway. Hum Mol Genet. 2009;18(23):4677–87.
Zheng J-S, Ja L, Sofianopoulou E, Imamura F, Stewart ID, Day FR, et al. Plasma vitamin C and type 2 diabetes: genome-wide association study and mendelian randomization analysis in European populations. Diabetes Care. 2021;44(1):98–106.
Manousaki D, Mitchell R, Dudding T, Haworth S, Harroud A, Forgetta V, et al. Luan Ja, Langenberg C, Timpson NJ: Genome-wide association study for vitamin D levels reveals 69 independent loci. Am J Hum Genet. 2020;106(3):327–37.
Major JM, Yu K, Wheeler W, Zhang H, Cornelis MC, Wright ME, et al. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum Mol Genet. 2011;20(19):3876–83.
Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375:n2233.
Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. Mendelian randomization. Nat Rev Methods Prim. 2022;2(1):1–21.
Dashti HS, Shea MK, Smith CE, Tanaka T, Hruby A, Richardson K, et al. Meta-analysis of genome-wide association studies for circulating phylloquinone concentrations. Am J Clin Nutr. 2014;100(6):1462–9.
Ng E, Lind PM, Lindgren C, Ingelsson E, Mahajan A, Morris A, et al. Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum Mol Genet. 2015;24(16):4739–45.
Jäger S, Cabral M, Kopp JF, Hoffmann P, Ng E, Whitfield JB, et al. Blood copper and risk of cardiometabolic diseases: a Mendelian randomization study. Hum Mol Genet. 2021;31(5):783–91. https://doi.org/10.1093/hmg/ddab275.
Manousaki D, Dudding T, Haworth S, Hsu Y-H, Liu C-T, Medina-Gómez C, et al. Low-frequency synonymous coding variation in CYP2R1 has large effects on vitamin D levels and risk of multiple sclerosis. Am J Hum Genet. 2017;101(2):227–38.
Shim H, Chasman DI, Smith JD, Mora S, Ridker PM, Nickerson DA, et al. A multivariate genome-wide association analysis of 10 LDL subfractions, and their response to statin treatment, in 1868 Caucasians. PloS One. 2015;10(4):e0120758.
Aschard H, Vilhjálmsson BJ, Joshi AD, Price AL, Kraft P. Adjusting for heritable covariates can bias effect estimates in genome-wide association studies. Am J Hum Genet. 2015;96(2):329–39.
Gagliano Taliun SA, VandeHaar P, Boughton AP, Welch RP, Taliun D, Schmidt EM, et al. Exploring and visualizing large-scale genetic associations by using PheWeb. Nat Genet. 2020;52(6):550–2.
FinnGen: FinnGen documentation of R6 release. 2022. https://finngen.gitbook.io/documentation/. Accessed 20 Feb 2022.
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner K, et al. FinnGen: unique genetic insights from combining isolated population and national health register data. medRxiv. 2022. https://doi.org/10.1101/2022.03.03.22271360.
UKBiobank HRC-imputed: 008: intestinal infection. 2018. https://pheweb.org/UKB-SAIGE/pheno/008. Accessed 6 Jan 2022.
UKBiobank HRC-imputed: 480.1: bacterial pneumonia. 2018. https://pheweb.org/UKB-SAIGE/pheno/480.1. Accessed 6 Jan 2022.
UKBiobank HRC-imputed: 591: urinary tract infection. 2018. https://pheweb.org/UKB-SAIGE/pheno/591. Accessed 6 Jan 2022.
FinnGen: intestinal infectious diseases. 2022. https://r6.finngen.fi/pheno/AB1_INTESTINAL_INFECTIONS. Accessed 20 Feb 2022.
FinnGen: bacterial pneumonia (organism specified). 2022. https://r6.finngen.fi/pheno/PNEUMOBACTKNOWN. Accessed 20 Feb 2022.
FinnGen: other disorders of urethra and urinary system. 2022. https://r6.finngen.fi/pheno/N14_URETHRAOTH. Accessed 20 Feb 2022.
Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–1.
Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64.
Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880–906.
Brion M-JA, Shakhbazov K, Visscher PM. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–501.
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–63.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14.
Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–98.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. elife. 2018;7:e34408.
Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–3.
Elsworth B, Lyon M, Alexander T, Liu Y, Matthews P, Hallett J, et al. The MRC IEU OpenGWAS data infrastructure. BioRxiv. 2020. https://doi.org/10.1101/2020.08.10.244293.
Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann Stat. 2020;48(3):1742–69.
Nudel R, Appadurai V, Schork AJ, Buil A, Bybjerg-Grauholm J, Børglum AD, et al. A large population-based investigation into the genetics of susceptibility to gastrointestinal infections and the link between gastrointestinal infections and mental illness. Hum Genet. 2020;139(5):593-604.
Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31(21):3555–7.
Focarelli F, Giachino A, Waldron KJ. Copper microenvironments in the human body define patterns of copper adaptation in pathogenic bacteria. PLoS Pathog. 2022;18(7):e1010617.
Berger MM, Spertini F, Shenkin A, Wardle C, Wiesner L, Schindler C, et al. Trace element supplementation modulates pulmonary infection rates after major burns: a double-blind, placebo-controlled trial. Am J Clin Nutr. 1998;68(2):365–71.
Muñoz C, López M, Olivares M, Pizarro F, Arredondo M, Araya M. Differential response of interleukin-2 production to chronic copper supplementation in healthy humans. Eur Cytokine Netw. 2005;16(4):261–5.
White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284(49):33949–56.
Çolak Y, Nordestgaard BG, Afzal S. Low vitamin D and risk of bacterial pneumonias: Mendelian randomisation studies in two population-based cohorts. Thorax. 2021;76(5):468–78.
Das RR, Singh M, Naik SS. Vitamin D as an adjunct to antibiotics for the treatment of acute childhood pneumonia. Cochrane Database Syst Rev. 2018;7(7):Cd011597.
Lassi ZS, Kurji J, Oliveira CS, Moin A, Bhutta ZA. Zinc supplementation for the promotion of growth and prevention of infections in infants less than six months of age. Cochrane Database Syst Rev. 2020;4(4):Cd010205.
Padhani ZA, Moazzam Z, Ashraf A, Bilal H, Salam RA, Das JK, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4(4):Cd013134.
Allingstrup M, Afshari A. Selenium supplementation for critically ill adults. Cochrane Database Syst Rev. 2015;2015(7):Cd003703.
Zhao Y, Yang M, Mao Z, Yuan R, Wang L, Hu X, et al. The clinical outcomes of selenium supplementation on critically ill patients: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2019;98(20):e15473.
Gill D, Benyamin B, Moore LSP, Monori G, Zhou A, Koskeridis F, et al. Associations of genetically determined iron status across the phenome: a mendelian randomization study. PLoS Med. 2019;16(6):e1002833.
Mohus RM, Flatby H, Liyanarachi KV, DeWan AT, Solligård E, Damås JK, et al. Iron status and the risk of sepsis and severe COVID-19: a two-sample Mendelian randomization study. Sci Rep. 2022;12(1):16157.
Hu Y, Cheng X, Mao H, Chen X, Cui Y, Qiu Z. Causal effects of genetically predicted iron status on sepsis: a two-sample bidirectional mendelian randomization study. Front Nutr. 2021;8:747547.
Ong J-S, Dixon-Suen SC, Han X, An J, Liyanage U, Dusingize J-C, et al. A comprehensive re-assessment of the association between vitamin D and cancer susceptibility using Mendelian randomization. Nat Commun. 2021;12(1):1–10.
Guo Y, Lu Y, Jin H. Appraising the role of circulating concentrations of micro-nutrients in epithelial ovarian cancer risk: a Mendelian randomization analysis. Sci Rep. 2020;10(1):1–9.
Fu Y, Xu F, Jiang L, Miao Z, Liang X, Yang J, et al. Circulating vitamin C concentration and risk of cancers: a Mendelian randomization study. BMC Med. 2021;19(1):1–14.
Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, Hartwig FP, Holmes MV, Minelli C, Relton CL, Theodoratou E. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2020;4:186. https://doi.org/10.12688/wellcomeopenres.15555.2.
Jiang X, O’Reilly PF, Aschard H, Hsu Y-H, Richards JB, Dupuis J, et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat Commun. 2018;9(1):1–12.
Acknowledgements
We want to acknowledge the participants and investigators of the UK Biobank and FinnGen study.
Funding
Open access funding provided by Norwegian University of Science and Technology. The present research used publicly available summary data, where no extra ethical approval is required. This work was supported by Samarbeidsorganet Helse Midt-Norge and the Norwegian University of Science and Technology, NTNU. The funders had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the interpretation of the data and critically revised the manuscript. All authors have approved the submitted final version and are to be personally accountable for their own contributions. The authors’ contributions were as follows. HMF wrote the paper taking into account the comments and suggestions of all the coauthors and had primary responsibility for the final content, conducted the literature search, and analyzed the data. AR, JKD, and ES advised on the analyses and drafted the paper. TR designed the study, advised on the analyses and visualization, and supervised the study. All authors revised the paper, interpreted the results, and read and approved the final manuscripts. The authors report no conflicts of interest.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. Only publicly available summary statistics were used.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
STROBE MR.
Additional file 2:
Additional Text – Exposure GWAS cohorts. Fig. S1. Scatter plot of secondary MR analysis of copper as risk factors on the risk of gastrointestinal infections. Table S2. ICD-10 codes for gastrointestinal infections in UK Biobank and FinnGen R6. Table S3. ICD-10 codes for pneumonia in UK Biobank, and FinnGen. Table S4. ICD-10 codes for urinary tract infection UK Biobank, and FinnGen. Table S6. Power calculations. Table S7. Genetic variants used as exposure for Mendelian randomization analyses. Table S8. Main mendelian randomization analyses of micronutrients as risk factors on the risk of gastrointestinal infections. Table S9. Main mendelian randomization analyses of micronutrients as risk factors on the risk of pneumonia. Table S10. Main mendelian randomization analyses of micronutrients as risk factors on the risk of urinary tract infections. Table S11. Secondary mendelian randomization analyses of micronutrients as risk factors on the risk of gastrointestinal infections, pneumonia and urinary tract infections suggestive-significant genetic instruments. Table S12. Secondary mendelian randomization analyses of copper as risk factors on the risk of gastrointestinal infections, where rs12582659 was removed. Table S21. IVW MR regression results for the leave one SNP out analysis in the Mendelian randomization analyses of micronutrients.
Additional file 3: Table S5.
Results from the meta-analysis for the genetic instruments used as the outcome in the Mendelian randomization analyses. Table S13. Phenome-wide association analysis of genetic instruments for copper. Table S14. Phenome-wide association analysis of genetic instruments for iron. Table S15. Phenome-wide association analysis of genetic instruments for selenium. Table S16. Phenome-wide association analysis of genetic instruments for zinc. Table S17. Phenome-wide association analysis of genetic instruments for beta carotene. Table S18. Phenome-wide association analysis of genetic instruments for vitamin B12. Table S19. Phenome-wide association analysis of genetic instruments for vitamin C. Table S20. Phenome-wide association analysis of genetic instruments for vitamin D.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Flatby, H.M., Ravi, A., Damås, J.K. et al. Circulating levels of micronutrients and risk of infections: a Mendelian randomization study. BMC Med 21, 84 (2023). https://doi.org/10.1186/s12916-023-02780-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-023-02780-3