Abstract
Background
Although guidelines recommend lipid injectable emulsions (ILEs) be used as a part of parenteral nutrition, many patients in Japan receive lipid-free parenteral nutrition. Furthermore, little is known about the effect of ILEs on clinical outcomes in medical inpatients managed with parenteral nutrition. The aim of this study was to investigate the clinical impact of ILEs on internal medicine inpatients receiving parenteral nutrition.
Methods
A propensity score matching (PSM) analysis was performed using a medical claims database covering 451 hospitals in Japan. Participants included the following internal medicine inpatients, ages ≥ 18 years, fasting > 10 days, and receiving exclusively parenteral nutrition, between 2011 and 2020. Participants were divided into 2 groups: those who did and did not receive ILEs. The primary endpoint was in-hospital mortality. The secondary endpoints included intravenous catheter infection, activities of daily living (ADL), hospital length of stay (LOS), and total medical costs. To adjust for energy doses, logistic or multiple regression analyses were performed using energy dose as an additional explanatory variable.
Results
After PSM, 19,602 matched pairs were formed out of 61,437 patients. The ILE group had significantly lower incidences than the non-ILE group of in-hospital mortality (20.3% vs. 26.9%; odds ratio [OR], 0.69; 95% confidence interval [CI], 0.66–0.72; p < 0.001), deteriorated ADL (10.8% vs. 12.5%; OR, 0.85; 95% CI, 0.79–0.92; p < 0.001), and shorter LOS (regression coefficient, − 0.8; 95% CI, − 1.6–0.0; p = 0.045). After adjusting for energy dose, these ORs or regression coefficients demonstrated the same tendencies and statistical significance. The mean total medical costs were $21,009 in the ILE group and $21,402 in the non-ILE group (p = 0.08), and the adjusted regression coefficient for the ILE vs. the non-ILE group was − $860 (95% CI, − $1252 to − $47).
Conclusions
ILE use was associated with improved clinical outcomes, including lower in-hospital mortality, in internal medicine inpatients receiving parenteral nutrition.
Similar content being viewed by others
Background
Lipid injectable emulsions (ILEs) serve as a source of essential fatty acids and energy-dense non-protein calories, as well as a principal part of parenteral nutrition [1, 2]. Nutritional support has been associated with improved clinical outcomes in hospitalized patients [3, 4]. Recent American Society for Parenteral and Enteral Nutrition (ASPEN) recommendations have advocated for the use of ILEs for those patients who require parenteral nutrition [5]. This recommendation is based on the potential clinical and biochemical benefits of the addition of ILEs to parenteral nutrition, which include modulation of inflammatory responses and reduction of immune suppression.
The value of using ILEs as part of parenteral nutrition for surgical and critically ill patients has been well established, with specific ILEs demonstrating both therapeutic and adverse effects [6]. On the other hand, recent retrospective surveys using medical claims databases [7, 8] have suggested that ILEs are not being widely used as part of parenteral nutrition in the current clinical practice in Japan, though this is not the case globally. Moreover, little is known about the effects of ILEs on internal medicine inpatients who are being managed with parenteral nutrition. In particular, real-world data on the actual impact of ILEs on clinical outcomes in internal medicine inpatients being managed with parenteral nutrition are lacking, and there have been no studies that have investigated the cost-effectiveness of using ILEs for these patients.
Clarifying the impact of ILEs on clinical outcomes in internal medicine inpatients may help promote the more appropriate management of parenteral nutrition in this patient population. The purpose of this study was to examine the impact of the use of ILEs on clinical outcomes (i.e., mortality, activities of daily living, and complications) and medical costs in adult internal medicine inpatients receiving parenteral nutrition, using a medical claims database.
Methods
Design and data source
A retrospective analysis was performed using data that was extracted from a medical claims database which included 451 hospitals and managed by the Medical Data Vision Co., Ltd. (MDV; Tokyo, Japan). The database uses the diagnosis procedure combination/per-diem payment system (DPC/PDPS), in which provider reimbursement is calculated on a flat-rate per-diem fee based on the diagnosis group. The study protocol was approved by the Ethics Committees of the Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences (No. 2108-041) and the Kurume University Graduate School of Medicine (No. 21139) and registered at the University Hospital Medical Information Network Clinical Trial Registry (UMIN000044962). Informed consent was not required, because all personal information used in this study was anonymized.
The database included information on dates of hospital admission and discharge, age at admission, sex, height, body weight, body mass index (BMI), number of beds in admission hospital, year and type of admission, primary diseases (coded using the International Statistical Classification of Diseases and Related Health Problems, 10th Revision [ICD-10]), comorbidities (used to determine the Charlson Comorbidity Index [CCI]) [9], activities of daily living (ADL) based on the Barthel Index (BI) [10], levels of consciousness based on the Japan Coma Scale (JCS) [11], malnutrition defined as having poor oral intake of at least 10 days and low body mass index according to the Global Leadership Initiative on Malnutrition (GLIM) criteria [12], medical treatments during hospitalization (using Japan-specific medical claims codes), and discharge outcome status, as well as other information not used in our study. The total daily doses of parenteral energy, amino acids, and ILE prescribed were calculated using the parenteral nutrition infusion product names and compositions along with the prescribed quantities of those products, as they appeared in the database. When recording these doses, day 1 was regarded as the day fasting started, day 2 as the second day after fasting started, and so on.
Patient population
This study included hospitalized adult patients ages 18 years or older who were fasting (receiving no oral or enteral nutrition) for more than 10 consecutive days and were managed with parenteral nutrition, between January 2011 and September 2020. Patients were excluded from the study who underwent surgery or entered the intensive care unit between the day of admission and the start of fasting, were suspected to be in the terminal disease phase (defined as prescribed mean energy doses < 10 kcal/kg or mean amino acid doses < 0.5 g/kg on days 4 through 10), or were considered to be overfed (which we based on prescribed mean energy doses ≥ 30 kcal/kg on days 4 through 10). The rationale for the use of days 4 through 10 was that the administration of parenteral nutrition usually involves a gradual increase in dose over the initial 3 to 4 days before reaching the full target dose [7, 8].
Clinical outcomes
The primary endpoint was in-hospital mortality. The secondary endpoints included intravenous catheter infection during hospitalization, deteriorated ADL at discharge, length of stay (LOS), readmission, and total medical costs. ADL at discharge, LOS, and readmission were recorded for only those patients who were discharged alive, whereas other data were recorded for all patients. Medical costs were calculated based on Japanese yen and were then converted to US dollar (US$) using the annual exchange rate of 2020 reported by the Organization for Economic Cooperation and Development (OECD) (US$1 = 107 Japanese yen) [13]. Patients were considered to have deteriorated ADL when their total BI scores were lower at the time of discharge than at the time of admission. Readmission was defined as being admitted to the same hospital again within 30 days of discharge.
Variables
The variables extracted from the database were categorized as follows: age at admission (18–59, 60–69, 70–79, 80–89, or ≥ 90 years), BMI (< 16.0, 16.0–18.5, 18.5–22.5, 22.5–25.0, or ≥ 25.0), number of beds admission hospital (< 200, 200–500, or ≥ 500), year of admission (2011–2012, 2013–2014, 2015–2016, 2017–2018, or 2019–2020), type of admission (elective or emergency), primary disease (by ICD-10 code), comorbidities (CCI of 0, 1, 2, or ≥ 3), ADL (BI of 0, 5–20, 25–40, 45–60, 65–95, or 100), levels of consciousness (JCS of 0 [alert], 1–3 [awake], 10–30 [arousable], or 100–300 [coma]), and nutritional status (malnutrition defined as BMI < 18.5 if < 70 years old or BMI < 20 if > 70 years old). Information about medical treatments (e.g., albumin infusion, blood transfusion, respirator use, dialysis, nutrition support team, and rehabilitation) ordered between the day of admission and day 10 was extracted from the database for each patient. Missing values for the type of admission, BI, and JCS were placed in an “unknown” category.
Prescribed doses of parenteral nutrition
The prescribed mean daily doses of energy, amino acids, and ILE for days 4 to 10 after the start of fasting were calculated for each patient based on the parenteral nutrition infusion product composition and prescribed quantity of that infusion and were based on the assumption that nutrient doses often take until day 4 to reach 100% of their target [14]. Prescribed daily doses of energy and amino acids were calculated as kilocalories (kcal) and grams (g), respectively, and reported per kilogram (kg) of body weight and prescribed daily doses of ILE were calculated and reported as both grams and the caloric percentage (%) of the total non-protein energy administered that day.
Statistical analysis
The data management and statistical analysis were performed by an independent third party (A2 Healthcare Corporation; Tokyo, Japan) in order to eliminate arbitrariness and ensure transparency. Categorical variables were summarized as numbers and percentages, and continuous variables were summarized as means and standard deviations (SD). Missing values were not included. First, patients eligible for the study were divided into 2 groups: the ILE group, who were prescribed ILEs during days 4 through 10, and the non-ILE group, who were not prescribed ILEs during days 4 through 10. Next, propensity score matching (PSM) was used to adjust for confounding factors [15]. The propensity score was estimated by multivariable logistic regression analysis with the ILE group as the objective variable and patient characteristics as the explanatory variables. PSM was conducted using a one-to-one nearest neighbor method and using the caliper width. The caliper value was 0.2, and matching was performed within the caliper values. To confirm the covariate balance between the groups, standardized differences were calculated before and after PSM. A standardized difference less than 10% was considered to represent a balanced covariate [16].
To compare the 2 groups for each outcome, both before and after PSM, the Student t-test was used for continuous variables and the chi-square test was used for categorical variables. To adjust for the differences in the prescribed mean daily parenteral energy doses between the 2 groups, even after PSM, multivariable logistic or multiple regression analyses, as appropriate, were performed, with the mean daily energy dose prescribed for days 4 through 10 added as an explanatory variable. In these analyses, odds ratios (ORs) or regression coefficients, as appropriate, along with 95% confidence intervals (CIs), were calculated, both before and after the adjustment for energy.
For in-hospital mortality, survival curves were generated for the 2 groups using the Kaplan-Meier method, and a log-rank test was performed. In addition, the Cox proportional hazard model was used to calculate a hazard ratio (HR), along with a 95% CI, of the ILE group to the non-ILE group, for in-hospital mortality. For these calculations, the patients who were discharged alive were censored on the day of discharge, and the inpatients who survived for 180 days or longer were censored on day 180. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), with a two-sided significance level of 5%.
Sensitivity analysis
Before modeling, the variance inflation factors (VIFs) of patient characteristics and prescribed mean daily parenteral nutrition doses were calculated to confirm that there was no multicollinearity between variables based on multiple regression analysis or multivariable logistic regression analysis [17].
To confirm the robustness of PSM, confounding factors were adjusted by multivariable logistic regression analysis or multiple regression analysis, and an adjustment analysis consisting of 2 explanatory variable groups (model 1, model 2) was performed. In model 1, the explanatory variables were the 2 groups and the patient characteristics. In model 2, the explanatory variables were those included in model 1 as well as the prescribed mean daily parenteral energy during days 4 to 10. Either ORs or regression coefficients, along with 95% CIs, were calculated for each model.
Results
Patient characteristics
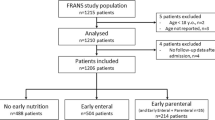
Following the screening of 295,464 medical inpatients, a total of 61,437 patients were eligible for the study (Fig. 1). Based on the GLIM criteria, malnutrition was found in 28,097 (45.7%) of the study patients (Table 1). Among all patients, 19,618 (31.9%) were in the ILE group and 41,819 (68.1%) were in the non-ILE group, and the mean (SD) parenteral nutrition duration for all patients was 24.4 (28.5) days. After PSM, 19,602 matched pairs of patients were formed. Of the 19,602 patients in the ILE group, 16,191 (82.6%) were 60 years or older, and 11,439 (58.4%) were males; in addition, the most common primary disease was digestive system malignancy in 6723 (34.3%) patients, followed by digestive system disease in 4865 (24.8%) patients.
Before PSM, the standardized differences between the 2 groups were greater than 10% for sex, BI, JCS, and medical treatments of albumin infusion and nutrition support team (Table 1). After PSM, there were no variables with standardized differences greater than 10%.
Mean daily nutrition doses for days 4 to 10
The prescribed mean daily doses of parenteral nutrition for days 4 to 10 were calculated for both groups, both before and after PSM (Table 2). In the ILE group, both before and after PSM, the mean (SD) non-protein calorie ratio of ILEs was 14.3 (11.5) %, and the mean dose of ILEs was 14.2 (10.9) g. After PSM, the mean (SD) energy dose was 16.5 (4.8) kcal/kg in the non-ILE group and 18.8 (5.1) kcal/kg in the ILE group, and this represented a significant difference (p < 0.001). Also after PSM, the mean (SD) daily dose of amino acids was 0.73 (0.17) g/kg in both the non-ILE and ILE groups.
Clinical outcomes
The results for primary and secondary endpoints, before and after PSM, as well as the ORs or regression coefficients before and after the adjustment for energy, are depicted in Table 3. Clinical outcome results reported below are those obtained after PSM, unless noted otherwise.
Primary endpoint
The unadjusted odds of in-hospital mortality were significantly lower for the ILE group than for the non-ILE group (OR, 0.69; 95% CI, 0.66–0.72; p < 0.001). After adjusting for the energy variable, the OR for in-hospital mortality showed the same trend (OR, 0.71; 95% CI, 0.68–0.75). The Kaplan-Meier curves demonstrated a significantly lower hazard for in-hospital mortality for the ILE group vs. the non-ILE group (HR, 0.76; 95% CI, 0.73–0.79; p < 0.001) (Fig. 2).
Kaplan-Meier survival curves for in-hospital mortality of internal medicine inpatients aged 18 years or older and fasting for more than 10 days in Japan, January 2011 to September 2020, after propensity score matching. The results expressed as the hazard ratio (95% confidence interval) of in-hospital mortality for the ILE group compared to the non-ILE group. Curves demonstrate a significantly lower hazard for in-hospital mortality for the ILE group vs. the non-ILE group (HR, 0.76; 95% CI, 0.73–0.79; p < 0.001). Abbreviations: ILE, lipid injectable emulsion (soybean oil-based)
Secondary endpoints
There was no significant difference between the 2 groups in terms of intravenous catheter infections (1.1% in the ILE group vs. 0.9% in the non-ILE group; unadjusted OR, 1.20; 95% CI, 0.98–1.46; p = 0.08). The unadjusted odds of deteriorated ADL in the patients who were discharged alive were significantly lower for the ILE group than for the non-ILE group (OR, 0.85; 95% CI, 0.79–0.92; p < 0.001). The unadjusted regression coefficient for LOS in the ILE vs. the non-ILE group was − 0.8 (95% CI, − 1.6–0.0; p = 0.045), and the coefficient adjusted for the mean daily energy dose variable was − 1.80 (95% CI, − 2.6 to − 1.0).
The mean (SD) total medical costs were $21,009 ($18,439) in the ILE group and $21,402 ($24,981) in the non-ILE group (p = 0.08), with the ILE group vs. non-ILE group unadjusted regression coefficient of − $393 (95% CI, − $822–$48). However, after adjustment for the prescribed mean daily energy dose on days 4 to 10, the regression coefficient of total medical costs was − 860 (95% CI, − $1252 to − $47).
Sensitivity analysis
The VIFs of patient characteristics and mean daily parenteral nutrition doses were all below 2.5, confirming that there was no multicollinearity between the variables (Additional file 1: Table S1). The ORs of in-hospital mortality and deteriorated ADL, after adjustment for patient characteristics (model 1), were 0.65 (95% CI, 0.62–0.68) and 0.77 (95% CI, 0.71–0.83), respectively (Additional file 1: Table S2). The regression coefficients for LOS were − 1.2 (95% CI, 2.0 to − 0.5) days in model 1 and − 2.1 (95% CI, − 2.8 to − 1.3) days after adding the adjustment for mean daily energy dose for days 4 to 10 in model 2, confirming the significant differences between the ILE group and non-ILE group in both models. As in the PSM analysis, there were no significant differences between the 2 groups in intravenous catheter infections and readmissions. Finally, the regression coefficients for total medical costs were − 411 (95% CI, − $776 to − $47) in model 1 and − $1244 (95% CI, − $1598 to − $850) in model 2, confirming that medical costs were significantly lower for the ILE group than for the non-ILE group.
Discussion
To the best of our knowledge, this is the first large-scale cohort study investigating the impact of parenteral ILE use on clinical outcomes in a population of fasting internal medicine inpatients. In this study, we found that the patients in this population who were prescribed parenteral ILEs had significantly lower odds of in-hospital mortality and of deteriorated ADL than those who were not prescribed ILEs. In addition, the ILE group had a significantly shorter LOS than the non-ILE group. In contrast, the odds of having intravenous catheter infections did not differ significantly between the groups. Finally, after adjustment for the energy dose, the mean total medical costs for the ILE group were $860 lower than for the non-ILE group.
Lipids are one of 3 essential macronutrients, along with carbohydrates and proteins. The inclusion of ILEs in parenteral nutrition has been recommended in several guidelines [5, 18]. Despite this, this study confirmed that ILEs are not being used consistently as part of parenteral nutrition in Japan, as has been previously reported [7, 8]. In fact, we observed that twice as many patients received parenteral nutrition without ILEs as patients who received it with ILEs in Japan. There are several possible reasons why ILEs are often not part of the parenteral nutrition given in Japan. First, the only commercially available ILEs in Japan come in the form of soybean oil-derived products. However, these products may promote inflammatory reactions because they contain high levels of n-6 polyunsaturated fatty acids [19], and they may lead to a deterioration in immune function and an increased risk of infectious complications because of their inhibitory effect on phagocytosis [20]. Also, whereas 3-in-1 or total nutrient admixtures are used as the standard globally [21], no product containing all 3 macronutrients is commercially available in Japan. Given these product limitations, the complexity of adding ILEs separately to parenteral nutrition, and the possibility of insufficient training related to ILE use, clinicians in Japan may have been hindered in their prescribing of ILEs as part of parenteral nutrition.
We believe that the results of this study should encourage more clinicians in Japan to prescribe ILEs and thus promote the expansion of the market for ILEs. Moreover, significant gaps in ILE administration relative to published recommendations have been reported in other countries, including the USA [22]. On top of that, there has been a lack of studies investigating the prevalence and clinical impact of ILE use and involving real-world databases. Accordingly, further large-scale investigations should be performed to enhance the understanding of ILE use in large groups of patients and in real-world settings.
The results of this study have suggested that the use of ILEs during parenteral nutrition can have a positive impact on clinical outcomes, including reducing in-hospital mortality, deterioration of ADLs, and hospital LOS, and the results suggest this can be accomplished without increasing the risk of intravenous catheter infections. Taken together, these findings should raise awareness that many fasting hospital inpatients in Japan are not being prescribed ILEs during parenteral nutrition and that there are substantial benefits to adding ILEs to parenteral nutrition in hospital-based clinical practice. There are possible explanations for how the addition of ILEs to parenteral nutrition may have contributed to the positive clinical outcomes in our study. First, the essential fatty acids contained in ILEs are important components of the cell membrane and are known to play an important part in maintaining biological and physiological functions and as precursors of physiologically active substances [18]. Second, most patients who are fasting and require parenteral nutrition have impaired glucose tolerance, and ILEs can serve as effective alternative sources of energy as well as substances that exert protein-sparing effects [23, 24]. This protein-sparing effect of ILEs has been indirectly associated with improved clinical outcomes in fasting medical patients [25].
PSM was used in this study to mitigate the potential confounding by other variables when investigating the impact of the addition ILEs on clinical outcomes. The prescribed mean daily parenteral energy dose was not included as one of the covariates in the initial propensity score estimation, because it was anticipated that the total energy doses prescribed would be higher in the patients who received ILEs than in those who did not (simply based on the extra calories from the ILEs). Indeed, the ILE group did have a higher prescribed mean daily energy dose than the non-ILE group, and this was the case even after PSM was applied. Therefore, additional multivariate logistic regression and multiple regression analyses were performed, using the prescribed mean daily energy dose on days 4 to 10 as an additional explanatory variable, and then PSM was applied again to reassess the impact of the addition of ILEs on clinical outcomes. After this adjustment for the prescribed mean daily energy dose, the resulting ORs and regression coefficients for the clinical outcomes showed the same tendencies and statistical significance. Importantly, these results suggested the earlier findings and demonstrated that the addition of parenteral ILEs, independent of their contribution to higher energy doses, had significant beneficial effects on in-hospital mortality, deterioration of ADLs, and hospital LOS.
Limitations
The present study has several limitations. First, this was a retrospective study. However, the sample size was large, and it can be challenging to perform a prospective, randomized controlled trial comparing outcomes in medical patients who received or did not receive ILEs. Second, despite efforts to control bias, unknown confounding factors or residual confounding might have been present. In the study, PSM was used to control for 17 potential confounding factors. However, residual confounding could have occurred, because data regarding disease severity and laboratory values could not be extracted from the database. Third, our findings were based on the information registered in a medical claims database. Because the database we used did not include information regarding the indications for parenteral nutrition for individual patients, we were unable to provide results concerning this characteristic in our patient population. Also, the database did not allow for a detailed cost analysis to investigate which factors enhanced cost-effectiveness. Moreover, the database had some missing data and may have contained entry errors. Finally, the use of ICD-10 codes to identify primary diseases is inferior to characterizing primary diseases prospectively. However, the use of CCI as a reliable and accurate measure of comorbidities has been validated in Japan [26]. Fourth, the ILEs prescribed to patients in this study were limited to soybean oil-based products because of the limited commercial availability of other products in Japan. Therefore, the effects of other ILEs, such as those derived from medium-chain fatty acids, olive oil, or fish oil, should be evaluated in future studies. Finally, the dose-dependent impact of ILEs on outcomes was not investigated as part of the study, because the mean daily doses of ILEs were too small. This issue should also be addressed in future studies.
Conclusions
The results of this study should raise awareness that many fasting internal medicine inpatients in Japan are not being prescribed ILEs as part of parenteral nutrition. The addition of ILEs to parenteral nutrition in internal medicine inpatients not only improved clinical outcomes but also led to enhanced cost-effectiveness. Verification of these findings in observational or prospective studies would be necessary to confirm a direct causal relationship between the use of ILEs and positive clinical outcomes.
Availability of data and materials
The datasets used in this study were purchased from Medical Data Vision Co., Ltd. (MDV). The authors cannot share the data with any third parties or make the data publicly available due to confidential protection over the sharing of personal data.
Abbreviations
- ADL:
-
Activities of daily living
- BI:
-
Barthel Index
- BMI:
-
Body mass index
- CCI:
-
Charlson Comorbidity Index
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- ILE:
-
Lipid injectable emulsion
- JCS:
-
Japan Coma Scale
- LOS:
-
Length of stay
- OR:
-
Odds ratio
- PSM:
-
Propensity score matching
- SD:
-
Standard deviations
- VIF:
-
Variance inflation factor
References
Mayer K, Klek S, Garcia-de-Lorenzo A, Rosenthal MD, Li A, Evans DC, Muscaritoli M, Martindale RG. Lipid use in hospitalized adults requiring parenteral nutrition. JPEN J Parenter Enteral Nutr. 2020;44(Suppl 1):S28–38.
Raman M, Almutairdi A, Mulesa L, Alberda C, Beattie C, Gramlich L. Parenteral nutrition and lipids. Nutrients. 2017;9(4).
Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, Kutz A, Tribolet P, Bregenzer T, Braun N, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. The Lancet. 2019;393(10188):2312–21.
Gomes F, Baumgartner A, Bounoure L, Bally M, Deutz NE, Greenwald JL, Stanga Z, Mueller B, Schuetz P. Association of nutritional support with clinical outcomes among medical inpatients who are malnourished or at nutritional risk: an updated systematic review and meta-analysis. JAMA Netw Open. 2019;2(11): e1915138.
Mirtallo JM, Ayers P, Boullata J, Gura KM, Plogsted S, Anderson CR, Worthington P, Seres DS, Nicolai E, Alsharhan M, et al. ASPEN lipid injectable emulsion safety recommendations, part 1: background and adult considerations. Nutr Clin Pract. 2020;35(5):769–82.
Sadu Singh BK, Narayanan SS, Khor BH, Sahathevan S, Abdul Gafor AH, Fiaccadori E, Sundram K, Karupaiah T. Composition and functionality of lipid emulsions in parenteral nutrition: examining evidence in clinical applications. Front Pharmacol. 2020;11:506.
Sasabuchi Y, Ono S, Kamoshita S, Tsuda T, Murano H, Kuroda A. A survey on total parenteral nutrition in 55,000 hospitalized patients: retrospective cohort study using a medical claims database. Clin Nutr ESPEN. 2020;39:198–205.
Maeda K, Murotani K, Kamoshita S, Horikoshi Y, Kuroda A. Nutritional management in inpatients with aspiration pneumonia: a cohort medical claims database study. Arch Gerontol Geriatr. 2021;95:104398.
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82.
Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–5.
Shigematsu K, Nakano H, Watanabe Y. The eye response test alone is sufficient to predict stroke outcome--reintroduction of Japan Coma Scale: a cohort study. BMJ Open. 2013;3(4).
Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, Barazzoni R, Blaauw R, Coats A, et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38(1):1–9.
OECD (Organisation for Economic Co-operation and Development Data). Exchange rates for 2020 [cited 30 December 2021]. Available from: https://data.oecd.org/conversion/exchange-rates.htm
Sasabuchi Y, Ono S, Kamoshita S, Tsuda T, Kuroda A. Clinical impact of prescribed doses of nutrients for patients exclusively receiving parenteral nutrition in Japanese hospitals: a retrospective cohort study. JPEN J Parenter Enteral Nutr. 2021;45(7):1514–22.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424.
Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Communications in Statistics - Simulation and Computation. 2009;38(6):1228–34.
Montgomery D, Peck E, Vining G. Introduction to linear regression analysis, 5th edition: Wiley; 2012.
Adolph M, Heller AR, Koch T, Koletzko B, Kreymann KG, Krohn K, Pscheidl E, Senkal M. Lipid emulsions - guidelines on parenteral nutrition, chapter 6. Ger Med Sci. 2009;7:Doc22.
Calder PC, Waitzberg DL, Klek S, Martindale RG. Lipids in parenteral nutrition: biological aspects. JPEN J Parenter Enteral Nutr. 2020;44(Suppl 1):S21–7.
Waitzberg DL, Lotierzo PH, Logullo AF, Torrinhas RS, Pereira CC, Meier R. Parenteral lipid emulsions and phagocytic systems. Br J Nutr. 2002;87(Suppl 1):S49–57.
Slattery E, Rumore MM, Douglas JS, Seres DS. 3-in-1 vs 2-in-1 parenteral nutrition in adults: a review. Nutr Clin Pract. 2014;29(5):631–5.
Christensen ML, Ayers P, Boullata JI, Guenter P, Gura KM, Holcombe B, Seres DS, Sacks GS; ASPEN PN Safety Committee. Lipid injectable emulsion survey with gap analysis. Nutr Clin Pract. 2017; 32(5):694–702.
Patkova A, Joskova V, Havel E, Kovarik M, Kucharova M, Zadak Z, Hronek M. Energy, protein, carbohydrate, and lipid intakes and their effects on morbidity and mortality in critically ill adult patients: a systematic review. Adv Nutr. 2017;8(4):624–34.
Nordenström J, Carpentier YA, Askanazi J, Robin AP, Elwyn DH, Hensle TW, Kinney JM. Metabolic utilization of intravenous fat emulsion during total parenteral nutrition. Ann Surg. 1982;196(2):221–31.
Tamiya H, Yasunaga H, Hosoi T, Yamana H, Matsui H, Fushimi K, Akishita M, Ogawa S. Association between protein intake and mortality in older patients receiving parenteral nutrition: a retrospective observational study. Am J Clin Nutr. 2021.
Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol. 2017;27(10):476–82.
Acknowledgements
Yoshinori Imokawa for the statistical programming support, Shigeki Omori and Yuko Otsuka for the support in writing the manuscript (A2 Healthcare Corporation), and Hiroko Inoue and Dr. Raymond K. Whalen (Whalen Medical Communications, PLLC, WA, USA) for the scientific editing support.
Funding
Otsuka Pharmaceutical Factory, Inc. was involved in designing, conducting, and reporting this study.
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows—all authors: concept and design of the study, acquisition, analysis or interpretation of the data, had full access to all of the data, and take responsibility for the integrity of the data and the accuracy of the data analysis; KT and SK: drafting of the manuscript; KM: statistical analysis; SK: administrative, technical, or material support; AK: supervision. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committees of the Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences (No. 2108-041) and the Kurume University Graduate School of Medicine (No. 21139) and registered at the University Hospital Medical Information Network Clinical Trial Registry (UMIN000044962). Informed consent was not required, because all personal information used in this study was anonymized.
Consent for publication
Not applicable.
Competing interests
Dr. Takagi and Dr. Murotani report grants from Otsuka Pharmaceutical Factory, Inc. during the conduct of the study. Satoru Kamoshita and Akiyoshi Kuroda are employees of Otsuka Pharmaceutical Factory, Inc. and hold stocks of Otsuka Holdings Co., Ltd.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Variance Inflation Factors (VIF) of patient characteristics and mean daily parenteral nutrition doses, for 61,437 internal medicine inpatients ages 18 years or older and fasting for more than 10 days in Japan, January 2011 to September 2020. Table S2. Sensitivity analysis of clinical outcomes of 61,437 internal medicine inpatients ages 18 years or older and fasting for more than 10 days in Japan, January 2011 to September 2020.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Takagi, K., Murotani, K., Kamoshita, S. et al. Clinical impact of lipid injectable emulsion in internal medicine inpatients exclusively receiving parenteral nutrition: a propensity score matching analysis from a Japanese medical claims database. BMC Med 20, 371 (2022). https://doi.org/10.1186/s12916-022-02568-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02568-x