Abstract
Background
Clinical complexity is increasingly prevalent among patients with atrial fibrillation (AF). The ‘Atrial fibrillation Better Care’ (ABC) pathway approach has been proposed to streamline a more holistic and integrated approach to AF care; however, there are limited data on its usefulness among clinically complex patients. We aim to determine the impact of ABC pathway in a contemporary cohort of clinically complex AF patients.
Methods
From the ESC-EHRA EORP-AF General Long-Term Registry, we analysed clinically complex AF patients, defined as the presence of frailty, multimorbidity and/or polypharmacy. A K-medoids cluster analysis was performed to identify different groups of clinical complexity. The impact of an ABC-adherent approach on major outcomes was analysed through Cox-regression analyses and delay of event (DoE) analyses.
Results
Among 9966 AF patients included, 8289 (83.1%) were clinically complex. Adherence to the ABC pathway in the clinically complex group reduced the risk of all-cause death (adjusted HR [aHR]: 0.72, 95%CI 0.58–0.91), major adverse cardiovascular events (MACEs; aHR: 0.68, 95%CI 0.52–0.87) and composite outcome (aHR: 0.70, 95%CI: 0.58–0.85). Adherence to the ABC pathway was associated with a significant reduction in the risk of death (aHR: 0.74, 95%CI 0.56–0.98) and composite outcome (aHR: 0.76, 95%CI 0.60–0.96) also in the high-complexity cluster; similar trends were observed for MACEs. In DoE analyses, an ABC-adherent approach resulted in significant gains in event-free survival for all the outcomes investigated in clinically complex patients. Based on absolute risk reduction at 1 year of follow-up, the number needed to treat for ABC pathway adherence was 24 for all-cause death, 31 for MACEs and 20 for the composite outcome.
Conclusions
An ABC-adherent approach reduces the risk of major outcomes in clinically complex AF patients. Ensuring adherence to the ABC pathway is essential to improve clinical outcomes among clinically complex AF patients.
Similar content being viewed by others
Background
In recent years, increasing awareness of clinical complexity has contributed to significant changes in the approach to the care of atrial fibrillation (AF) patients. Multimorbidity, polypharmacy and frailty can be seen as three different expressions of clinical complexity, and although these often coexist and overlap, each of these phenomena has a specific role in influencing prognosis [1]. All of these have been repeatedly described in AF patients, and several reports have outlined their detrimental effects in terms of quality of care and major outcomes [2,3,4,5,6].
Recent guidelines on AF [7, 8] have recommended appropriate characterization and evaluation of patients [9], followed by a holistic or integrated care approach to AF management, based on the ‘Atrial fibrillation Better Care’ (ABC) pathway approach to streamline a comprehensive and holistic management of AF patients [10]. The ABC pathway approach has three pillars: ‘A’, Anticoagulation/avoid stroke; ‘B’, Better symptom management; and ‘C’, Cardiovascular risk factors and Comorbidities optimization [10]. Adherence to the ABC pathway in patients with AF is associated with a lower risk of major outcomes and health-related costs in real-world observational studies [11,12,13,14,15], confirmed by the prospective mAFA-II randomized controlled trial [16] and highlighted in a systematic review and meta-analysis [17]. The latter showed that adherence to an ABC pathway was associated with lower risk of all major outcomes (including all-cause death, stroke, and major bleeding) among AF patients.
To date, there are few contemporary data on the effectiveness of the ABC pathway in specific high-risk subgroups of AF patients, particularly in clinically complex subjects. In this analysis from the European Society of Cardiology (ESC) EURObservational Research Programme (EORP) Atrial Fibrillation General Long-Term Registry, we explored whether adherence to the ABC management strategy would be associated with reduced risk of adverse outcomes in clinically complex patients, defined as those with frailty, multimorbidity and/or polypharmacy.
Methods
For the purpose of this analysis, we used data from the ESC-EHRA EURObservational Research Programme (EORP) Atrial Fibrillation General Long-Term Registry, which is a prospective, observational, multicentre registry, held by the ESC and endorsed by the EHRA. The study enrolled consecutive AF inpatients and outpatients in 250 cardiology practices, across 27 countries. Details on study design, baseline characteristics, outcomes adjudication and follow-up are reported elsewhere [18, 19].
Briefly, all patients enrolled had AF, documented in the 12 months preceding enrolment. All patients were aged ≥18 years and provided written informed consent. Enrolment was undertaken from October 2013 to September 2016, with planned 1-year and 2-year follow-up. Patient data were collected after the signing of a written informed consent by each patient, and following the approval of the study protocol by an Institutional Review Board/Ethic Committee. The study was first approved by the National Coordinators’ main institutions (listed in the Acknowledgements section) and subsequently authorized by each site under the responsibility of the lead contact and study team (all listed in the Acknowledgements section), as per the specific national and local regulation. Any details regarding approval numbers for the study protocol regarding any specific site could be obtained from the corresponding authors, upon reasonable request. The study was performed according to the European Union Note for Guidance on Good Clinical Practice CPMP/ECH/135/95 and the Declaration of Helsinki.
Symptomatic status was defined according to EHRA score [8], while thromboembolic and bleeding risk were assessed according to CHA2DS2-VASc and HAS-BLED scores, computed according to the original schemes [8]. We defined high thromboembolic risk when CHA2DS2-VASc was ≥2 in males and ≥3 in females, and high bleeding risk when HAS-BLED was ≥3. Frailty was assessed according to a 40-item frailty index (FI) (Additional file 1: Table S1), built according to the cumulative deficits model, as proposed by Rockwood and Mitnitski [20, 21]. Calculation of FI was performed as the ratio of the total deficits found for each patient over the total number of possible deficits examined. According to the usual clinical use, a FI ranging from 0.10 to <0.25 defined the presence of pre-frailty, while a FI ≥0.25 defined the presence of frailty [22]. Multimorbidity was defined as the presence of ≥2 comorbidities. Number of drugs received at baseline was used to assess polypharmacy, which was defined as the concomitant use of ≥5 drugs [23].
Adherence to the Atrial fibrillation Better Care (ABC) pathway was evaluated at baseline and defined as per previously published study [14] according to three criteria [10]:
-
‘A’ Criterion: Patients were considered ‘adherent’ to the ‘A’ criterion if properly prescribed with oral anticoagulant (OAC) according to their thromboembolic risk. Specifically, we considered adherent males with CHA2DS2-VASc≥1 and females with CHA2DS2-VASc≥2, treated with either vitamin K antagonist (VKA) (with a time in therapeutic range ≥70%) or a non-vitamin K antagonist oral anticoagulant (NOAC); patients not receiving OAC and with low thromboembolic risk (i.e. CHA2DS2-VASc=0 in males or =1 in females) were also considered adherent.
-
‘B’ Criterion: As this criterion refers to the actual symptom control, rather than the attempt, we considered ‘adherent’ those patients with an EHRA score of I (no symptoms) or II (mild symptoms) at baseline.
-
‘C’ Criterion: For this criterion, we considered the comorbidities most frequently found in AF patients: hypertension, coronary artery disease (CAD), peripheral artery disease (PAD), heart failure (HF), previous stroke/transient ischaemic attack (TIA) and diabetes mellitus. Any patient with ≥1 of these conditions and treated according to ‘optimal medical treatment’ (defined according to the current clinical guidelines) was considered adherent to this criteria. Optimal treatment was defined as follows: (i) hypertension: if blood pressure at baseline was ≤140/90 mmHg; (ii) CAD: treatment with angiotensin-converting enzyme (ACE) inhibitors, beta-blockers and statins; (iii) PAD: treatment with statins; (iv) previous stroke/TIA: treatment with statins; (v) HF: treatment with ACE inhibitors or angiotensin receptor blockers, and beta-blockers; and (vi) diabetes mellitus: treatment with insulin or oral antidiabetics. Patients with 2 or more of the above conditions needed to be optimally treated for all to be considered adherent to the ‘C’ criterion.
Patients who met all three criteria were considered adherent to the ABC pathway; otherwise, they were considered ABC-non adherent.
Major adverse events
For this analysis, we considered the following major adverse events: (i) all-cause death; (ii) major adverse cardiovascular events (MACEs), as the composite of any thromboembolic events, any acute coronary syndrome and cardiovascular death; and (iii) a composite outcome of all-cause death and MACE.
Statistical analysis
Continuous variables were expressed as mean (Standard Deviation, SD) or median [interquartile range, IQR]; differences across groups were evaluated with appropriate parametric and non-parametric tests, respectively. Categorical variables were expressed as counts and percentages; differences across groups were assessed through chi-square test.
Cox regression models were fitted to evaluate the impact of ABC pathway (in terms of full adherence, number of criteria fulfilled and each additional criteria) in clinically complex patients (defined as having at least one complexity criteria among frailty, multimorbidity and polypharmacy), and separately in those with frailty, multimorbidity and polypharmacy, after adjustment for age, sex, type of atrial fibrillation and components of CHA2DS2-VASc (previous thromboembolism, coronary artery disease, congestive heart failure, hypertension, diabetes, peripheral artery disease).
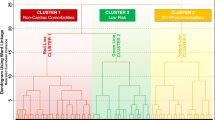
To explore the interplay between the different domains of clinical complexity (i.e. frailty, multimorbidity, polypharmacy), the ABC pathway and the risk of outcomes, we performed a K-medoids cluster analysis with the use of the partition around medoids cluster algorithm. Optimal number of clusters was selected according to the average silhouette width. We included 4 pre-determined variables in the cluster-analysis (age, frailty index, number of comorbidities and number of drugs taken), to reflect the different domains of clinical complexity; each of these variables was scaled before clustering. For each cluster, we reported baseline characteristics and compared categorical and continuous variables as already specified. We also evaluated the impact of ABC pathway adherence in each cluster identified through Kaplan-Meier and Cox-regression analyses.
To further analyse the impact of adherence to the ABC pathway on the risk of outcomes, we performed a quantile regression to estimate the delay of event (DoE) [24,25,26] attained in the ABC-adherent group of patients at 6 months, 1 year and 2 years of follow-up. Finally, for the clinical complexity group and the high clinical complexity cluster, we calculated the number needed to treat (NNT) along with 95% confidence interval (CI) based on absolute risk reduction at 1 year of follow-up. A two-sided p < 0.05 was considered statistically significant. All analyses were performed using R 4.1.2 (R Core Team, Vienna, Austria) for Windows.
Results
Among the 11,096 patients enrolled in the ESC-EHRA EORP-AF General Long-Term Registry, a total of 9966 (89.8%) with complete data available on FI, number of comorbidities and number of drugs received were included in this analysis.
Overall, 8289 (83.1%) patients were defined as clinically complex (presenting with at least one of frailty, multimorbidity and/or polypharmacy). Baseline characteristics of the overall cohort and of the clinically complex, frailty (n=2108), multimorbidity (n=7894) and polypharmacy (n=5366) subgroups are shown in Additional file 1: Table S2. Overall, higher age and burden of comorbidities were associated with each of the complexity domains explored. Among patients with complete data on ABC adherence (n=6091), less than one-third clinically complex patients were treated as ABC-pathway adherent, with frail individuals showing lowest figures for each ABC criterion, across the clinically complexity domains explored.
Outcomes in clinically complex and subgroups of frail, multimorbid and polypharmacy AF patients
Outcomes according to clinical complexity status and in subgroups of frail, multimorbid and polypharmacy patients during a median follow-up of 730 [IQR: 701–749] days are reported in Additional file 1: Table S3. Clinically complex AF patients showed increased risk of all-cause death (adjusted Hazard Ratio [aHR]: 1.97, 95% CI: 1.40–2.76), MACEs (aHR: 1.49, 95% CI: 1.07–2.06) and composite outcome (aHR: 1.76, 95% CI: 1.36–2.28), after adjustment for age, sex, type of AF, anticoagulation and components of CHA2DS2-VASc score. Among the clinical complexity subgroups, the highest increase in risk for all outcomes was observed for frail patients (compared to robust ones), with a 3-fold higher risk of death and composite outcome, and more than double risk of MACEs.
Multivariate Cox regression models are summarized in Table 1. Among clinically complex patients, full adherence to the ABC pathway reduced the risk of all-cause death (aHR: 0.72, 95% CI: 0.58–0.91), MACE (aHR: 0.68, 95% CI: 0.52–0.87) and composite outcome (aHR: 0.70, 95% CI: 0.58–0.85). When compared to patients’ adherent to 0 criteria, incremental risk reductions were observed as the number of ABC-adherent criteria increased.
We observed consistent trends in the multimorbidity and frailty group, consistent with the primary analysis above. In frail patients, adherence to each additional criterion was associated with a significant reduction in all-cause death (HR: 0.67, 95%CI: 0.56–0.80), MACE (HR: 0.75, 95%CI 0.61–0.92) and the composite outcome (HR: 0.74, 95%CI: 0.63–0.87). In the polypharmacy group, we did not observe any statistically significant associations between complete adherence to ABC pathway and outcomes; however, patients that were adherent to at least 2 ABC criteria were at lower risk of all-cause death (HR: 0.68, 95%CI: 0.53–0.88) and the composite outcome (HR: 0.72, 95%CI 0.57–0.90), but not MACE (HR 0.83, 95%CI: 0.61–1.12).
Cluster analysis
Using a k-medoids cluster analysis, we identified 2 as the number of optimal clusters, according to the average silhouette width method. Baseline characteristics of the population according to the cluster grouping are shown in Additional file 1: Table S4.
Cluster 1 included patients with ‘high clinical complexity’, with 45% of patients being frail (median FI: 0.24, [IQR 0.20–0.29]), while cluster 2 included patients with ‘moderate clinical complexity’, with 64% being pre-frail (median FI: 0.12, IQR [0.09–0.16]). Prevalence of multimorbidity and polypharmacy was higher in cluster 1 (number of comorbidities: median (IQR), 5 [4–6] vs. 2 [1–3], p < 0.001; numbers of drugs 6 [5–7] vs. 4 [3–5], p < 0.001). Patients in the high clinical complexity cluster were older and more likely female, with higher thromboembolic and bleeding risks, and were less ABC adherent (23.4% vs. 35.1%, p < 0.001).
Kaplan-Meier curves for all-cause death, MACE and the composite outcome according to clusters are shown in Figure 1 and Additional File 1: Figure S1 and S2, respectively. Patients in the high clinical complexity cluster showed higher rates of all the outcomes investigated (p<0.001 for all; Table 2). Compared to the moderate complexity cluster, patients in the high clinical complexity cluster showed an increased risk of all-cause death (aHR 1.92, 95%CI 1.59–2.32), MACEs (aHR 1.95, 95%CI 1.57–2.42) and the composite outcome (aHR 1.87, 95%CI: 1.59–2.19), after adjustment for age, sex, components of CHA2DS2-VASc score, use of anticoagulant and type of AF.
Cox regression analyses (Table 2) showed that in the high clinical complexity cluster, adherence to ABC pathway was associated with reduced risk of all-cause death (aHR: 0.74, 95%CI: 0.56–0.98) and the composite outcome (aHR: 0.76, 95%CI: 0.60–0.96) but not MACE. Adherence to an increasing number of ABC criteria was associated with risk reductions for all outcomes. Adherence to at least 2 ABC criteria was associated with a lower risk of all events when compared to subjects’ adherent to 0 or 1 criteria.
In the moderate complexity cluster, full ABC pathway adherence was associated with a significant reduction in the risk of MACE and composite outcome, but not all-cause death; however, adherence to at least 2 ABC criteria was associated with a significant reduction of the risk of all-cause death when compared to patients adherent to 0–1 criteria.
Delay of event analysis and NNT
In clinically complex patients, ABC-adherent management resulted in a significant delay of all events investigated: at 1-year follow-up, ABC adherent patients gained 402 [95%CI: 242–1018] days of survival, 396 [95%CI: 57–573] days of MACE-free time and 385 [95%CI: 303–491] days of composite outcome-free time (Table 3). Based on absolute risk reduction at 1 year of follow-up, the NNTs for ABC pathway adherence was 24 (95%CI: 18–37) for all-cause death, 31 (95%CI: 22–56) for MACEs and 20 (95%CI: 15–31) for the composite outcome. Similar trends were noted in the different subgroups of frailty, multimorbidity and polypharmacy.
When analysing the DoEs, individuals in the high clinical complexity cluster who were adherent to the ABC-pathway showed gain of event-free survival for all the outcomes at most time-points investigated (Figure 2 for all-cause mortality and Additional File 1: Figure S3 and S4 for MACE and composite outcome, respectively). At 1 year of follow-up, the ABC-adherent NNT in the high clinical complexity cluster was 21 (95%CI: 14–44) for all-cause death, 34 (95%CI: 19–250) for MACEs, and 22 (95%CI: 13–67) for the composite outcome.
Discussion
Our results show that clinically complex AF patients have a poor prognosis, encompassed by an increased risk of all the outcomes investigated, including death, MACE and composite outcome. Our principal findings were as follows: (i) adherence to the ABC pathway in the clinically complex group reduced the risk of all-cause death, MACE and the composite outcome; (ii) these findings were further confirmed by the cluster analysis, and the DoE data whereby an ABC-adherent approach resulted in significant gains in event-free survival for all the outcomes investigated; and (iii) the NNTs for ABC pathway adherence was 24 for all-cause death, 31 for MACEs and 20 for the composite outcome at 1 year of follow-up (Fig. 3).
In this study, we provide the first systematic assessment of the efficacy of a comprehensive and integrated approach (the ABC pathway) for the management of AF patients, in clinically complex patients. Consistent with our ‘proof of concept’ post hoc analysis from an (old) AFFIRM trial dataset [15], ABC-adherent management reduced the risk of all-cause death, MACE and composite outcome among clinically complex patients, and the magnitude of the effect increased with the number of the ABC criteria fulfilled. With the use of K-medoids cluster analysis, we were able to show the beneficial impact of the ABC adherent approach according to different levels of overall complexity, reflecting the real-world scenario of patients with interacting frailty, multimorbidity and polypharmacy. Also, using a DoE analysis approach, ABC-adherent clinically complex AF patients had a meaningful gain in event-free survival.
For our primary analysis, we defined clinical complexity according to three domains (i.e. frailty, multimorbidity and/or polypharmacy). Our manuscript is also the first to analyse the efficacy of an integrated care approach among three domains of clinical complexity, which are closely but not interchangeable [1]; these three entities capture different expressions of clinical complexity, which often coexist and act synergistically in real-world patients, leading to worse outcomes. The strength of this approach is to consider clinical complexity as a whole entity, encompassing not only the presence of multiple risk factors, but also the accumulation of deficits and the complexities arising from polypharmacy, an often-underestimated issue in AF patients.
In the real world, the presence of frailty, multimorbidity and/or polypharmacy tend to cluster, and the results of our cluster analysis are particularly important given that AF patients often present with a multifaceted interplay between different complexity criteria, leading to a synergistic detrimental effect. Indeed, the high clinical complexity cluster identified a group of AF patients (45% frail, virtually all burdened by multimorbidity, and the vast majority (83%) treated with 5 or more drugs) for whom—given the poor prognosis—there is an urgent need for effective interventions.
Management according to the ABC pathway provided approximately 30% reduction of the risk of all outcomes; the magnitude of the effect was even greater when comparing patients who were adherent to all the ABC criteria with those who were completely non-adherent, with a 65 to 71% reduction in the risk of death, MACE and composite outcome. Indeed, based on absolute risk reduction at 1 year of follow-up, the NNTs for ABC pathway adherence show how implementation of an ABC approach can avoid one death or the composite outcome in approximately every 20 patients who were clinically complex or in the high complexity cluster, while slightly higher figures were found for MACE events. The usefulness of the ABC approach is further reinforced by the DoE analysis. Indeed, an ABC-adherent approach resulted in more than 12 months delay of all outcomes in the clinically complex group at 1 year of follow-up, even in the high complexity cluster.
Our results are aligned with the findings of the main analysis on the effect of the ABC-pathway in the ESC-EHRA EORP Long Term Registry [14] and reinforce the importance of a comprehensive management in the care of clinically complex AF patients [15]. To date, awareness of the effect of frailty [5, 27], multimorbidity [4, 28, 29] and polypharmacy [3] in AF patients is increasing, with compelling evidence on their detrimental effects on quality of care, efficacy of treatment and finally adverse outcomes [2, 4, 29]. However, there is still limited data on how to handle complexity in clinical practice. In recent years, there has been a shift from the focus on single comorbidities (while important in determining the risk of outcomes in AF patients [30,31,32]), to that on multimorbidity in influencing clinical management and outcomes [4, 6, 29, 33]. In this real-world analysis from a large contemporary European AF cohort, we show that the main issue is not exclusively the burden of diseases (nor the impairment of functionality or the burden of medications), but the overall higher clinical complexity, irrespective of which are the main components, in directly influencing the prognosis of AF patients.
Our findings have several important clinical implications. First, clinical complexity is common among the general AF population and needs awareness and specific strategies to tackle the higher risks associated with this state. Second, the proportion of clinically complex patients adherent to the ABC pathway was unsatisfactory among all the clinical complexity domains explored, especially so in the high complexity cluster. Given the beneficial clinical outcomes with ABC pathway adherenc e[17], this should lead to proactive strategies to improve the adoption of integrated care management in these subgroups of patients, as advocated in other complex chronic long-term conditions [34, 35]. Third, adherence to the ABC pathway was effective in reducing the risk of major outcomes, with a clear trend towards greater benefit with increasing ABC criteria attained, resulting in longer event-free survival with ABC-adherence. These improvements would impact on quality of life and healthcare-associated costs, which are highly relevant given the increasing burden of AF on healthcare systems [36].
Limitations
Our analysis has some limitations. The observational design and the limited power to evaluate subgroups that were not specified in the original study design may limit the generalizability of our findings; however, we have analysed more than 6000 patients with complete data on the ABC pathway, this contributing to the reliability of our estimates. Furthermore, we have provided extensive adjustment of our analyses on the evaluation of the effect of the ABC pathway on the risk of outcomes. While we cannot exclude the contribution of other unaccounted bias, which may have contributed to the results presented, our results are consistent with previous evidence on the effect of ABC pathway in similar scenarios, this further reinforcing our results [15, 37]. Finally, our cohort was established across European countries and may not completely reflect other AF populations; therefore, our results may not be immediately applicable to other cohorts and require further evaluation in other geographical settings.
Conclusions
An ABC-adherent approach reduces the risk of major outcomes in AF patients characterized by clinical features of complexity (i.e. frailty, multimorbidity and polypharmacy). Ensuring adherence to the ABC pathway is essential to improve clinical outcomes among clinically complex AF patients.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request, and after approval of all other co-investigators.
Abbreviations
- ABC:
-
Atrial fibrillation Better Care
- ACE:
-
Angiotensin-converting enzyme
- AF:
-
Atrial fibrillation
- CAD:
-
Coronary artery disease
- CI:
-
Confidence interval
- DoE:
-
Delay of event
- EHRA:
-
European Heart Rhythm Association
- EORP:
-
EURObservational Research Programme
- ESC:
-
European Society of Cardiology
- FI:
-
Frailty index
- HF:
-
Heart failure
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- MACE:
-
Major adverse cardiovascular event
- NNT:
-
Number needed to treat
- NOAC:
-
Non-vitamin K antagonist oral anticoagulant
- OAC:
-
Oral anticoagulant
- PAD:
-
Peripheral artery disease
- SD:
-
Standard deviation
- TIA:
-
Transient ischaemic attack
- VKA:
-
Vitamin K antagonist
References
Woolford SJ, Aggarwal P, Sheikh CJ, Patel HP. Frailty, multimorbidity and polypharmacy. Medicine (United Kingdom). 2021;49:166–72.
Gallagher C, Nyfort-Hansen K, Rowett D, Wong CX, Middeldorp ME, Mahajan R, et al. Polypharmacy and health outcomes in atrial fibrillation: a systematic review and meta-analysis. Open Heart. 2020;7:e001257.
Chen N, Alam AB, Lutsey PL, Maclehose RF, Claxton JS, Chen LY, et al. Polypharmacy, adverse outcomes, and treatment effectiveness in patients ≥75 with atrial fibrillation. J Am Heart Assoc. 2020;9:15089.
Jani BD, Nicholl BI, McQueenie R, Connelly DT, Hanlon P, Gallacher KI, et al. Multimorbidity and co-morbidity in atrial fibrillation and effects on survival: findings from UK Biobank cohort. Europace. 2018;20:f329–36.
Proietti M, Cesari M. Describing the relationship between atrial fibrillation and frailty: clinical implications and open research questions. Exp Gerontol. 2021;152:111455.
Proietti M, Marzona I, Vannini T, Tettamanti M, Fortino I, Merlino L, et al. Long-term relationship between atrial fibrillation, multimorbidity and oral anticoagulant drug use. Mayo Clin Proc. 2019;94:2427–36.
Chao TF, Joung B, Takahashi Y, Lim TW, Choi EK, Chan YH, et al. 2021 Focused update consensus guidelines of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemost. 2022;122:20-47.
Hindricks G, Potpara T, Dagres N, Bax JJ, Boriani G, Dan GA, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498.
Potpara TS, Lip GYH, Blomstrom-Lundqvist C, Boriani G, van Gelder IC, Heidbuchel H, et al. The 4S-AF scheme (stroke risk; symptoms; severity of burden; substrate): a novel approach to in-depth characterization (rather than classification) of atrial fibrillation. Thromb Haemost. 2021;121:270–8.
Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14:627–8.
Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Improved outcomes by integrated care of anticoagulated patients with atrial fibrillation using the simple ABC (Atrial Fibrillation Better Care) pathway. Am J Med. 2018;131:1359–1366.e6.
Yoon M, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. Improved population-based clinical outcomes of patients with atrial fibrillation by compliance with the simple ABC (Atrial Fibrillation Better Care) pathway for integrated care management: a nationwide cohort study. Thromb Haemost. 2019;119:1695–703.
Pastori D, Farcomeni A, Pignatelli P, Violi F, Lip GY. ABC (Atrial fibrillation Better Care) pathway and healthcare costs in atrial fibrillation: the ATHERO-AF study. Am J Med. 2019;132:856–61.
Proietti M, Lip GYH, Laroche C, Fauchier L, Marin F, Nabauer M, et al. Relation of outcomes to ABC (Atrial Fibrillation Better Care) pathway adherent care in European patients with atrial fibrillation: An analysis from the ESC-EHRA EORP Atrial Fibrillation General Long-Term (AFGen LT) Registry. Europace. 2021;23:174–83.
Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Comprehensive management with the abc (Atrial fibrillation better care) pathway in clinically complex patients with atrial fibrillation: A post hoc ancillary analysis from the affirm trial. J Am Heart Assoc. 2020;9:e014932.
Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, et al. Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol. 2020;75:1523–34.
Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D, et al. Adherence to the “Atrial Fibrillation Better Care” pathway in patients with atrial fibrillation: impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb Haemost. 2022;122:406–14.
Boriani G, Proietti M, Laroche C, Fauchier L, Marin F, Nabauer M, et al. Contemporary stroke prevention strategies in 11 096 European patients with atrial fibrillation: a report from the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) Long-Term General Registry. Europace. 2018;20:747–57.
Boriani G, Proietti M, Laroche C, Fauchier L, Marin F, Nabauer M, et al. Association between antithrombotic treatment and outcomes at 1-year follow-up in patients with atrial fibrillation: The EORP-AF General Long-Term Registry. Europace. 2019;21:1013–22.
Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–36.
Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol - Ser A Biol Sci Med Sci. 2007;62:722–7.
Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10.
Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
Koenker R. Quantile Regression. Cambridge: Cambridge University Press; 2005.
Hong HG, Christiani DC, Li Y. Quantile regression for survival data in modern cancer research: expanding statistical tools for precision medicine. Precis Clin Med. 2019;2:90–9.
Lytsy P, Berglund L, Sundström J. A proposal for an additional clinical trial outcome measure assessing preventive effect as delay of events. Eur J Epidemiol. 2012;27:903–9.
Proietti M, Romiti GF, Raparelli V, Diemberger I, Boriani G, Dalla Vecchia LA, et al. Frailty prevalence and impact on outcomes in patients with atrial fibrillation: a systematic review and meta-analysis of 1,187,000 patients. Ageing Res Rev. 2022;79:101652.
Kozieł M, Simovic S, Pavlovic N, Kocijancic A, Paparisto V, Music L, et al. Impact of multimorbidity and polypharmacy on the management of patients with atrial fibrillation: insights from the BALKAN-AF survey. Ann Med. 2021;53:17–25.
Proietti M, Esteve-Pastor MA, Rivera-Caravaca JM, Roldán V, Roldán Rabadán I, Muñiz J, et al. Relationship between multimorbidity and outcomes in atrial fibrillation. Exp Gerontol. 2021;153:111482.
Proietti M, Lane DA, Lip GYH. Chronic kidney disease, time in therapeutic range and adverse clinical outcomes in anticoagulated patients with non-valvular atrial fibrillation: Observations from the SPORTIF Trials. EBioMedicine. 2016;8:309–16.
Violi F, Davì G, Proietti M, Pastori D, Hiatt WR, Corazza GR, et al. Ankle-Brachial Index and cardiovascular events in atrial fibrillation: the ARAPACIS study. Thromb Haemost. 2016;115:856–63.
Romiti GF, Corica B, Pipitone E, Vitolo M, Raparelli V, Basili S, et al. Prevalence, management and impact of chronic obstructive pulmonary disease in atrial fibrillation: a systematic review and meta-Analysis of 4,200,000 patients. Eur Heart J. 2021;42:3541–3554C.
Alexander KP, Brouwer MA, Mulder H, Vinereanu D, Lopes RD, Proietti M, et al. Outcomes of apixaban versus warfarin in patients with atrial fibrillation and multi-morbidity: Insights from the ARISTOTLE trial. Am Heart J. 2019;208:123–31.
Lip GYH, Ntaios G. Novel clinical concepts in thrombosis: integrated care for stroke management-easy as ABC. Thromb Haemost. 2021. https://doi.org/10.1055/a-1632-1777.
Field M, Kuduvalli M, Torella F, McKay V, Khalatbari A, Lip GYH. Integrated care systems and the aortovascular hub. Thromb Haemost. 2021. https://doi.org/10.1055/a-1591-8033.
Burdett P, Lip GYH. Atrial fibrillation in the UK: predicting costs of an emerging epidemic recognizing and forecasting the cost drivers of atrial fibrillation-related costs. Eur Heart J - Qual Care Clin Outcomes. 2020. https://doi.org/10.1093/ehjqcco/qcaa093.
Yao Y, Guo Y, Lip GYH. The effects of implementing a mobile health-technology supported pathway on atrial fibrillation-related adverse events among patients with multimorbidity: the mAFA-II randomized clinical trial. JAMA Netw Open. 2021;4:2140071.
Acknowledgements
EORP Oversight Committee, Executive and Steering Committees (National Coordinators) of the EURObservational Research Programme (EORP)—Atrial Fibrillation General Long-Term (EORP-AFGen LT) Registry of the European Society of Cardiology (ESC). Data collection was conducted by the EORP department by Patti-Ann McNeill as Project Officer and Viviane Missiamenou as Data Manager. Overall activities were coordinated and supervised by Doctor Aldo P. Maggioni (EORP Scientific Coordinator).
EURObservational Research Programme Atrial Fibrillation (EORP-AF) Long-Term General Registry Investigators
Executive committee: G. Boriani (Chair), G.Y.H. Lip, L. Tavazzi, A. P. Maggioni, G-A. Dan, T. Potpara, M. Nabauer, F. Marin, Z. Kalarus, L. Fauchier, R. Ferrari, A. Shantsila.
Steering Committee (National Coordinators): A. Goda, University Hospital Center ‘Mother Tereza’, Tirana, Albania; G. Mairesse, Cliniques du Sud-Luxembourg, Arlon, Belgium; T. Shalganov, National Heart Hospital, Sofia, Bulgaria; L. Antoniades, Nicosia General Hospital, Latsia, Cyprus; M. Taborsky, University Hospital Olomouc, Olomouc, Czech Republic; S. Riahi, Aalborg University Hospital, Aalborg, Denmark; P. Muda, University of Tartu, Tartu, Estonia; I. García Bolao, Navarra Institute for Health Research, Pamplona, Spain; O. Piot, Centre Cardiologique du Nord, Saint-Denis, France; M. Nabauer, Ludwig-Maximilians-University, Munich, Germany; K. Etsadashvili, G. Chapidze Emergency Cardiology Center, Tbilisi, Georgia; EN. Simantirakis, University Hospital of Heraklion, School of Medicine, University of Crete, Heraklion, Crete, Greece; M. Haim, Soroka Medical Center, Beer Sheva, Israel; A. Azhari, J. Najafian, Cardiovascular Research Institute, Isfahan University of Medical Sciences, Isfahan, Iran; M. Santini, San Filippo Neri Hospital, Rome, Italy; E. Mirrakhimov, National Center of Cardiology and Internal Medicine, Bishkek, Kyrgyzstan; K. Kulzida, Scientific-Research Institute of Cardiology and Internal Diseases, Almaty, Republic of Kazakhstan; A. Erglis, Pauls Stradins Clinical University Hospital University of Latvia Riga Latvia; L. Poposka, University Clinic of Cardiology, Faculty of Medicine, Ss Cyril and Methodius University of Skopje, Skopje, Republic of Macedonia; MR. Burg, Mater Dei Hospital, Triq Dun Karm Psaila, Malta; H. Crijns, Ö. Erküner, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Centre, Maastricht, The Netherlands; D. Atar, Oslo University Hospital Ullevål and Institute of Clinical Sciences, University of Oslo, Oslo, Norway; R. Lenarczyk, Silesian Center for Heart Disease, Zabrze, Poland; M. Martins Oliveira, Hospital Santa Marta, Lisbon, Portugal; D. Shah, Department of Medicine Specialities, University Hospital Geneva, Geneva, Switzerland; G-A. Dan, Colentina University Hospital, Bucharest, Romania; E. Serdechnaya, Northern State Medical University, Arkhangelsk, Russia; T. Potpara, Cardiology Clinic, Clinical Center of Serbia, Belgrade, Serbia; E. Diker, Başakşehir Çam and Sakura City Hospital, Istanbul, Turkey; G.Y.H. Lip, D. Lane; City Hospital, University of Birmingham, Birmingham, United Kingdom.
Investigators : Albania—Durrës: E. Zëra; Tirana: U. Ekmekçiu, V. Paparisto, M. Tase; Tirana: H. Gjergo, J. Dragoti, A. Goda. Belgium—Bastogne: M. Ciutea, N. Ahadi, Z. el Husseini, M. Raepers; Gilly: J. Leroy, P. Haushan, A. Jourdan; Haine Saint Paul: C. Lepiece; Hasselt: L. Desteghe, J. Vijgen, P. Koopman, G. Van Genechten, H. Heidbuchel; Kortrijk: T. Boussy, M. De Coninck, H. Van Eeckhoutte, N. Bouckaert; La Louviere: A. Friart, J. Boreux, C. Arend; Liege: P. Evrard; Liège: L. Stefan, E. Hoffer, J. Herzet, M. Massoz; Liège: C. Celentano, M. Sprynger, L. Pierard; Liège: P. Melon; Overpelt: B. Van Hauwaert, C. Kuppens, D. Faes, D. Van Lier, A. Van Dorpe; Waremme: A. Gerardy; Yvoir: O. Deceuninck, O. Xhaet, F. Dormal, E. Ballant, D. Blommaert. Bulgaria—Pleven: D. Yakova, M. Hristov, T. Yncheva, N. Stancheva, S. Tisheva; Plovdiv: M. Tokmakova, F. Nikolov, D. Gencheva; Sofia: T. Shalganov, B. Kunev, M. Stoyanov; Sofia: D. Marchov, V. Gelev, V. Traykov; Varna: A. Kisheva, H. Tsvyatkov, R. Shtereva, S. Bakalska-Georgieva, S. Slavcheva, Y. Yotov. Czech Republic—Ústí nad Labem: M. Kubíčková. Denmark—Aalborg: A. Marni Joensen, A. Gammelmark, L. Hvilsted Rasmussen, P. Dinesen, S. Riahi, S. Krogh Venø, B. Sorensen, A. Korsgaard, K. Andersen, C. Fragtrup Hellum; Esbjerg: A. Svenningsen, O. Nyvad, P. Wiggers; Herning: O. May, A. Aarup, B. Graversen, L. Jensen, M. Andersen, M. Svejgaard, S. Vester, S. Hansen, V. Lynggaard. Estonia— Tallinn: R. Vettus; Tartu: P. Muda. Elche, Alicante: A. Maestre; Toledo: S. Castaño. France—Abbeville: S. Cheggour; Abbeville: J. Poulard, V. Mouquet, S. Leparrée; Aix-en-Provence: J. Bouet, J. Taieb; Amiens: A. Doucy, H. Duquenne; Angers: A. Furber, J. Dupuis, J. Rautureau; Aurillac: M. Font, P. Damiano; Avignon Cedex: M. Lacrimini; Brest: J. Abalea, S. Boismal, T. Menez, J. Mansourati; Chartres: G. Range, H. Gorka, C. Laure, C. Vassalière; Creteil: N. Elbaz, N. Lellouche, K. Djouadi; Montpellier: F. Roubille, D. Dietz, J. Davy; Nimes: M. Granier, P. Winum, C. Leperchois-Jacquey; Paris: H. Kassim, E. Marijon, J. Le Heuzey; Paris: J. Fedida, C. Maupain, C. Himbert, E. Gandjbakhch, F. Hidden-Lucet, G. Duthoit, N. Badenco, T. Chastre, X. Waintraub, M. Oudihat, J. Lacoste, C. Stephan; Pau: H. Bader, N. Delarche, L. Giry; Pessac: D. Arnaud, C. Lopez, F. Boury, I. Brunello, M. Lefèvre, R. Mingam, M. Haissaguerre; Rennes: M. Le Bidan, D. Pavin, V. Le Moal, C. Leclercq; Saint Denis: O. Piot, T. Beitar; Saint Etienne: I. Martel, A. Schmid, N. Sadki, C. Romeyer-Bouchard, A. Da Costa; Tours: I. Arnault, M. Boyer, C. Piat, L. Fauchier. FYR Macedonia—Bitola: N. Lozance, S. Nastevska; Ohrid: A. Doneva, B. Fortomaroska Milevska, B. Sheshoski, K. Petroska, N. Taneska, N. Bakrecheski; Skopje: K. Lazarovska, S. Jovevska, V. Ristovski, A. Antovski; Skopje: E. Lazarova, I. Kotlar, J. Taleski, L. Poposka, S. Kedev; Skopje: N. Zlatanovik; Štip: S. Jordanova, T. Bajraktarova Proseva, S. Doncovska. Georgia—Tbilisi: D. Maisuradze, A. Esakia, E. Sagirashvili, K. Lartsuliani, N. Natelashvili, N. Gumberidze, R. Gvenetadze; Tbilisi: K. Etsadashvili, N. Gotonelia, N. Kuridze; Tbilisi: G. Papiashvili, I. Menabde. Germany—Aachen: S. Glöggler, A. Napp, C. Lebherz, H. Romero, K. Schmitz, M. Berger, M. Zink, S. Köster, J. Sachse, E. Vonderhagen, G. Soiron, K. Mischke; Bad Reichenhall: R. Reith, M. Schneider; Berlin: W. Rieker; Biberach: D. Boscher, A. Taschareck, A. Beer; Boppard: D. Oster; Brandenburg: O. Ritter, J. Adamczewski, S. Walter; Chemnitz: A. Frommhold, E. Luckner, J. Richter, M. Schellner, S. Landgraf, S. Bartholome; Chemnitz: R. Naumann, J. Schoeler; Dachau: D. Westermeier, F. William, K. Wilhelm, M. Maerkl; Detmold: R. Oekinghaus, M. Denart, M. Kriete, U. Tebbe; Ebersbach: T. Scheibner; Erlangen: M. Gruber, A. Gerlach, C. Beckendorf, L. Anneken, M. Arnold, S. Lengerer, Z. Bal, C. Uecker, H. Förtsch, S. Fechner, V. Mages; Friedberg: E. Martens, H. Methe; Göttingen: T. Schmidt; Hamburg: B. Schaeffer, B. Hoffmann, J. Moser, K. Heitmann, S. Willems, S. Willems; Hartmannsdorf: C. Klaus, I. Lange; Heidelberg: M. Durak, E. Esen; Itzehoe: F. Mibach, H. Mibach; Kassel: A. Utech; Kirchzarten: M. Gabelmann, R. Stumm, V. Ländle; Koblenz: C. Gartner, C. Goerg, N. Kaul, S. Messer, D. Burkhardt, C. Sander, R. Orthen, S. Kaes; Köln: A. Baumer, F. Dodos; Königsbrück: A. Barth, G. Schaeffer; Leisnig: J. Gaertner, J. Winkler; Leverkusen: A. Fahrig, J. Aring, I. Wenzel; Limburg: S. Steiner, A. Kliesch, E. Kratz, K. Winter, P. Schneider; Ludwigsburg: A. Haag, I. Mutscher, R. Bosch; Markkleeberg: J. Taggeselle, S. Meixner; Meissen: A. Schnabel; Meppen: A. Shamalla, H. Hötz, A. Korinth; Merzig: C. Rheinert; Moosburg: G. Mehltretter; Mühldorf: B. Schön, N. Schön, A. Starflinger, E. Englmann; Munich: G. Baytok, T. Laschinger, G. Ritscher; Munich: A. Gerth; Münster: D. Dechering, L. Eckardt; Nienburg: M. Kuhlmann, N. Proskynitopoulos; Paderborn: J. Brunn, K. Foth; Pirna: C. Axthelm, H. Hohensee, K. Eberhard, S. Turbanisch; Plauen: N. Hassler, A. Koestler; Riesa: G. Stenzel; Riesa: D. Kschiwan, M. Schwefer, S. Neiner, S. Hettwer; Rotenburg a.d. Fulda: M. Haeussler-Schuchardt, R. Degenhardt, S. Sennhenn, S. Steiner; Starnberg: M. Brendel; Westerstede: A. Stoehr, W. Widjaja, S. Loehndorf, A. Logemann, J. Hoskamp, J. Grundt; Zorneding: M. Block; Zwiesel: R. Ulrych, A. Reithmeier, V. Panagopoulos. Italy—Bologna: C. Martignani, D. Bernucci, E. Fantecchi, I. Diemberger, M. Ziacchi, M. Biffi, P. Cimaglia, J. Frisoni, G. Boriani; Firenze: I. Giannini, S. Boni, S. Fumagalli, S. Pupo, A. Di Chiara, P. Mirone; Modena: E. Fantecchi, G. Boriani, F. Pesce, C. Zoccali, V.L. Malavasi. Kazakhstan—Almaty: A. Mussagaliyeva, B. Ahyt, Z. Salihova, K. Koshum-Bayeva. Kyrgyzstan—Bishkek: A. Kerimkulova, A. Bairamukova, E. Mirrakhimov. Latvia—Riga: B. Lurina, R. Zuzans, S. Jegere, I. Mintale, K. Kupics, K. Jubele, A. Erglis, O. Kalejs. Malta—Birkirkara: K. Vanhear, M. Burg, M. Cachia, E. Abela, S. Warwicker, T. Tabone, R. Xuereb. Montenegro—Podgorica: D. Asanovic, D. Drakalovic, M. Vukmirovic, N. Pavlovic, L. Music, N. Bulatovic, A. Boskovic. Netherlands—Almere: H. Uiterwaal, N. Bijsterveld; Amsterdam: J. De Groot, J. Neefs, N. van den Berg, F. Piersma, A. Wilde; Delfzijl: V. Hagens; Enschede: J. Van Es, J. Van Opstal, B. Van Rennes, H. Verheij, W. Breukers; Heerenveen: G. Tjeerdsma, R. Nijmeijer, D. Wegink, R. Binnema; Hengelo: S. Said; Maastricht: Ö. Erküner, S. Philippens, W. van Doorn, H. Crijns; Rotterdam: T. Szili-Torok, R. Bhagwandien, P. Janse, A. Muskens; s-Hertogenbosch: M. van Eck, R. Gevers, N. van der Ven; Venlo: A. Duygun, B. Rahel, J. Meeder. Norway—Oslo: A. Vold, C. Holst Hansen, I. Engset, D. Atar. Poland—Bytom: B. Dyduch-Fejklowicz, E. Koba, M. Cichocka; Cieszyn: A. Sokal, A. Kubicius, E. Pruchniewicz; Gliwice: A. Kowalik-Sztylc, W. Czapla; Katowice: I. Mróz, M. Kozlowski, T. Pawlowski, M. Tendera; Katowice: A. Winiarska-Filipek, A. Fidyk, A. Slowikowski, M. Haberka, M. Lachor-Broda, M. Biedron, Z. Gasior; Kielce: M. Kołodziej, M. Janion; Kielce: I. Gorczyca-Michta, B. Wozakowska-Kaplon; Łódź: M. Stasiak, P. Jakubowski, T. Ciurus, J. Drozdz; Łódź: M. Simiera, P. Zajac, T. Wcislo, P. Zycinski, J. Kasprzak; Nysa: A. Olejnik, E. Harc-Dyl, J. Miarka, M. Pasieka, M. Ziemińska-Łuć, W. Bujak; Opoczno: A. Śliwiński, A. Grech, J. Morka, K. Petrykowska, M. Prasał; Opole: G. Hordyński, P. Feusette, P. Lipski, A. Wester; Radlin: W. Streb; Rzeszów: J. Romanek, P. Woźniak, M. Chlebuś, P. Szafarz, W. Stanik; Szczecin: M. Zakrzewski, J. Kaźmierczak; Szczecin: A. Przybylska, E. Skorek, H. Błaszczyk, M. Stępień, S. Szabowski, W. Krysiak, M. Szymańska; Tarnów: J. Karasiński, J. Blicharz, M. Skura; Warsaw: K. Hałas, L. Michalczyk, Z. Orski, K. Krzyżanowski, A. Skrobowski; Warsaw: L. Zieliński, M. Tomaszewska-Kiecana, M. Dłużniewski; Warsaw: M. Kiliszek, M. Peller, M. Budnik, P. Balsam, G. Opolski, A. Tymińska, K. Ozierański, A. Wancerz; Warsaw: A. Borowiec, E. Majos, R. Dabrowski, H. Szwed; Zabrze: A. Musialik-Lydka; Zabrze: A. Leopold-Jadczyk, E. Jedrzejczyk-Patej, M. Koziel, R. Lenarczyk, M. Mazurek, Z. Kalarus; Zabrze: K. Krzemien-Wolska, P. Starosta, E. Nowalany-Kozielska; Zakopane: A. Orzechowska, M. Szpot, M. Staszel. Portugal—Almada: S. Almeida, H. Pereira, L. Brandão Alves, R. Miranda, L. Ribeiro; Carnaxide Lisboa: F. Costa, F. Morgado, P. Carmo, P. Galvao Santos, R. Bernardo, P. Adragão; Santarém: G. Ferreira da Silva, M. Peres, M. Alves, M. Leal; Vila Real: A. Cordeiro, P. Magalhães, P. Fontes, S. Leão; Viseu: A. Delgado, A. Costa, B. Marmelo, B. Rodrigues, D. Moreira, J. Santos, L. Santos. Romania—Arad: A. Terchet, D. Darabantiu, S. Mercea, V. Turcin Halka, A. Pop Moldovan; Brasov: A. Gabor, B. Doka, G. Catanescu, H. Rus, L. Oboroceanu, E. Bobescu; Bucharest: R. Popescu, A. Dan, A. Buzea, I. Daha, G. Dan, I. Neuhoff; Bucharest: M. Baluta, R. Ploesteanu, N. Dumitrache, M. Vintila; Bucharest: A. Daraban, C. Japie, E. Badila, H. Tewelde, M. Hostiuc, S. Frunza, E. Tintea, D. Bartos; Bucharest: A. Ciobanu, I. Popescu, N. Toma, C. Gherghinescu, D. Cretu, N. Patrascu, C. Stoicescu, C. Udroiu, G. Bicescu, V. Vintila, D. Vinereanu, M. Cinteza, R. Rimbas; Iași: M. Grecu; Oradea: A. Cozma, F. Boros, M. Ille, O. Tica, R. Tor, A. Corina, A. Jeewooth, B. Maria, C. Georgiana, C. Natalia, D. Alin, D. Dinu-Andrei, M. Livia, R. Daniela, R. Larisa, S. Umaar, T. Tamara, M. Ioachim Popescu; Târgu Mureș: D. Nistor, I. Sus, O. Coborosanu; Timișoara: N. Alina-Ramona, R. Dan, L. Petrescu; Timișoara: G. Ionescu, I. Popescu, C. Vacarescu, E. Goanta, M. Mangea, A. Ionac, C. Mornos, D. Cozma, S. Pescariu. Russian Federation—Arkhangelsk: E. Solodovnicova, I. Soldatova, J. Shutova, L. Tjuleneva, T. Zubova, V. Uskov; Arkhangelsk: D. Obukhov, G. Rusanova; Arkhangelsk: I. Soldatova, N. Isakova, S. Odinsova, T. Arhipova; Arkhangelsk: E. Kazakevich, E. Serdechnaya, O. Zavyalova; Saint-Petersburg: T. Novikova; Saint-Petersburg: I. Riabaia, S. Zhigalov; Saint-Petersburg: E. Drozdova, I. Luchkina, Y. Monogarova; Vladivostok: D. Hegya, L. Rodionova, L. Rodionova, V. Nevzorova; Vladivostok: I. Soldatova, O. Lusanova. Serbia—Belgrade: A. Arandjelovic, D. Toncev, M. Milanov, N. Sekularac; Belgrade: M. Zdravkovic, S. Hinic, S. Dimkovic, T. Acimovic, J. Saric; Belgrade: M. Polovina, T. Potpara, B. Vujisic-Tesic, M. Nedeljkovic; Belgrade: M. Zlatar, M. Asanin; Belgrade: V. Vasic, Z. Popovic; Belgrade: D. Djikic, M. Sipic, V. Peric, B. Dejanovic, N. Milosevic; Belgrade: A. Stevanovic, A. Andric, B. Pencic, M. Pavlovic-Kleut, V. Celic; Kragujevac: M. Pavlovic, M. Petrovic, M. Vuleta, N. Petrovic, S. Simovic, Z. Savovic, S. Milanov, G. Davidovic, V. Iric-Cupic; Niška Banja: D. Simonovic, M. Stojanovic, S. Stojanovic, V. Mitic, V. Ilic, D. Petrovic, M. Deljanin Ilic, S. Ilic, V. Stoickov; Pirot: S. Markovic; Šabac: S. Kovacevic. Spain—Alicante: A. García Fernandez; Benalmadena: A. Perez Cabeza; Córdoba: M. Anguita; Elche, Alicante: A. Maestre; Toledo: S. Castaño Granada: L. Tercedor Sanchez; Huarte: E. Mau, J. Loayssa, M. Ayarra, M. Carpintero; Madrid: I. Roldán Rabadan; Murcia: M. Leal; Murcia: M. Gil Ortega; Murcia: A. Tello Montoliu, E. Orenes Piñero, S. Manzano Fernández, F. Marín, A. Romero Aniorte, A. Veliz Martínez, M. Quintana Giner; Madrid: M. Ciudad; Pamplona: G. Ballesteros, M. Palacio, O. Alcalde, I. García-Bolao; San Juan de Alicante: V. Bertomeu Gonzalez; Santiago de Compostela: F. Otero-Raviña, J. García Seara, J. Gonzalez Juanatey. Switzerland—Geneva: N. Dayal, P. Maziarski, P. Gentil-Baron, D. Shah. Turkey—Adana: M. Koç; Afyon: E. Onrat, I. E. Dural; Ankara: K. Yilmaz, B. Özin; Ankara: S. Tan Kurklu, Y. Atmaca; Ankara: U. Canpolat, L. Tokgozoglu; Ankara: A. K. Dolu, B. Demirtas, D. Sahin; Ankara: O. Ozcan Celebi, E. Diker; Antalya: G. Gagirci; Bayraklı/Izmir: U.O.Turk; Bursa: H. Ari; Diyarbakır: N. Polat, N. Toprak; Gaziantep: M. Sucu; Görükle-Bursa: O. Akin Serdar; Istanbul: A. Taha Alper; Istanbul: A. Kepez; Istanbul: Y. Yuksel; Kurupelit - Samsun: A. Uzunselvi, S. Yuksel, M. Sahin; Merkez/Düzce: O. Kayapinar; Mersin: T. Ozcan; Sivas: H. Kaya, M. B. Yilmaz; Trabzon: M. Kutlu; Yüreğir-Adana: M. Demir. UK—Barnstaple: C. Gibbs, S. Kaminskiene, M. Bryce, A. Skinner, G. Belcher, J. Hunt, L. Stancombe, B. Holbrook, C. Peters, S. Tettersell; Birmingham: A. Shantsila, D. Lane, K. Senoo, M. Proietti, K. Russell, P. Domingos, S. Hussain, J. Partridge, R. Haynes, S. Bahadur, R. Brown, S. McMahon, G. Y H Lip; Blackburn: J. McDonald, K. Balachandran, R. Singh, S. Garg, H. Desai, K. Davies, W. Goddard; Blackpool: G. Galasko, I. Rahman, Y. Chua, O. Payne, S. Preston, O. Brennan, L. Pedley, C. Whiteside, C. Dickinson, J. Brown, K. Jones, L. Benham, R. Brady; Carlisle: L. Buchanan, A. Ashton, H. Crowther, H. Fairlamb, S. Thornthwaite, C. Relph, A. McSkeane, U. Poultney, N. Kelsall, P. Rice, T. Wilson; Chertsey: M. Wrigley, R. Kaba, T. Patel, E. Young, J. Law; Cramlington: C. Runnett, H. Thomas, H. McKie, J. Fuller, S. Pick; Exeter: A. Sharp, A. Hunt, K. Thorpe, C. Hardman, E. Cusack, L. Adams, M. Hough, S. Keenan, A. Bowring, J. Watts; Great Yarmouth: J. Zaman, K. Goffin, H. Nutt; Harrogate: Y. Beerachee, J. Featherstone, C. Mills, J. Pearson, L. Stephenson; Huddersfield: S. Grant, A. Wilson, C. Hawksworth, I. Alam, M. Robinson, S. Ryan; Macclesfield: R. Egdell, E. Gibson, M. Holland, D. Leonard; Maidstone: B. Mishra, S. Ahmad, H. Randall, J. Hill, L. Reid, M. George, S. McKinley, L. Brockway, W. Milligan; Manchester: J. Sobolewska, J. Muir, L. Tuckis, L. Winstanley, P. Jacob, S. Kaye, L. Morby; Nottingham: A. Jan, T. Sewell; Poole: C. Boos, B. Wadams, C. Cope, P. Jefferey; Portsmouth: N. Andrews, A. Getty, A. Suttling, C. Turner, K. Hudson, R. Austin, S. Howe; Redhill: R. Iqbal, N. Gandhi, K. Brophy, P. Mirza, E. Willard, S. Collins, N. Ndlovu; Rhyl: E. Subkovas, V. Karthikeyan, L. Waggett, A. Wood, A. Bolger, J. Stockport, L. Evans, E. Harman, J. Starling, L. Williams, V. Saul; Salisbury: M. Sinha, L. Bell, S. Tudgay, S. Kemp, J. Brown, L. Frost; Shrewsbury: T. Ingram, A. Loughlin, C. Adams, M. Adams, F. Hurford, C. Owen, C. Miller, D. Donaldson, H. Tivenan, H. Button; South Shields: A. Nasser, O. Jhagra, B. Stidolph, C. Brown, C. Livingstone, M. Duffy, P. Madgwick; Southampton: P. Roberts, E. Greenwood, L. Fletcher, M. Beveridge, S. Earles; Taunton: D. McKenzie, D. Beacock, M. Dayer, M. Seddon, D. Greenwell, F. Luxton, F. Venn, H. Mills, J. Rewbury, K. James, K. Roberts, L. Tonks; Torquay: D. Felmeden, W. Taggu, A. Summerhayes, D. Hughes, J. Sutton, L. Felmeden; Watford: M. Khan, E. Walker, L. Norris, L. O'Donohoe; Weston-super-Mare: A. Mozid, H. Dymond, H. Lloyd-Jones, G. Saunders, D. Simmons, D. Coles, D. Cotterill, S. Beech, S. Kidd; Wolverhampton: B. Wrigley, S. Petkar, A. Smallwood, R. Jones, E. Radford, S. Milgate, S. Metherell, V. Cottam; Yeovil: C. Buckley, A. Broadley, D. Wood, J. Allison, K. Rennie, L. Balian, L. Howard, L. Pippard, S. Board, T. Pitt-Kerby.
Funding
Since the start of EORP, the following companies have supported the programme: Abbott Vascular Int. (2011–2021), Amgen Cardiovascular (2009–2018), AstraZeneca (2014–2021), Bayer (2009–2018), Boehringer Ingelheim (2009–2019), Boston Scientific (2009–2012), The Bristol Myers Squibb and Pfizer Alliance (2011–2016), The Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2011–2017), Edwards (2016–2019), Gedeon Richter Plc. (2014–2017), Menarini Int. Op. (2009–2012), MSD-Merck & Co. (2011–2014), Novartis Pharma AG (2014–2020), ResMed (2014–2016), Sanofi (2009–2011), SERVIER (2010–2021), Vifor (2019–2022).
Author information
Authors and Affiliations
Consortia
Contributions
GFR, MP and GYHL conceived and design the analysis; GFR and MP analysed, interpreted the data and drafted the manuscript; MV, NB, AMF, WYD, LF, FM, MN, GAD, TSP, GB and GYHL revised the manuscript and gave relevant intellectual contribution. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Patient data were obtained after the signing of a written informed consent by each patient, following the approval of study protocol by an Institutional Review Board/Ethic Committee. The study was first approved by the National Coordinators’ main institutions (listed in the Acknowledgements section) and subsequently authorized by each peripheral site, under the responsibility of the lead contact and study team (all listed in the Acknowledgements section), according to the specific national and local regulation. Any details regarding approval numbers for the study protocol regarding any specific site could be obtained from the corresponding authors, upon reasonable request. The study was performed according to the European Union Note for Guidance on Good Clinical Practice CPMP/ECH/135/95 and the Declaration of Helsinki.
Consent for publication
Not applicable
Competing interests
MP is an investigator of the AFFIRMO project on multimorbidity in AF, which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 899871. LF has been a consultant and speaker for AstraZeneca, Bayer, BMS/Pfizer, Boehringer Ingelheim, Medtronic, Novartis, Novo, XO and Zoll. FM is a consultant for Bayer and Boehringer-Ingelheim; FM received small research’s fees from AFNET, Ferrer, Astra-Zeneca, Boehringer-Ingelheim and Bayer; FM is an investigator of the AFFIRMO project on multimorbidity in AF, which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 899871. TSP is a consultant for Bayer and Pfizer, with no fees received personally; GB a received small speaker’s fees from Medtronic, Boston, Boehringer Ingelheim and Bayer; GYHL is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally. GYHL is co-principal investigator of the AFFIRMO project on multimorbidity in AF, which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 899871.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Items Included into the Frailty Index. Table S2. Baseline Characteristics of the Cohort. Table S3. Cox Regression for the risk of major outcomes according to clinical complexity and subgroups. Table S4. Baseline characteristics according to cluster allocation. Figure S1. Kaplan Meier Curves for the risk of MACE according to cluster analysis. Figure S2. Kaplan Meier Curves for the risk of composite outcome according to cluster analysis. Figure S3. Delay of Event analysis for MACE, ABC adherent vs. non-adherent in cluster 1 subgroup. Figure S4. Delay of Event analysis for Composite Outcome, ABC adherent vs. non-adherent in cluster 1 subgroup.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Romiti, G.F., Proietti, M., Vitolo, M. et al. Clinical complexity and impact of the ABC (Atrial fibrillation Better Care) pathway in patients with atrial fibrillation: a report from the ESC-EHRA EURObservational Research Programme in AF General Long-Term Registry. BMC Med 20, 326 (2022). https://doi.org/10.1186/s12916-022-02526-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02526-7