Abstract
Background
In China, colorectal cancer (CRC) incidence and mortality have been steadily increasing over the last decades. Risk models to predict incident CRC have been developed in various populations, but they have not been systematically externally validated in a Chinese population.
This study aimed to assess the performance of risk scores in predicting CRC using the China Kadoorie Biobank (CKB), one of the largest and geographically diverse prospective cohort studies in China.
Methods
Nine models were externally validated in 512,415 participants in CKB and included 2976 cases of CRC. Model discrimination was assessed, overall and by sex, age, site, and geographic location, using the area under the receiver operating characteristic curve (AUC). Model discrimination of these nine models was compared to a model using age alone. Calibration was assessed for five models, and they were re-calibrated in CKB.
Results
The three models with the highest discrimination (Ma (Cox model) AUC 0.70 [95% CI 0.69–0.71]; Aleksandrova 0.70 [0.69–0.71]; Hong 0.69 [0.67–0.71]) included the variables age, smoking, and alcohol. These models performed significantly better than using a model based on age alone (AUC of 0.65 [95% CI 0.64–0.66]). Model discrimination was generally higher in younger participants, males, urban environments, and for colon cancer. The two models (Guo and Chen) developed in Chinese populations did not perform better than the others. Among the 10% of participants with the highest risk, the three best performing models identified 24–26% of participants that went on to develop CRC.
Conclusions
Several risk models based on easily obtainable demographic and modifiable lifestyle factor have good discrimination in a Chinese population. The three best performing models have a higher discrimination than using a model based on age alone.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is the third most diagnosed cancer and the second most common cause of cancer-related death worldwide [1]. In China, CRC incidence and mortality have been steadily increasing over the last decades [2]. As a result, strategies to identify those at higher risk are needed in China to improve early detection of CRC and reduce the burden of disease. In many high-income countries, decisions around screening for CRC are based on age alone. The US Preventative Services Task Force recommends screening for CRC in all adults aged 50–75 years [3]. In the UK, all adults aged 56–74 are offered a faecal immunochemical test (FIT) every 2 years and those with abnormal FIT results are referred to colonoscopy, the gold-standard test for diagnosing CRC [4]. These screening programmes have shown to be effective in reducing mortality of CRC [5, 6]. In China, there is currently no nationwide screening programme [7, 8]. While there is some screening regionally and for those at high risk (defined as having a first degree relative with colorectal cancer, history of cancer, or history of bowel conditions), it is not standardised and uptake is limited [9]. It is not clear whether using age as the only factor to screen for CRC would be an effective screening strategy in China. Moreover, it would be useful to know whether an age-based screening strategy could be improved upon by adding other modifiable risk factors to enhance prediction of CRC. Prognostic risk prediction models, based on easily obtainable demographic, medical history, and lifestyle variables, can be used to stratify the population to identify high-risk individuals, guide referral to screening, and motivate behavioural changes that could reduce risk [10].
Previous systematic reviews have identified risk prediction models for CRC developed in various populations, but they have not been systematically externally validated in a Chinese population [11]. In this study, we aimed to assess the performance of risk scores in predicting CRC using the China Kadoorie Biobank (CKB), one of the largest and geographically diverse prospective cohort studies in China. Specifically, we aim to externally validate published risk scores for predicting CRC based on lifestyle and demographic information and determine how these models compare to using an age threshold alone as a screening strategy.
Methods
Selection of risk prediction models
We identified nine risk prediction models for either CRC, colon cancer, or rectal cancer that met our inclusion criteria (Fig. 1) by updating a previous systematic review from November 2016 to June 2021 (Additional File 1: Page S1) [11]. We excluded 12,645 articles based on their title and abstract, screened 56 full-text articles. From the full text, we excluded 22 models that included genetic or biochemical biomarkers, nine that included family history, and eight articles that did not include a risk score but described risk factor associations with CRC (Fig. 1). Five models assessed prognosis of those diagnosed with CRC and were excluded, and three models were excluded for containing procedural variables. Eight articles were included in our study, including a total of nine models (Ma article developed two different models [12]). We performed an external validation of these risk models following the TRIPOD (Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis) guideline (Additional File 1: Page S2) [13].
Study population
Data from the China Kadoorie Biobank (CKB), a large, prospective population-based cohort in China, were used to externally validate the risk models. The details of the CKB design, survey methods, and population characteristics are described elsewhere [14]. In brief, 512,726 participants aged 30–79 years were recruited into the study between 2004 and 2008 from 10 geographically defined localities (5 urban and 5 rural) in China. Central ethical approvals were obtained from Oxford University and the China National Centre for Disease Control and Prevention (CDC). Approvals were also obtained from institutional research boards at the local CDCs in the 10 areas. At local assessment centres, participants completed an interviewer-administered laptop-based questionnaire on sociodemographic characteristics, smoking, alcohol consumption, diet, physical activity, personal and family medical history, and current medication. A range of physical measurements were recorded by trained technicians, including height, weight, hip and waist circumference, blood pressure, and heart rate, using calibrated instruments with standard protocols. A description of how the variables in CKB were ascertained is given in Additional File 1: Page S3 [15, 16].
Follow-up for cancer incidence and mortality
The vital status of each participant was determined periodically through China CDC’s Disease Surveillance Points (DSP) system and national health insurance system, supplemented by regular checks against local residential and administrative records and by annual active confirmation through street committees or village administrators. In addition, information about major diseases and any episodes of hospitalisation was collected through linkage, via each participant’s unique national identification number, with cancer registries, national health insurance claims databases, and death registries. Data on colorectal cancer incidence and mortality was available for each participant up to December 31, 2017. All death, diagnosis, or hospitalisation events were coded using International Classification of Disease 10th Revision (ICD-10) by trained staff who were blinded to baseline information [14]. Information on cancer histological subtypes was also collected for a subset of the cases through cancer registries or reviews of hospital medical notes as part of the ongoing outcome adjudication for major diseases.
Risk model prediction variables and outcome variables
For each risk factor, we used data collected from the baseline questionnaire. Variables from the CKB dataset were matched as closely as possible to the variables used in each model. If there was not an exact equivalent, proxy variables were derived. Full details of the risk factor definitions, how they were operationalised in the CKB dataset, and how missing data were handled are given in Additional File 1: Page S4 [17,18,19]. The outcome for each risk model was a composite outcome based on incidence or death from colorectal cancer (ICD C18-20), colon cancer (ICD C18), and rectal cancer (C19-20), as well as right-sided colon cancer (ICD 18.0–18.3) and left-sided colon cancer (ICD C18.5–18.7) using data from linked cancer registries, health insurance records, and death registries.
Statistical methods
The discrimination and calibration of risk prediction models were computed. Participants were followed-up from study entry to diagnosis or death from CRC, loss to follow-up, death from other causes, or 10 years since study entry, whichever occurred first. Because the aetiology of site-specific CRCs is hypothesised to differ, model discrimination was also assessed in cancers of the colon and rectum separately, as well as in right- and left-sided colon cancer [20, 21]. Discrimination was also assessed separately in males and females (even if the model was developed only from males), urban and rural populations (because the increasing incidence of CRC in China has been hypothesised to be linked to more “western” urban lifestyles [22, 23]), and in those age 56 years or older versus younger than age 56 to determine how the full models perform in older and younger participants. Discrimination among the nine models was also compared to a model based on age alone, by comparing to the national UK colorectal screening age cut-offs (adults between 56 and 74 are screened using FIT every 2 years). A model was fit with the only explanatory variable being age 56 or older versus younger than age 56 to determine how the multivariable models perform compared to a model just using an age cut-off. The primary outcome was incidence or mortality from CRC, and the discriminative capability of the models was compared using receiver operating characteristic curves (ROC) and areas under the ROC (AUC). Sensitivity, specificity, and both positive and negative predictive values were computed for the 10% and 25% of participants with the highest risk of CRC, as determined by each model. In addition, the difference in ROC analyses between the age only model and the models that included lifestyle risk factors was quantified using the Delong method [24].
Calibration was assessed graphically for models that included estimations of absolute risk of CRC at different risk score levels in the published article presenting the model. Observed risk was plotted against expected risk of developing CRC over the 10-year period in groups based on quantiles of expected risk and the slope and intercept were estimated. Most models predicted risk over 10 years, other than the Driver [25] model, which predicted risk over 20 years and required converting the predicted risk to over 10 years by assuming an exponential distribution. To re-calibrate the models, the predicted scores were split at their deciles and the slope and intercept of the observed risk plotted against expected risk graph was computed. Each score value (corresponding to a decile of risk) was multiplied by the slope and the intercept was added, to produce a new re-calibrated set of estimates of absolute risk of CRC.
Several sensitivity analyses were carried out. Discrimination analyses were performed by fitting a Cox regression and comparing the C-statistic, which considers the time of CRC onset, to the ROC analysis, which is based on a binary outcome variable. The rationale for using the ROC analysis is both to compare results to existing literature, and to compare model performance in different cohorts. Finally, because the aspirin variable is only available for a subsection of the population in CKB (those with a history of coronary heart disease), we removed the aspirin variable from the two models that contained it (Imperiale [26] and Hong [27] models) and compared their performance.
Analyses were done using R version 3.6.3 and packages pROC (version 1.18), stringi (version 1.7.5), tidyverse (version 1.3.1), and table 1 (version 1.1).
Results
Because most risk models predict the 10-year risk of developing CRC, we included up to 10 years of follow-up for each participant. The 311 participants previously diagnosed with intestinal cancer, and two participants with missing BMI, were excluded. This resulted in 512,415 participants included in the primary analyses. Among those, there were 2976 cases of incident CRC (which includes cancer anywhere from the caecum to the rectum, including cancer in both the colon and rectum), 1720 cases of incident colon cancer, and 1772 cases of incident rectal cancer. Characteristics of participants with and without incident CRC are given in Table 1. Those with CRC were more likely to be older, male, current or ex-regular smokers, weekly alcohol drinkers, have a diagnosis of diabetes, and do less physical activity.
Characteristics of included models
Of the nine included models, all had CRC as the main outcome and two articles, including three models (Driver [25], Ma Point [12], Ma Cox [12]), additionally considered colon or rectal cancer as separate outcomes. Information about these models, including the study size, variables used in the model, and cancer outcomes included, are given in Table 2. All articles published either a point score, Cox proportional hazards model, or a logistic regression, other than the Ma article, which had both a point score and Cox model. Four models (Driver [25], Ma Point [12], Ma Cox [12], and Guo [28]) were developed only in a male population; the rest were developed in both male and female populations. Two models (Guo [28] and Chen [29]) were developed in Chinese populations. Five models were developed using < 500 cases of CRC, the two Ma [12] models used 543 cases, the Hong [27] model was developed from 1117 cases, and Aleksandrova [30] from 3645 cases of CRC.
Variables included in the risk scores are given in Table 3. Age was included in all models and all models (other than Guo [28] and Aleksandrova [30]) included either sex or BMI. The other most frequently included variables were smoking, alcohol, and physical activity. Three models included dietary variables and two included aspirin intake. Details for the full equations of the risk models are given in Additional File 1: Page S5.
Model discrimination
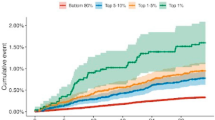
Figure 2A shows the AUC for colorectal cancer in males and females and separated by sex (Fig. 2B for males and 2c for females). The three models with the highest discrimination (Ma Cox AUC 0.70 [95% CI 0.69–0.71]; Aleksandrova 0.70 [0.69–0.71]; Hong 0.69 [0.67–0.71]) include age, smoking, and alcohol in their models. In addition, Ma Cox [12] and Aleksandrova [30] included BMI or waist circumference and Hong [27] included sex. In contrast, Driver [25], Imperiale [26], and Chen [29] had the lowest discrimination (Driver AUC 0.61 [95% CI 0.59–0.63]; Imperiale 0.60 [0.58–0.63]; Chen 0.62 [0.61–0.63]). The age threshold model had an AUC of 0.65 [0.64–0.66], which was statistically significantly lower than the three best performing models when compared using the Delong method (p < 0.001 for the Ma Cox, Aleksandrova, and Hong model, respectively). In terms of sex differences, the Driver [25] and Imperiale [26] models performed better in females, the Hong [27] model performed the same, and all other models performed similarly by sex. Of the four models that were developed in males, all performed better in males except Driver [25]; however, four of the five developed in both males and females also performed better in males.
Figure 3A shows colon cancer and Fig. 3B shows rectal cancer outcomes. The overall discrimination for colon cancer was similar compared to CRC (AUC range 0.61–0.70) and discrimination was generally similar for predicting rectal cancer (AUC range 0.59–0.69). The discrimination of models was generally similar for right-sided colon cancer, compared to the combined colon cancer outcome (Fig. 3C, A, respectively), but both were lower than the left-sided colon cancer outcome (Fig. 3D). Figure 4 shows the discrimination of the models in predicting CRC by comparing those younger than 56 to those aged 56 or older (Fig. 4A, B, respectively) as well as those in urban and rural environments (Fig. 4C, D, respectively). In general, models performed better in younger participants than in older ones; in older participants, all models had an AUC lower than 0.60, whereas in younger participants, six models had an AUC higher than 0.60. When comparing models evaluated on participants from urban and rural environments, all models performed better in urban environments than in rural ones, except for the Imperiale [26] model which performed the same in both environments.
Model discrimination for 10-year risk of developing colorectal cancer by age and geographic location. Area under the receiver operating characteristic curve for the risk models in A younger participants (age < 56 years), B older participants (age ≥ 56), C participants from urban settings, and D participants from rural settings
Table 4 shows sensitivity, specificity, and positive and negative predictive values for the 10% and 25% of participants with the highest risk, predicted by each model. Among the 10% of participants with the highest risk, the Ma Cox [12] and Aleksandrova [30] models identified 25.9% and 25.2% of participants, respectively, that went on to develop CRC. In contrast, the Chen [29], Guo [28], and Driver [25] models only identified 6.8%, 7.6%, and 9.2% of those that went on to develop CRC. Among the 25% of participants with the highest risk, the Ma Cox [12] and Aleksandrova [30] models identified 52.7% and 51.8% of participants, respectively, that went on to develop CRC. In contrast, the Imperiale [26], Betes [31], and Driver [25] models only identified 32.7%, 35.5%, and 36.8% of those that went on to develop CRC. The specificity was above 90% for all models for the 10% of participants with the highest risk. The Hong [27] and Aleksandrova [30] models had the lowest specificity (90.1%) and the Guo [28] and Chen [29] models had the highest of (96.3% and 95.6%, respectively). Among the 25% of participants with the highest risk, the model specificity ranged from 75.1% in the Aleksandrova [30] and Hong [27] models to 84% in the Betes [31] model. The positive predictive values for the 10% with the highest risk ranged from 1.2% (Guo model) to 2.3% (Driver model). The negative predictive values for the 10% with the highest risk ranged from 99.0% (Driver and Imperiale models) to 99.6% (Aleksandrova model).
Model calibration
Five models contained estimations of absolute risk of CRC in the published articles and their calibration could be assessed in CKB (Ma Point [12], Imperiale [26], Driver [25], Guo [28], and Hong [27]). Figure 5 shows the observed and expected 10-year risk of CRC for those models in males and females combined. Three models (Ma Point [12], Driver [25], and Hong [27] models) overestimated risk and two models (Imperiale [26] and Guo [28] models) underestimated risk, especially at higher levels of observed risk. Models were recalibrated and the slope and intercept were adjusted to match the observed and expected risks. The recalibrated expected 10-year risks for each model fitted to CKB data are given in Additional File 2: Table S1 and the recalibrated curves can be found in Additional File 3: Figure S1.
Sensitivity analyses
The results from the sensitivity analyses were consistent with the main results. When a Cox proportional hazards model was compared to a ROC curve analysis, all models had a slightly higher discrimination using Cox compared to ROC, but how the models performed relative to each other remained the same (Additional File 3: Figure S2). The two best performing models were still the Aleksandrova [30] and Ma Cox [12] models which both had C-statistics of 0.71 [95% CI 0.70–0.72]. The aspirin variable was only available for a subsection of the population, and when the aspirin variable was removed, the AUC slightly dropped in Hong [27] but remained the same for Imperiale [26] (Hong AUC dropped from 0.69 [95% CI 0.67–0.71] to 0.68 [0.67–0.69] and Imperiale AUC remained the same at 0.60 [0.58–0.63]).
Discussion
In this study of CRC model performance assessed in a large prospective cohort study in China, performance of published risk models based on demographic and easily obtainable lifestyle variables varied substantially but models had good overall discrimination. Of the nine models assessed, the Ma Cox [12] and Aleksandrova [30] models had the highest discrimination (AUC 0.70), which is similar to that of the derivation cohort in the published articles (0.70 and 0.71 for the Ma and Aleksandrova models, respectively). The data from this study show that using these models, the 10% with the highest risk would include approximately one-quarter of people that go on to develop CRC. The 25% with the highest risk would include approximately half of those that develop CRC. These models were better at predicting incident CRC than simply using an age cut-off of 56 years, which had an AUC of 0.65. This study showed that several existing models could offer better efficacy in identifying those at higher risk of CRC than using age 56 as the only criteria.
The finding that most models performed better in males than in females in this Chinese population is consistent with external validations of CRC models in other populations [32]. This could relate to a difference in reporting of risk factors or a difference in the aetiology of CRC between males and females. For example, more females present with right sided colon cancer than males and differences in the association between dietary factors (such as meat and fibre consumption) and CRC by sex has been reported [20, 33]. Moreover, female hormonal factors are likely to contribute to differences in risk. Previous and current hormone replacement therapy (HRT), especially combined oestrogen-progesterone therapy, is associated with a lower risk of CRC [34, 35]. However, because fewer females in China are on HRT compared to in “western” populations, it is not clear if exogenous hormonal factors are significant contributors to developing CRC [36].
The finding that models developed in Chinese populations did not perform better than those developed in European or North American populations was unexpected but could be related to the small number of CRC cases that the models were based on, limiting their generalisability. The two Chinese models were developed based on 138 and 353 cases of CRC, compared to > 1000 cases for the Hong [27] model and > 3000 for the Aleksandrova [30] model, two of the best performing models. While other studies have shown that country-specific risk models can perform better due to differences in the distribution and impact of risk factors, this study’s findings suggest that models based on a large number of CRC cases may have more generalisability across different populations than country-specific models based on small populations.
To address whether lifestyle information is important for absolute risk beyond age alone, we assessed model performance in younger (< 56 years) and older (56 years and older) age groups. The results showed that, for most models, discrimination was higher at younger ages. Although we cannot be certain why models performed better in younger populations, it is possible that earlier onset CRC is more likely to have a heritable component where the risk factors play a bigger part in the disease pathogenesis. This finding highlights the potential utility of using risk prediction models for screening high-risk younger participants, who could be motivated to change behaviours that may influence risk over decades. Moreover, three models that included age and other medical history and lifestyle variables had a higher discrimination than the UK age cut-off model for predicting CRC risk, highlighting the relevance of modifiable ‘lifestyle’ factors to CRC risk prediction beyond age alone.
Discrimination analyses were performed using the AUC, a standard measure of discrimination, which allowed for comparison to model validation in other populations (using a C-statistic produced similar results). Compared to the discrimination of risk models for other cancers, the discrimination of the best performing models was only slightly lower than risk models for breast (0.72–0.76), melanoma (0.70–0.79), and kidney cancer (> 0.70) [37,38,39]. The AUC results from this study were similar but slightly lower than those from the internal validation studies for the Driver, Imperiale, Hong, Guo, and Chen models. The published Ma Cox article was the only one that performed a validation in an external population in Japan and the AUC was lower than in this study (CKB AUC was 0.70 [0.69–0.72] compared to external validation AUC of 0.64 [0.61–0.67]). A similar study externally validated risk prediction models for CRC in UK Biobank (UKB), the largest population-based cohort in the UK [32, 40]. Three models that were used in this study (Driver, Ma Point, Ma Cox) were also externally validated in UKB and they performed similarly in both cohorts (Additional File 2: Table S2).
This was the first study to externally validate multiple risk prediction models in the China Kadoorie Biobank (CKB), a large prospective cohort of 0.5 million participants from geographically diverse areas. The strengths of CKB include a prospective design, a large and diverse study population, large numbers of CRC cases by sex and by anatomical site, wider regional variation than studies used for existing scores, completeness of data, linkage to national cancer registries, and over 10 years of follow-up. A strength of this study is the direct comparison of multiple models in the same population and the re-calibration of five models to better predict risk in a Chinese population. A limitation of this study is that models were excluded from the systematic review that required family history of CRC or genetic information, as these details were not available for the main study population. Although family history of CRC may be a useful risk factor in the absence of other information, it may not be a main contributor to risk if other lifestyle information is available [26, 41]. In addition, including genetic information to risk scores for CRC has been shown to improve discrimination and calibration, but reduces their generalisability [42,43,44]. In a country like China, without a national screening programme, having a risk model based on easily obtainable data (like age, smoking status, BMI, alcohol consumption) could be preferable.
Future work should explore how risk models can help make recommendations about type of screening that should be offered to those at high risk, the age to begin screening, and screening intervals. An ongoing randomised controlled trial in China (TARGET-C) is comparing the effectiveness and cost-effectiveness of three different screening strategies in adults aged 50–74 (one-time colonoscopy, annual FIT, and annual risk score screening) [45]. Interim analysis has shown a high participation rate for risk-score screening and its diagnostic yield was superior to that of FIT [46]. The outcome of this trial, and others like it, will provide valuable information about the feasibility of obtaining risk factor data for use in risk scores and the potential benefits of incorporating a risk-based stratification approach along with other methods of screening into clinical practice.
Conclusions
In conclusion, this was the first study to externally validate risk prediction models for CRC in the China Kadoorie Biobank, a large, geographically diverse prospective cohort study in China. Nine risk models were found to have good discrimination; the two best performing models included easily obtainable variables based on demographic, medical history, and lifestyle information. This study showed that three models had a higher discrimination than using the UK CRC screening age cut-off of 56. Five models have been recalibrated to better fit a Chinese population. These should be evaluated alongside other screening modalities (such as colonoscopy and FIT) to establish how these risk scores can be used to identify high risk individuals and improve screening uptake in China.
Availability of data and materials
The China Kadoorie Biobank (CKB) is a global resource for the investigation of lifestyle, environmental, blood biochemical, and genetic factors as determinants of common diseases [14]. The CKB study group is committed to making the cohort data available to the scientific community in China, the UK, and worldwide to advance knowledge about the causes, prevention, and treatment of disease. For detailed information on what data is currently available to open access users and how to apply for it, visit: http://www.ckbiobank.org/site/Data+Access.
Researchers who are interested in obtaining the raw data from the China Kadoorie Biobank study that underlines this paper should contact ckbaccess@ndph.ox.ac.uk. A research proposal will be requested to ensure that any analysis is performed by bona fide researchers and—where data is not currently available to open access researchers—is restricted to the topic covered in this paper.
Abbreviations
- AUC:
-
Area under the receiver operator curve
- BMI:
-
Body mass index
- China CDC:
-
China National Centre for Disease Control and Prevention
- CKB:
-
China Kadoorie Biobank
- CRC:
-
Colorectal cancer
- DSP:
-
Disease Surveillance Points system
- FIT:
-
Faecal immunochemical test
- HRT:
-
Hormone replacement therapy
- ICD:
-
International Classification of Disease
- ROC:
-
Receiver operating characteristic curve
- UK:
-
United Kingdom
- UKB:
-
UK Biobank
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32.
Haghighat S, Sussman DA, Deshpande A. US Preventive services task force recommendation statement on screening for colorectal cancer. JAMA. 2021;326(13):1328.
England PH. Bowel cancer screening: programme overview GOV.UK2021 [Available from: https://www.gov.uk/guidance/bowel-cancer-screening-programme-overview#target-population.
Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, von Wagner C, et al. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut. 2012;61(10):1439–46.
Hewitson P, Glasziou P, Irwig L, Towler B, Watson E. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev. 2007:2007(1):CD001216. https://doi.org/10.1002/14651858.CD001216.pub2. PMID: 17253456; PMCID: PMC6769059.
Cao M, Li H, Sun D, He S, Yu Y, Li J, et al. Cancer screening in China: the current status, challenges, and suggestions. Cancer Lett. 2021;506:120–7.
National Clinical Research Center for Digestive D, National Early Gastrointestinal-Cancer P, Treatment Center A, Chinese Society of Digestive E, Chinese Society of Health M, Digestive Endoscopy Professional Committee of Chinese Endoscopist A, et al. [Chinese consensus of early colorectal cancer screening (2019, Shanghai)]. Zhonghua Nei Ke Za Zhi. 2019;58(10):736–44.
Chen H, Li N, Ren J, Feng X, Lyu Z, Wei L, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut. 2019;68(8):1450–7.
Gaziano TA, Young CR, Fitzmaurice G, Atwood S, Gaziano JM. Laboratory-based versus non-laboratory-based method for assessment of cardiovascular disease risk: the NHANES I Follow-up Study cohort. Lancet. 2008;371(9616):923–31.
Usher-Smith JA, Walter FM, Emery JD, Win AK, Griffin SJ. Risk prediction models for colorectal cancer: a systematic review. Cancer Prev Res (Phila). 2016;9(1):13–26.
Ma E, Sasazuki S, Iwasaki M, Sawada N, Inoue M, Shoichiro T, et al. 10-Year risk of colorectal cancer: development and validation of a prediction model in middle-aged Japanese men. Cancer Epidemiol. 2010;34(5):534–41.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD). Ann Intern Med. 2015;162(10):735–6.
Chen Z, Chen J, Collins R, Guo Y, Peto R, Wu F, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40(6):1652–66.
Wang L, Jin G, Yu C, Lv J, Guo Y, Bian Z, et al. Cancer incidence in relation to body fatness among 0.5 million men and women: findings from the China Kadoorie Biobank. Int J Cancer. 2020;146(4):987–98.
Im PK, Millwood IY, Chen Y, Guo Y, Du H, Kartsonaki C, et al. Problem drinking, wellbeing and mortality risk in Chinese men: findings from the China Kadoorie Biobank. Addiction. 2020;115(5):850–62.
Tian X, Du H, Li L, Bennett D, Gao R, Li S, et al. Fruit consumption and physical activity in relation to all-cause and cardiovascular mortality among 70,000 Chinese adults with pre-existing vascular disease. PLoS One. 2017;12(4):e0173054.
Millwood IY, Li L, Smith M, Guo Y, Yang L, Bian Z, et al. Alcohol consumption in 0.5 million people from 10 diverse regions of China: prevalence, patterns and socio-demographic and health-related correlates. Int J Epidemiol. 2013;42(3):816–27.
Kalinowski A, Humphreys K. Governmental standard drink definitions and low-risk alcohol consumption guidelines in 37 countries. Addiction. 2016;111(7):1293–8.
Hansen IO, Jess P. Possible better long-term survival in left versus right-sided colon cancer - a systematic review. Dan Med J. 2012;59(6):A4444.
Stefansson T, Moller PH, Sigurdsson F, Steingrimsson E, Eldon BJ. Familial risk of colon and rectal cancer in Iceland: evidence for different etiologic factors? Int J Cancer. 2006;119(2):304–8.
Zhu J, Tan Z, Hollis-Hansen K, Zhang Y, Yu C, Li Y. Epidemiological trends in colorectal cancer in China: an ecological study. Dig Dis Sci. 2017;62(1):235–43.
Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, et al. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381(9882):1987–2015.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45.
Driver JA, Gaziano JM, Gelber RP, Lee IM, Buring JE, Kurth T. Development of a risk score for colorectal cancer in men. Am J Med. 2007;120(3):257–63.
Imperiale TF, Monahan PO, Stump TE, Ransohoff DF. Derivation and validation of a predictive model for advanced colorectal neoplasia in asymptomatic adults. Gut. 2021;70(6):1155–61.
Hong SN, Son HJ, Choi SK, Chang DK, Kim YH, Jung SH, et al. A prediction model for advanced colorectal neoplasia in an asymptomatic screening population. PLoS One. 2017;12(8):e0181040.
Guo L, Chen H, Wang G, Lyu Z, Feng X, Wei L, et al. Development of a risk score for colorectal cancer in Chinese males: a prospective cohort study. Cancer Med. 2020;9(2):816–23.
Chen G, Mao B, Pan Q, Liu Q, Xu X, Ning Y. Prediction rule for estimating advanced colorectal neoplasm risk in average-risk populations in southern Jiangsu Province. Chin J Cancer Res. 2014;26(1):4–11.
Aleksandrova K, Reichmann R, Kaaks R, Jenab M, Bueno-de-Mesquita HB, Dahm CC, et al. Development and validation of a lifestyle-based model for colorectal cancer risk prediction: the LiFeCRC score. BMC Med. 2021;19(1):1.
Betes M, Munoz-Navas MA, Duque JM, Angos R, Macias E, Subtil JC, et al. Use of colonoscopy as a primary screening test for colorectal cancer in average risk people. Am J Gastroenterol. 2003;98(12):2648–54.
Usher-Smith JA, Harshfield A, Saunders CL, Sharp SJ, Emery J, Walter FM, et al. External validation of risk prediction models for incident colorectal cancer using UK Biobank. Br J Cancer. 2018;118(5):750–9.
Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21(17):5167–75.
Lin KJ, Cheung WY, Lai JY, Giovannucci EL. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J Cancer. 2012;130(2):419–30.
Bae JM, Kim JH, Cho NY, Kim TY, Kang GH. Prognostic implication of the CpG island methylator phenotype in colorectal cancers depends on tumour location. Br J Cancer. 2013;109(4):1004–12.
Huang KE, Xu L, I NN, Jaisamrarn U. The Asian Menopause Survey: knowledge, perceptions, hormone treatment and sexual function. Maturitas. 2010;65(3):276–83.
Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102(10):680–91.
Usher-Smith JA, Emery J, Kassianos AP, Walter FM. Risk prediction models for melanoma: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1450–63.
Harrison H, Thompson RE, Lin Z, Rossi SH, Stewart GD, Griffin SJ, et al. Risk Prediction Models for Kidney Cancer: A Systematic Review. Eur Urol Focus. 2021;7(6):1380-1390.
Allen N, Sudlow C, Downey P, Peakman T, Danesh J, Elliott P, Gallacher J, Green J, Matthews P, Pell J, Sprosen T, Collins R. UK Biobank: Current status and what it means for epidemiology. Health Policy Technol. 2012;1(3):123–6.
Imperiale TF, Monahan PO, Stump TE, Glowinski EA, Ransohoff DF. Derivation and validation of a scoring system to stratify risk for advanced colorectal neoplasia in asymptomatic adults: a cross-sectional study. Ann Intern Med. 2015;163(5):339–46.
McGeoch L, Saunders CL, Griffin SJ, Emery JD, Walter FM, Thompson DJ, et al. Risk prediction models for colorectal cancer incorporating common genetic variants: a systematic review. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1580–93.
Saunders CL, Kilian B, Thompson DJ, McGeoch LJ, Griffin SJ, Antoniou AC, et al. External validation of risk prediction models incorporating common genetic variants for incident colorectal cancer using UK Biobank. Cancer Prev Res (Phila). 2020;13(6):509–20.
Hayward J, Bishop M, Rafi I, Davison V. Genomics in routine clinical care: what does this mean for primary care? Br J Gen Pract. 2017;67(655):58–9.
Chen H, Li N, Shi J, Ren J, Liu C, Zhang Y, et al. Comparative evaluation of novel screening strategies for colorectal cancer screening in China (TARGET-C): a study protocol for a multicentre randomised controlled trial. BMJ Open. 2019;9(4):e025935.
Chen H, Lu M, Liu C, Zou S, Du L, Liao X, et al. Comparative evaluation of participation and diagnostic yield of colonoscopy vs fecal immunochemical test vs risk-adapted screening in colorectal cancer screening: interim analysis of a multicenter randomized controlled trial (TARGET-C). Am J Gastroenterol. 2020;115(8):1264–74.
Acknowledgements
The chief acknowledgment is to the China Kadoorie Biobank participants, the project staff based at Beijing, Oxford, and the 10 regional centres, the China National Centre for Disease Control and Prevention (CDC) and its regional offices for assisting with the fieldwork. The study is funded by the Kadoorie Charitable Foundation in Hong Kong and Wellcome Trust in the UK. Further support is also provided by the Chinese Ministry of Science and Technology and Natural Science Foundation, and the UK MRC, BHF and CRUK.
Funding
Baseline survey: Kadoorie Charitable Foundation, Hong Kong. Long-term continuation: UK Wellcome Trust (088158/Z/09/Z, 104085/Z/14/Z), Chinese Ministry of Science and Technology (2011BAI09B01, 2012–14), and Chinese National Natural Science Foundation (81390541). For the purpose of Open Access, the author has applied a CC-BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
REA and CK conceived and designed the study and analysed the data. REA and CK carried out the statistical analysis. BT was involved with interpretation of results. LY, IM, YG, JL, PP, PW, CY, and YC were involved in the data acquisition and interpretation of results. REA drafted the manuscript. XY and DA were involved in the data acquisition and management. JL, LL, and ZC conceived, designed, manage, and obtain funding for CKB. All authors reviewed, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval from the Oxford University Tropical Research Ethics Committee, the Chinese Centre for Disease Control and Prevention (CDC) Ethical Review Committee, and the local CDC of each study area were obtained, and all participants provided written informed consent. The study was performed in accordance with the Declaration of Helsinki.
Ethical approval references:
UK
Kadoorie Study of Chronic disease in China (KSCDC)—Baseline, Oxford Tropical Research Ethics Committee (OxTREC) (2005) OxTREC Ref: 025–04.
China Kadoorie Biobank (A prospective study of environmental and genetic causes of premature death in 500,000 Chinese adults) – Second resurvey (2013) OXTREC Reference: 1024–13.
Objective measurement of physical activity in urban and rural Chinese: A feasibility study (2017) OxTREC Reference: 514–17.
Assessing health risks associated with exposure to household and ambient air pollution in rural and urban China. (2017) OxTREC Reference: 5109–17.
China Kadoorie Biobank – Third resurvey (2019) OxTREC Ref: 18–19.
China
Kadoorie Study of Chronic disease in China (KSCDC) – Baseline, Chinese Centre for Disease Control and Prevention. Ethical Review Committee (2004) Approval Notice 005/2004.
Kadoorie Study of Chronic disease in China (KSCDC) – Second Phase, Chinese Academy of Medical sciences/Peking Union Medical College, Ethical Committee (2010) 24th December 2010.
Kadoorie Study of Chronic disease in China (KSCDC) – Second Resurvey, Chinese Academy of Medical Sciences/Peking Union Medical College, Ethical Committee (2013) Ref: X1303262001.
Members of the China Kadoorie Biobank collaborative group:
International Steering Committee: Junshi Chen, Zhengming Chen (PI), Robert Clarke, Rory Collins, Yu Guo, Liming Li (PI), Chen Wang, Jun Lv, Richard Peto, Robin Walters.
International Co-ordinating Centre, Oxford: Daniel Avery, Ruth Boxall, Derrick Bennett, Ka Hung Chan, Yumei Chang, Yiping Chen, Zhengming Chen, Robert Clarke, Huaidong Du, Zammy Fairhurst-Hunter, Wei Gan, Simon Gilbert, Alex Hacker, Parisa Hariri, Mike Hill, Michael Holmes, Pek Kei Im, Andri Iona, Maria Kakkoura, Christiana Kartsonaki, Rene Kerosi, Kuang Lin, Iona Millwood, Qunhua Nie, Alfred Pozarickij, Paul Ryder, Sam Sansome, Dan Schmidt, Paul Sherliker, Rajani Sohoni, Becky Stevens, Iain Turnbull, Robin Walters, Lin Wang, Neil Wright, Ling Yang, Xiaoming Yang, Pang Yao.
National Co-ordinating Centre, Beijing: Yu Guo, Xiao Han, Can Hou, Chun Li, Chao Liu, Jun Lv, Pei Pei, Canqing Yu.
10 Regional Co-ordinating Centres:
Guangxi Provincial CDC: Naying Chen, Duo Liu, Zhenzhu Tang. Liuzhou CDC: Ningyu Chen, Qilian Jiang, Jian Lan, Mingqiang Li, Yun Liu, Fanwen Meng, Jinhuai Meng, Rong Pan, Yulu Qin, Ping Wang, Sisi Wang, Liuping Wei, Liyuan Zhou. Gansu Provincial CDC: Caixia Dong, Pengfei Ge, Xiaolan Ren. Maiji CDC: Zhongxiao Li, Enke Mao, Tao Wang, Hui Zhang, Xi Zhang. HainanProvincial CDC: Jinyan Chen, Ximin Hu, Xiaohuan Wang. Meilan CDC: Zhendong Guo, Huimei Li, Yilei Li, Min Weng, Shukuan Wu. Heilongjiang Provincial CDC: Shichun Yan, Mingyuan Zou, Xue Zhou. Nangang CDC: Ziyan Guo, Quan Kang, Yanjie Li, Bo Yu, Qinai Xu. Henan Provincial CDC: Liang Chang, Lei Fan, Shixian Feng, Ding Zhang, Gang Zhou. Huixian CDC: Yulian Gao, Tianyou He, Pan He, Chen Hu, Huarong Sun, Xukui Zhang. Hunan Provincial CDC: Biyun Chen, Zhongxi Fu, Yuelong Huang, Huilin Liu, Qiaohua Xu, Li Yin. Liuyang CDC: Huajun Long, Xin Xu, Hao Zhang, Libo Zhang. Jiangsu Provincial CDC: Jian Su, Ran Tao, Ming Wu, Jie Yang, Jinyi Zhou, Yonglin Zhou. Suzhou CDC: Yihe Hu, Yujie Hua, Jianrong Jin Fang Liu, Jingchao Liu, Yan Lu, Liangcai Ma, Aiyu Tang, Jun Zhang. Qingdao Qingdao CDC: Liang Cheng, Ranran Du, Ruqin Gao, Feifei Li, Shanpeng Li,Yongmei Liu, Feng Ning, Zengchang Pang, Xiaohui Sun, Xiaocao Tian, Shaojie Wang, Yaoming Zhai, Hua Zhang, Licang CDC: Wei Hou, Silu Lv, Junzheng Wang. Sichuan Provincial CDC: Xiaofang Chen, Xianping Wu, Ningmei Zhang, Weiwei Zhou. Pengzhou CDC: Xiaofang Chen, Jianguo Li, Jiaqiu Liu, Guojin Luo, Qiang Sun, Xunfu Zhong. Zhejiang Provincial CDC: Weiwei Gong, Ruying Hu, Hao Wang, Meng Wan, Min Yu. Tongxiang CDC: Lingli Chen, Qijun Gu, Dongxia Pan, Chunmei Wang, Kaixu Xie, Xiaoyi Zhang.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Page S1.
Systematic review search strategy for colorectal cancer risk models. Page S2. TRIPOD checklist for colorectal cancer risk models. Page S3. Ascertainment of anthropometric measurements, covariates, and alcohol intake in the China Kadoorie Biobank. Page S4. Derivation of colorectal cancer risk model variables in the China Kadoorie Biobank, and Page S5. Full equations of the colorectal cancer risk models used for external validation in the China Kadoorie Biobank.
Additional file 2: Table S1.
Recalibration of five colorectal cancer risk prediction models to the China Kadoorie Biobank, and Table S2. Comparison of three colorectal risk models validated in UK Biobank and China Kadoorie Biobank.
Additional file 3: Figure S1.
Recalibrated curves of observed and expected 10-year risk of colorectal cancer in men and women, and Figure S2. Discrimination of colorectal cancer models comparing ROC curve analysis and cox regression.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abhari, R.E., Thomson, B., Yang, L. et al. External validation of models for predicting risk of colorectal cancer using the China Kadoorie Biobank. BMC Med 20, 302 (2022). https://doi.org/10.1186/s12916-022-02488-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02488-w