Abstract
Background
Factors contributing to the lack of understanding of research studies include poor reporting practices, such as selective reporting of statistically significant findings or insufficient methodological details. Systematic reviews have shown that prognostic factor studies continue to be poorly reported, even for important aspects, such as the effective sample size. The REMARK reporting guidelines support researchers in reporting key aspects of tumor marker prognostic studies. The REMARK profile was proposed to augment these guidelines to aid in structured reporting with an emphasis on including all aspects of analyses conducted.
Methods
A systematic search of prognostic factor studies was conducted, and fifteen studies published in 2015 were selected, three from each of five oncology journals. A paper was eligible for selection if it included survival outcomes and multivariable models were used in the statistical analyses. For each study, we summarized the key information in a REMARK profile consisting of details about the patient population with available variables and follow-up data, and a list of all analyses conducted.
Results
Structured profiles allow an easy assessment if reporting of a study only has weaknesses or if it is poor because many relevant details are missing. Studies had incomplete reporting of exclusion of patients, missing information about the number of events, or lacked details about statistical analyses, e.g., subgroup analyses in small populations without any information about the number of events. Profiles exhibit severe weaknesses in the reporting of more than 50% of the studies. The quality of analyses was not assessed, but some profiles exhibit several deficits at a glance.
Conclusions
A substantial part of prognostic factor studies is poorly reported and analyzed, with severe consequences for related systematic reviews and meta-analyses. We consider inadequate reporting of single studies as one of the most important reasons that the clinical relevance of most markers is still unclear after years of research and dozens of publications. We conclude that structured reporting is an important step to improve the quality of prognostic marker research and discuss its role in the context of selective reporting, meta-analysis, study registration, predefined statistical analysis plans, and improvement of marker research.
Similar content being viewed by others
Background
As in many other fields of medicine, deficiencies in the reporting of tumor marker prognostic factor studies have long been recognized [1,2,3]. The Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) guidelines were developed and subsequently discussed in detail in an “explanation and elaboration” (E&E) paper [4, 5]. Prognostic factors are clinical factors used to help predict an individual patient’s risk of a future outcome, such as disease recurrence after primary treatment. Many initially promising findings of prognostic factors for cancer have failed to replicate, and very few have emerged as being clinically useful [6]. A large body of work has identified major areas of concern about the quality of much prognostic factor research, including that studies are often poorly analyzed [7] and/or selectively reported [3, 8, 9].
As highlighted in The Lancet Reduce waste, increase value series (e.g., [10, 11]), similar deficiencies are widespread across many fields of biomedical research. Reporting guidelines, which have been developed for a range of study designs [12], typically describe a minimum set of information that should be clearly reported, provide examples of guideline-consistent reporting, and include a checklist to facilitate compliance [13]. Adherence to reporting guidelines ensures that readers are provided with sufficient details to enable them to critically appraise a study. Good reporting also promotes greater transparency and standardization, which enhances the ability to compare and synthesize the results of different studies and thus facilitates the process of evidence synthesis and meta-analysis [14].
Unfortunately, there is convincing evidence that the publication of REMARK has not resulted in a major improvement in the quality and completeness of reporting of tumor marker prognostic factor studies [8, 14]. In a recent systematic review, Kempf et al. [9] investigated 98 prognostic factor studies published in 17 high-impact oncology journals in 2015. Almost all displayed evidence of selective reporting (i.e., the failure to present the results of all planned analyses), and most were incompletely reported (e.g., omitted essential information such as reporting a hazard ratio without its associated confidence interval). A particularly common occurrence was focusing solely on significant results in the conclusions, despite multivariable modeling revealing at least one non-significant prognostic factor effect. The presence of reporting and/or publication bias in favor of statistically significant results had already been noted over a decade ago [15].
The purpose of this paper is a structured display, “REMARK Profile,” to improve the reporting of statistical analyses conducted in tumor marker prognostic studies. This profile consists of two parts: (A) patients, treatment, and variables and (B) statistical analysis of survival outcomes. The REMARK profile is complementary to the REMARK guidelines, and a prior version was proposed and discussed in the E&E paper [5], extended with a specific example of the prognostic ability of the Nottingham Prognostic Index for breast cancer [16], and also advocated in the recent abridged version of the E&E paper written to encourage the dissemination and uptake of REMARK [17]. Our intention is to provide clear and simple examples and demonstrate how the creation of such profiles enhances the presentation and transparency of statistical analyses. The importance of transparent reporting of statistical analyses is particularly germane for observational studies (as is typical of tumor marker prognostic studies), especially where multiple exploratory analyses are included that increase the chance of spurious findings [18]. Although the REMARK guidelines focus primarily on studies of single prognostic markers, the value of a structured profile is likely to apply equally to other types of prognostic studies, including studies of multiple markers and studies of markers to predict response to treatment. Similarly, it is equally relevant to specialties other than cancer, as reflected in the fact that the REMARK guidelines have been used more widely (e.g., [19, 20]).
In this study, we produce and evaluate REMARK profiles for a selection of tumor marker prognostic studies published in five clinical journals on cancer research in 2015 (three papers from each). The paper is organized as follows. In the “Methods” section, we begin by describing the REMARK profile in greater detail, and we outline how the papers were selected and coded for analysis. In the Results section the findings are presented in two ways. First, we chose two studies which we considered to be well-reported and two studies which we considered to be less well-reported, and highlight pertinent features of each with reference to their profile. Second, we summarize and discuss the key aspects of the reporting quality of all 15 selected studies. In the Discussion section we mention several issues related to the broader role of structured reporting. We conclude that structured reporting is an important step to improve quality of prognostic marker research. A REMARK profile template is also provided with guidance to help the authors prepare profiles for their own study, ideally prospectively.
Methods
The REMARK profile
The REMARK profile is a structured display of relevant information designed to help authors to summarize key aspects of a tumor marker prognostic study, primarily to improve the completeness and transparency of the reporting of statistical analyses. It is intended to enable readers to quickly and accurately understand the aims of the paper, the patient population, and all statistical analyses that were carried out. The profile, if created retrospectively as in this study, can aid in assessing how well-reported a study is, identifying severe weaknesses and omissions that may call into question certain aspects of the study’s findings. Yet, ideally, if created prospectively by the authors, it might be invaluable in helping to ensure that errors and omissions do not occur in the first place. If published as Table x or as an online supplement, it could summarize relevant information without compromising the articles’ flow of reading. The profile includes much needed metadata beneficial for identifying whether a specific study fulfills inclusion and exclusion criteria for systematic reviews or meta-analyses, and the widespread use of such profiles will improve the quality and inclusiveness of primary research and reviews.
The REMARK profile consists of two sections. The first section provides information about the patient population, inclusion and exclusion criteria, the number of eligible patients and events for each outcome in the full data, how the marker of interest was handled in the analysis, and additional variables available.
The second section of the profile gives a sequential overview of all of the analyses conducted, including the variables included in each, the sample size, and the number of outcome events. It is important to also include the initial data analyses (IDA), which are a key step in the analysis workflow and aid in the correct presentation and interpretation of model results [21]. The original proposal for such a REMARK profile [5] was later extended [16] to provide more detail about the entire analysis process, including checks of important assumptions. For illustration, it is displayed in Table 1. Obviously, each study has different aspects and details of a profile differ. A simple generic profile is shown in Table 2.
Selected papers
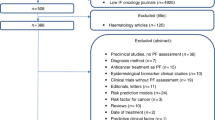
Papers were selected from five clinical journals reporting on prognostic studies in cancer research. These were Breast Cancer Research and Treatment (BCRT), Cancer, European Journal of Cancer (EJC), International Journal of Cancer (IJC), and Journal of Clinical Oncology (JCO). The choice of these journals was based on the earlier paper about the assessment of adherence to REMARK [14]. Four journals were already included in this study and here we added EJC. A search was conducted with the search terms “cancer” in the title and “prognostic” in the title, abstract, or keywords. From each journal, three original research papers, published in 2015, were identified and reviewed, with the most recently published papers considered for eligibility first. A publication was eligible if it was a prognostic study with survival outcomes, and multivariable models were used in the statistical analysis. The exclusion criteria were randomized trials, laboratory studies, reviews, meta-analyses, methods papers, and letters. If a paper was not eligible for inclusion, the next most recent paper from that journal was selected.
The publications were summarized, including the number of patients assessed, number of patients excluded, and number of patients and events reported in the final models. Each statistical model was assessed with respect to which variables were included, number of events for the primary outcome, and whether the number of events was reported for each model or subgroup analysis. For studies that included a training and validation data set, only the training data set was considered for this summary. The studies were graded according to the completeness of information on exclusions of subjects as follows: 3, exclusion criteria and number of exclusions known; 2, exclusion criteria listed, but number of excluded patients unknown; and 1, exclusion criteria not listed.
Originally, continuous marker variables are often categorized or dichotomized for the purpose of analysis. While they technically do not represent a “new” marker, we decided to include them in the marker section of part A of the profiles for reasons of clarity and comprehensibility. An example can be seen in Martin et al. [22] with “M1” being the continuous version of the marker, and “M1(10)” or “M1(5)” describing categorized versions of the same marker with ten and five categories, respectively.
Results
Fifteen studies from five journals were included in this review. To illustrate how REMARK profiles help readers to better understand the analysis steps in a study, we will present two positive examples in which the analyses were reported in detail and were easily understandable. Here, profiles can help readers to quickly identify that a study is well-reported and find the information needed to properly evaluate the findings. However, more frequently reporting of many important parts of the analyses is insufficient, which we will illustrate by also presenting two poorly reported studies. All fifteen profiles are available in the web appendix (Additional file 1). In the second part of the “Results” section, we will summarize our findings from them.
Selected profiles to illustrate weaknesses of current reporting and advantages of the REMARK profile
Examples of better-reported studies
Xing et al [23]
This REMARK profile (Table 3), for a paper examining the association between BRAF V600E mutation and recurrence of papillary thyroid cancer (PTC) in eight countries between 1978 and 2011 shows at a glance that the analysis involved both univariable and multivariable analyses and employed both Cox regression (PTC regression expressed as a proportion) and Poisson regression (PTC recurrence expressed as rate per 1000 person-years). It also involves a number of subgroup analyses, including by type of PTC, and also restricting the sample to low-risk patients, defined variously as tumor stage 1, tumor stage 2, and tumor size ≤ 1.0 cm. It shows that the sample size and the effective sample size (number of events) were reported for each of these analyses. It shows that the proportional hazards assumption was checked and that a violation of this assumption resulted in the decision to stratify multivariable analyses by medical center. It shows that three nested predictive models were applied, both in analyses involving the overall sample and those restricted to subgroups: an unadjusted model including only the marker of interest (BRAF V600E mutation), a multivariable model adjusting for age and sex and stratifying by medical center, and a full model adjusting for 5 additional variables.
The profile also reveals two minor reporting deficiencies. The number of patients assessed for eligibility is not provided, nor is the number of exclusions (or indeed whether there were any exclusion criteria). There is also no mention of missing data, though it appears that there may have been none.
Huzell et al [24]
This profile (Table 4) summarizes a paper exploring the effect of oral contraceptive use on breast cancer events and distant metastasis among Swedish patients diagnosed with primary breast cancer between 2002 and 2011 and followed up for a median of 3 years. The analyses are complex, with the marker categorized in 5 different ways and a number of subgroups explored. In general, however, the profile shows that the reporting of key information is quite good, with the n’s and number of outcome events for each analysis known (with the exception of the subgroup analyses in which distant metastasis was the outcome) and clear statements on missing data in Tables 1 and 2 of Ref. [22]. The profile is particularly valuable as many analyses were conducted and some were only briefly mentioned in the text of the results section. For some analyses (e.g., A1 and A4), no data are provided. Thus, the profile greatly helps to clarify what was done, including to clarify which covariates were included in each analysis.
Examples of inadequately reported studies
Thurner et al. [25]
This profile (Table 5) summarizes an analysis of the effect of pre-treatment C-reactive protein on three clinical outcomes (cancer-specific survival, overall survival, and disease-free survival) in prostate cancer patients. All received 3D radiation therapy and were followed up for a median of 80 months. Five clinical variables are included in models as potential covariates, while a sixth (risk group) is used in subgroup analyses. The numbers of patients both initially assessed and subsequently excluded are not provided, as is clear from the profile.
The marker variable (C-reactive protein) is initially dichotomized on the basis of a ROC curve analysis (no details given), and a series of univariable and multivariable models are applied to the full data set. Dichotomization, although known to have severe weaknesses [7], is used in the overall population and in subgroups (IDA2, IDA3). Unsurprisingly, different cutpoints were identified in different populations. While the amount of missing data for individual variables is provided, the number of patients included in multivariable models including combinations of these variables is not provided, and consequently, the number of outcome events for these analyses is not known. In subgroup analyses by risk group, the number of outcome events is never provided. Overall, the profile effectively communicates the complexity of the analyses, much of the detail of which is hidden in the text of the results section rather than reported in any tables (see the remarks for A6, A7, and A8), and the omission of important data on the number of outcome events in all subgroup analyses.
Schirripa et al. [26]
This study evaluated the role of NRAS mutations as a prognostic marker in metastatic colorectal cancer (mCRC), among 786 patients treated at the University Hospital of Pisa from 2009 to 2012. Patients were categorized as having a NRAS mutation, KRAS mutation, BRAF mutation, or none of the above (all wild type). The primary outcome was overall survival, without any information about follow-up time. A number of demographic and clinical variables were examined for their relation to overall survival, some of which were selected for inclusion in multivariable models. These survival models compared the three types of mutation with the wild-type category.
The REMARK profile prepared for this paper (Table 6) reveals a number of important omissions and questionable practices. As well as the failure to specify the follow-up period, the number of events was unspecified for the overall survival. It is also unstated whether all patients with mCRC with available data and treated in the specified time period were included in the analysis, or whether there were other exclusion criteria. There were missing data for some of the covariates (see Table 1 of Ref. [26]), and as a result, an unstated number of observations are excluded in each of the multivariable models presented; that is, for each model, both the number of observations and the number of outcome events are unknown.
The paper is also an example of two problems which are widespread in the literature. The first is only reporting univariable analyses which were statistically significant and omitting information about the other variables investigated. For example, it cannot be ascertained whether variable v7 (nodal involvement) was not investigated, or whether it was simply non-significant. The second problem is the use of the results of univariable analyses to select variables for inclusion in multivariable models, which is not recommended, mainly because it can lead to the exclusion of important covariates [27]. Finally, the statistical software used to carry out the analyses is not specified.
Summary of the quality of reporting
While the final number of patients included in the analyses was consistently reported (though incorrectly in one publication), complete information on how many patients were assessed or excluded was missing in 67% (10 of 15) of the publications (Table 7). Four studies (27%) did not provide the time period over which patients were selected for inclusion.
The number of events for the primary outcome among the total number of included patients was missing in 40% (6 of 15) of the publications (Table 8). More frequently, however, the number of events for multivariable models could not be ascertained because of missing data for one or more covariates. While for such models the number of observations was generally reported, it was often not known whether the exclusions were event cases or non-events. Of the 9 publications which reported the total number of events, five [22, 25, 28,29,30] were affected by this problem.
Follow-up was commonly reported as the median follow-up, while some authors included minimum, maximum, or range of follow-up. In 3 publications (20%), the duration of follow-up was not reported.
Sample sizes and number of events were often missing for subgroup analyses. Of the 10 studies with subgroup analyses, only 5 stated both the sample size and the number of events for at least one of the subgroup analyses. A further publication provided the sample size, but the number of events was not reported.
The type and version of the statistical software used in the analysis were mentioned in 10 of the 15 papers.
Discussion
Nearly forty years ago Altman et al. [38] proposed statistical guidelines for the contributor to medical journals; about a decade later, Lang and Secic [39] published a book about how to report statistics in medicine, and Lang and Altman [40] published the SAMPL (Statistical Analyses and Methods in the Published Literature) guidelines. They state “The truth is that the problem of poor statistical reporting is long-standing, widespread, potentially serious, concerns mostly basic statistics and yet is largely unsuspected by most readers of the biomedical literature,” and in a study assessing reporting quality of about 400 research papers, Diong et al. [41] conclude that there is no evidence that reporting practices improved following the publication of editorial advice. Obviously, severe improvement is urgently needed. Suitable ideas, such as tables to replace text [42] and a list of key points giving guidance for conducting confirmatory prognostic factor studies [43], can be helpful.
Reporting guidelines have been published and it has been proposed to summarize key issues of a study, including all steps of the analysis, in a REMARK profile [4, 5, 17]. Our review of 15 prognostic factor studies demonstrated poor reporting of analyses, with relevant information, such as years of patient selection, number of patients assessed, years of follow-up, and number of events, missing. Furthermore, if available, this information may not have been clearly presented or easy to find in the paper. REMARK profiles augment the more detailed REMARK guidelines and empower researchers to prospectively report sequential analyses to provide sufficient information in a brief and clear structure. We present several reasons why this format should be adopted by researchers.
Structured profiles to improve reporting bias and related consequences for meta-analyses
Weaknesses of analyses have been known for a long time from seminal papers about statistical aspects and methodological challenges of prognostic factor studies [44, 45]. With an emphasis on all statistical analyses conducted, we summarized the information according to the principles of the REMARK profile [5] and some extensions [16]. In a book providing a broad overview and summarizing the major reporting guidelines in health research, Altman et al. stressed the importance of structured reporting and selected the REMARK profile as one of their creators’ preferred bits [46, 47]. Two reviews of prognostic factor studies showed that adherence to the REMARK reporting guidelines is lacking [14, 48], but according to our knowledge, this is the first study that provides structured profiles for a group of systematically selected study publications. Unfortunately, we must assume that most of the studies lacked a prospective statistical analysis plan (SAP), and it is likely that many more analyses were conducted in many studies and that the reporting bias is therefore strong.
It is well-known that problems from the design, analysis, and reporting from single studies cause severe problems for subsequent systematic reviews and meta-analyses, specifically in the context of observational studies. Already 20 years ago, Doug Altman [49] stated As a consequence of the poor quality of research, prognostic markers may remain under investigation for many years after initial studies without any resolution of the uncertainty. Multiple separate and uncoordinated studies may actually delay the process of defining the role of prognostic markers. Subsequent research and empirical evaluations have shown his concerns were justified. In a large systematic review of tumor markers for neuroblastoma, Riley et al. [1] identified 130 different markers in 260 studies. They identified severe problems in both statistical analysis and presentation which restricted both the extraction of data and the meta-analysis of results from the primary studies. In a paper entitled Prognostic Factors – confusion caused by bad quality of design, analysis and reporting of many studies, Sauerbrei [50] discussed several critical issues in data analysis and summary assessment of a prognostic factor. It is well accepted that the concept of evidence-based medicine is a key part of research and decision-making for the assessment and comparison of treatments. As EBM requires suitable systematic reviews and meta-analyses, it is still a long way until this concept becomes reality for the use of prognostic markers in patient handling [51].
This unfortunate situation is also well known to many clinicians and it is frustrating to witness that several markers are investigated for a long time without being able to assess their clinical utility. Malats et al. [52] reviewed 168 publications from 117 studies assessing the value of P53 as a prognostic marker for bladder cancer. They conclude After 10 years of research, evidence is not sufficient to conclude whether changes in P53 act as markers of outcome in patients with bladder cancer’ and state That a decade of research on P53 and bladder cancer has not placed us in a better position to draw conclusions relevant to the clinical management of patients is frustrating.
The cited papers were published at the beginning of the century and REMARK guidelines, which were published in 2005, were still unknown to the authors. Since then, there have been many important proposals to improve prognostic marker research (see below), but it is still not uncommon that systematic reviews and meta-analyses of prognostic markers have severe weaknesses and do not provide evidence-supported knowledge about the clinical value of a marker. In a systematic review, Papadakis et al. [53] identified 20 studies investigating BAG-1 as a marker in early breast cancer prognosis. They assessed the quality of reporting according to the REMARK guidelines and conducted three meta-analyses. Sauerbrei and Haeussler [54] criticized several major weaknesses in the quality of reporting and meta-analyses and concluded that results and inferences from the study were not justified by the assessments and analyses presented. An inadequate assessment of the quality of reporting according to REMARK is the first issue they mention.
Only a small number of markers accepted and used in practice
It is often critiqued that only a small number of markers is generally accepted and used in practice [2]. Weaknesses of bad reporting of single studies are among the main reasons for this unfortunate situation. Bad reporting causes severe problems to conduct a systematic review followed by an informative meta-analysis, which aims to provide an unbiased estimate of the effect of a variable. Many markers could not show their value in a meta-analysis, and we should be pleased that they are hardly accepted and used in practice.
Kyzas et al. [3] published a meta-analysis of the tumor suppressor protein TP53 as a prognostic factor in head and neck cancer. The authors provide compelling empirical evidence that selective reporting biases are a major impediment to conducting meaningful meta-analyses of prognostic marker studies. In a related editorial, McShane et al. [2] discuss that these biases have serious implications, not only for meta-analyses but also for the interpretation of the cancer prognostic literature as a whole. They summarize The number of cancer prognostic markers that have been validated as clinically useful is pitifully small …, and 2 years later Real and Malats [55] state The saga of replication failures in prognostic-marker studies is frustrating: no new molecular markers have yet been incorporated into clinical practice for bladder cancer. The messages from educational and methodological papers were very clear, but publishing reporting guidelines was not sufficient to help improve this unfortunate situation. Seven years after the publication of the REMARK guidelines, Kern [56] states in a paper entitled Why your new cancer biomarker may never work: recurrent patterns and remarkable diversity in biomarker failures that less than 1% of published cancer biomarkers actually enter clinical practice. He also discusses systematic attempts to improve marker development and adoption but who’s listening, a question asked in the more general context of reducing waste in biomedical research [57].
Guidelines for different study designs and the consequences of insufficient reporting
The development of reporting guidelines started with CONSORT for randomized trials [58], which were updated several times. The CONSORT statement is required in many journals and has led to more clarity and details in the reporting of such studies. It provides more background to readers to appropriately evaluate the significance of the studies and helps to better assess the reported results. Realizing the advantages further guidelines were developed for many types of observational studies [59, 60], with the EQUATOR network [61] serving as a coordinating center [12]. Meanwhile, hundreds of reporting guidelines have been developed. To improve and partly standardize this process, Moher et al. [62] proposed guidance for developing a reporting guideline in health research.
For the reporting of systematic reviews, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was published, with an updated version (the PRISMA 2020 statement) recently [63]. Systematic reviews and meta-analyses are the key parts of evidence-based medicine and consequently also for decision-making in patient handling, clearly illustrating the importance of the guideline for practice.
To extend REMARK to a reporting guideline for multivariable prediction models, where several prognostic covariates are combined to make individualized predictions, the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) initiative published the TRIPOD statement with a corresponding explanation and elaboration paper [64, 65]. To assess the completeness of reporting of prediction model studies published just before the introduction of the TRIPOD statement, Heus et al. [66] conducted a review in journals with high impact factors. They found that more than half of the items considered essential for transparent reporting were not fully addressed in publications and that essential information for using a model in individual risk prediction, i.e., model specifications and model performance, was incomplete for more than 80% of the models. For (nearly) all common diseases, many prediction models and sometimes even related tools are developed, but most of them are never used in practice [67, 68]. A quarter of a century ago, Wyatt and Altman [69] published a commentary entitled Prognostic models: Clinically useful or quickly forgotten? The empirical evidence of poor reporting provides one of the explanations that many prediction models cannot be used in practice and are quickly forgotten.
For a systematic review of prediction models, the Checklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS) was developed [70]. These guidelines were used to assess the methodological quality of prognostic models applied to resectable pancreatic cancer [71]. The authors provide evidence of severe weaknesses, and for improvement in the future, they highlight issues relating to general aspects of model development and reporting, applicability of models, and sources of bias. Due to a lack of standardization of reporting of outcomes, a meta-analysis could not be performed.
Consequences of bad reporting and the severity of problems it causes in the assessment of prediction models for COVID-19 were recently illustrated. Wynants et al. [72] conducted a systematic review and critical appraisal (up to 5 May 2020) of prediction models for diagnosis and prognosis. Their summary is shattering “…proposed models are poorly reported, at high risk of bias, and their reported performance is probably optimistic. Hence, we do not recommend any of these reported prediction models for use in current practice.” This sentiment is echoed in an Editorial by Sperrin et al. [73] who argue that the urgency of the situation cannot excuse “methodological shortcuts and poor adherence to guidelines,” as hastily developed models might “do more harm than good.”
REMARK and TRIPOD were developed for markers and models based on clinical data, having not more than some dozens of potential predictors in mind. Obviously, problems of analysis and reporting are more severe in high-dimensional data, which provide many new opportunities for clinical research and patient handling. In order to extract the relevant information from such complex data sets, machine learning, artificial intelligence, and more complicated statistical methods are often used to analyze the data. Obviously, it is important that techniques used adhere to established methodological standards already defined in prognostic factor and prediction model research [74]. Concerning patients’ benefit from the use of machine learning and artificial intelligence techniques, Vollmer et al. [75] ask 20 critical questions on transparency, replicability, ethics, and effectiveness. To present machine learning model information, a model facts label was recently proposed [76]. If adopted widely, it can become an important instrument to severely improve the clinical usefulness of machine learning models.
Including in the supplemental information, a reproducible report (Markdown or Jupyter Notebook) with all the code for the statistical analyses would be another suitable way to report analyses of gene expression data and all associated statistical analyses. This was done by Birnbaum et al. [77] who derived a 25-gene classifier for overall survival in resectable pancreatic cancer.
Selective reporting and risk of bias
Reporting bias is a problem known for many years. In the context of diagnostic and prognostic studies, Rifai et al. [78] clearly stated that there is time for action, and a brief overview is given in a box entitled “Selective reporting” in the E&E paper of REMARK [5]. Ioannidis raised awareness for possible drivers for the lack of reliability of published biomedical research and the large number of false-positive results [79], including small sample sizes, small effect sizes, selective reporting of statistically significant results, or exploratory and hypothesis-generating research. This is also noted by Andre et al. [80] who discuss publication bias and hidden multiple-hypothesis testing distorting the assessment of the true value of markers. Hidden multi-hypotheses testing arises when several markers are tested by different teams using the same samples. The more hypotheses (i.e., marker association with outcome) that are tested, the greater the risk of false-positive findings. They stress the importance of a comprehensive marker study registry. Yavchitz et al. [81] identified 39 types of spin, which they classify and rank according to the severity. It is also known that many studies are started and that researchers do not finalize the study because they lose interest due to unsatisfactory early results. Empirical evidence of a “loss of interest bias” is given in [82]. In a systematic review of prognostic factors in oncology journals with an impact factor above 7, overinterpretation and misreporting were assessed in high-impact journals [9]. The authors identified misleading reporting strategies that could influence how readers interpret study findings. Doussau et al. [83] compared protocols and publications for prognostic and predictive marker studies. Not surprisingly, they found that protocols are often not accessible or not used for these studies and publications were often explicitly discordant with protocols.
In the section above, we referred to the critical appraisal of COVID prediction models by Wynants et al. [72]. Statements and the related editorial refer to the first publication of this “living systematic review” which included 232 prediction models in the third update. The authors had used the CHARMS checklist and assessed the risk of bias using PROBAST (Prediction Model Risk of Bias Assessment Tool) [70, 84]. The latter is organized into 4 domains: participants, predictors, outcome, and analysis. These domains contain a total of 20 signaling questions to facilitate structured judgment of risk of bias, which is defined to occur when shortcomings in study design, conduct, or analysis lead to systematically distorted estimates of model predictive performance. Wynants et al. [72] found that All models reported moderate to excellent predictive performance, but all were appraised to have high risk of bias owing to a combination of poor reporting and poor methodological conduct for participant selection, predictor description, and statistical methods used. We agree that the risk of bias has to be assessed as “high” if a study is badly reported. More detailed reporting would allow to assess the quality of the analysis and some of the 232 prediction models may have received a more positive assessment by Wynants et al [72].
Barriers to better reporting, steps in the right direction, and more action needed
Above, we discuss that problems from single studies transfer to related meta-analyses and give several examples illustrating that the prognostic value of many markers is still unclear after more than a decade after the first publications, followed by hundreds of publications from other groups. Obviously, as for areas like treatment comparisons and (unbiased) estimate of treatment effects, evidence synthesis is also needed in prognosis research [85]. Debray et al. [85] discuss a number of key barriers of quantitative synthesis of data from prognosis studies. This includes lack of high-quality meta-data due to poor reporting of study designs, lack of uniformity in statistical analysis across studies, lack of agreement on relevant statistical measures, and lack of meta-analytical guidance for the synthesis of prognosis study data and emphasize also that there is relatively little guidance on how to do the actual meta-analysis of results from prognosis studies. They describe statistical methods for the meta-analysis of aggregate data, individual participant data and a combination thereof. The ideal would be the availability of individual participant data from all relevant studies. Such analyses become more popular and a review identified already 48 individual participants’ data MAs of prognostic factor studies published until March 2009. However, it is obvious that such projects face numerous logistical and methodological obstacles, and their conduct and reporting can often be substantially improved [86]. We refer to [87, 88] for more recent examples but there are several barriers for individual participant data meta-analysis studies [85, 89], and they are still rare exceptions in prognosis research. Meta-analyses based on aggregate data are common but can they provide suitable assessments of the value of prognostic markers? Obviously, inadequate reporting of the original studies is an important reason that the answer is a clear “no.” A number of other critical issues are briefly discussed by Sauerbrei and Haeussler [54].
There are several important steps which help to improve prognosis research. Starting in 2004, Richard Riley, Doug Altman, and several colleagues initiated the Cochrane Prognosis Methods Group [90]. The group brought together researchers and clinicians with an interest in generating the best evidence to improve the pathways of prognostic research and facilitate evidence-based prognosis results to inform research, service development, policy, and more [91, 92]. In 2010, Riley, Hemingway, and Altman formed the PROGRESS (PROGnosis RESearch Strategy) partnership [93]. This group published several papers about prognosis research, with a paper giving recommendations for improving transparency in prognosis research as the most relevant for this discussion [94]. A related book was published [95], including a chapter on “Ten principles to strengthen prognosis research” [96], some of the principles refer to specific issues of analyses but more guidance for analysis is needed. Providing accessible and evidence-based guidance for key topics in the design and analysis of observational studies is the main objective of the STRengthening Analytical Thinking for Observational Studies (STRATOS) initiative [97]. The topic group “Initial data analysis” emphasizes the importance of providing more details about the steps on the data of a study between the end of the data collection and the start of those statistical analyses that address research questions. In a recent review, they showed that early steps of analyses are often not mentioned and they provide recommendations for improvement [98]. Already in the REMARK E&E paper [5], it was stressed that data manipulations and pre-modeling decisions could have a substantial impact on the results and should be reported. Despite its importance, reporting of initial data analysis steps is usually not done.
Recently, Dwivedi and Shukla [99] proposed the statistical analysis and methods in biomedical research (SAMBR) checklist, but it needs to be seen whether this proposal finds wider acceptance. Anyhow, more generally accepted guidance for the design and analysis of prognostic factor studies would certainly help to standardize analyses and the quality of reporting would improve [92]. Several other relevant steps have been proposed, but adherence is still bad. Registration of prognosis studies and publishing protocols to reduce selective reporting, improve transparency, and promote data sharing was often proposed during the last decade [80, 94, 100, 101] but is hardly followed. Sauerbrei et al. [17] proposed that journals require a REMARK checklist for the first submission of a new paper. Such a checklist would help reviewers and editors in the submission process and also readers when checking for specific issues in a paper. A checklist would help authors to realize which parts of the analysis are missing or may need extensions. We refer to Tomar et al. [102] for a nice example but altogether this easy task to improve prognosis research is hardly used.
Further issues are discussed in a paper about Doug Altman as the driving force of critical appraisal and improvements in the quality of methodological and medical research. Sauerbrei et al. [92] summarize Doug Altman’s message concerning (1) education for statistics in practice, (2) reporting of prognosis research, (3) structured reporting and study registration, and (4) standardization and guidance for analysis. Using COVID-19 research as an example, Van Calster et al. [103] provide reliable and accessible evidence that the scandal of poor medical research, as denounced by Altman in 1994 [104], persists today. In three tables, they summarize (1) issues which lead to research waste, (2) practices which result in prioritizing publication appearance over quality, and (3) examples of initiatives to improve the methodology and reproducibility of research.
Conclusions
We consider inadequate reporting of single studies as one of the most important reasons that the clinical relevance of most markers is still unclear after years of research and dozens of publications. As it is clear from the examples of inadequately reported studies, there is an urgent need to improve the completeness and reporting quality of all parts of the analyses conducted.
We propose to summarize the key information from a prognostic factor study in a structured profile, ideally prospectively created and registered. Defining all details of the analysis part when designing a study would correspond to a detailed statistical analysis plan. Obviously, an SAP may have to be modified, for example, if important assumptions are violated. Any such changes should be described in the paper’s corresponding REMARK profile and readers would then see all analyses and would be able to distinguish between preplanned analyses, data-dependent modifications, and additional subgroup or sensitivity analyses, if performed. Such a severe improvement in the reporting of single studies will have an impact on related systematic reviews and meta-analyses and therefore on the quality of prognosis research. The concept of structured reporting can be easily transferred to many other types of studies to improve reporting and transparency of analyses in medical and methodological research.
Availability of data and materials
The data (our REMARK profiles) are published in the appendix.
Abbreviations
- BCRT:
-
Breast Cancer Research and Treatment
- CHARMS:
-
Checklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies
- CI:
-
Confidence interval
- CONSORT:
-
Consolidated Standards of Reporting Trials
- E&E:
-
Explanation and elaboration
- EJC:
-
European Journal of Cancer
- HR:
-
Hazard ratio
- IJC:
-
International Journal of Cancer
- JCO:
-
Journal of Clinical Oncology
- MA:
-
Meta-analysis
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROBAST:
-
Prediction Model Risk of Bias Assessment Tool
- PROGRESS:
-
PROGnosis RESearch Strategy
- PTC:
-
Papillary thyroid cancer
- REMARK:
-
Reporting Recommendations for Tumor Marker Prognostic Studies
- ROC:
-
Receiver operating curve
- SAMBR:
-
Statistical Analysis and Methods in Biomedical Research
- SAMPL:
-
Statistical Analyses and Methods in the Published Literature
- SAP:
-
Statistical analysis plan
- STRATOS:
-
STRengthening Analytical Thinking for Observational Studies
- TRIPOD:
-
Transparent Reporting of a multivariable prediction models for Individual Prognosis Or Diagnosis
References
Riley R, Abrams K, Sutton A, Lambert P, Jones D, Heney D, et al. Reporting of prognostic markers: current problems and development of guidelines for evidence-based practice in the future. Br J Cancer. 2003;88(8):1191–8 https://doi.org/10.1038/sj.bjc.6600886.
McShane L, Altman D, Sauerbrei W. Identification of clinically useful cancer prognostic factors: what are we missing? J National Cancer Institute. 2005;97(14):1023–5 https://doi.org/10.1093/jnci/dji193.
Kyzas P, Loizou K, Ioannidis J. Selective reporting biases in cancer prognostic factor studies. J National Cancer Institute. 2005;97(14):1043–55 https://doi.org/10.1093/jnci/dji184.
McShane L, Altman D, Sauerbrei W, Taube S, Gion M, Clark G. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK). J National Cancer Institute. 2005;97(16):1180–4 https://doi.org/10.1093/jnci/dji237.
Altman D, McShane L, Sauerbrei W, Taube S. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): explanation and elaboration. PLoS Med. 2012;9(5):e1001216 https://doi.org/10.1371/journal.pmed.1001216.
Riley R, Hayden J, Steyerberg E, Moons K, Abrams K, Kyzas P, et al. Prognosis Research Strategy (PROGRESS) 2: prognostic factor research. PLoS Med. 2013;10(2):e1001380 https://doi.org/10.1371/journal.pmed.1001380.
Holländer N, Sauerbrei W. On statistical approaches for the multivariable analysis of prognostic marker studies. Advances in Statistical Methods for the Health Sciences:19-38. https://doi.org/10.1007/978-0-8176-4542-7_2.
Jankova L, Dent O, Molloy M, Chan C, Chapuis P, Howell V, et al. Reporting in studies of protein biomarkers of prognosis in colorectal cancer in relation to the REMARK guidelines. PROTEOMICS - Clin Applications. 2015;9(11-12):1078–86 https://doi.org/10.1002/prca.201400177.
Kempf E, de Beyer J, Cook J, Holmes J, Mohammed S, Nguyên T, et al. Overinterpretation and misreporting of prognostic factor studies in oncology: a systematic review. Br J Cancer. 2018;119(10):1288–96 https://doi.org/10.1038/s41416-018-0305-5.
Glasziou P, Altman D, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet. 2014;383(9913):267–76 https://doi.org/10.1016/S0140-6736(13)62228-X.
Ioannidis J, Greenland S, Hlatky M, Khoury M, Macleod M, Moher D, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet. 2014;383(9912):166–75 https://doi.org/10.1016/S0140-6736(13)62227-8.
Simera I, Moher D, Hirst A, Hoey J, Schulz K, Altman D. Transparent and accurate reporting increases reliability, utility, and impact of your research: reporting guidelines and the EQUATOR Network. BMC Med. 2010;8(1) https://doi.org/10.1186/1741-7015-8-24.
Thombs B, Levis B, Rice D, Wu Y, Benedetti A. Reducing waste and increasing the usability of psychiatry research: the family of EQUATOR Reporting Guidelines and one of ts newest members: the PRISMA-DTA Statement. Can J Psychiatry. 2018;63(8):509–12 https://doi.org/10.1177/0706743718773705.
Sekula P, Mallett S, Altman D, Sauerbrei W. Did the reporting of prognostic studies of tumour markers improve since the introduction of REMARK guideline? A comparison of reporting in published articles. PLoS One. 2017;12(6):e0178531 https://doi.org/10.1371/journal.pone.0178531.
Kyzas P, Denaxa-Kyza D, Ioannidis J. Almost all articles on cancer prognostic markers report statistically significant results. Eur J Cancer. 2007;43(17):2559–79 https://doi.org/10.1016/j.ejca.2007.08.030.
Winzer K, Buchholz A, Schumacher M, Sauerbrei W. Improving the prognostic ability through better use of standard clinical data - the Nottingham Prognostic Index as an example. PLoS One. 2016;11(3):e0149977 https://doi.org/10.1371/journal.pone.0149977.
Sauerbrei W, Taube S, McShane L, Cavenagh M, Altman D. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): an abridged explanation and elaboration. J National Cancer Institute. 2018;110(8):803–11 https://doi.org/10.1093/jnci/djy088.
McShane L, Hayes D. Publication of tumor marker research results: the necessity for complete and transparent reporting. J Clin Oncol. 2012;30(34):4223–32 https://doi.org/10.1200/JCO.2012.42.6858.
Hemingway H, Philipson P, Chen R, Fitzpatrick N, Damant J, Shipley M, et al. Evaluating the quality of research into a single prognostic biomarker: a systematic review and meta-analysis of 83 studies of C-reactive protein in stable coronary artery disease. PLoS Med. 2010;7(6):e1000286 https://doi.org/10.1371/journal.pmed.1000286.
Sigounas D, Tatsioni A, Christodoulou D, Tsianos E, Ioannidis J. New prognostic markers for outcome of acute pancreatitis. Pancreas. 2011;40(4):522–32 https://doi.org/10.1097/MPA.0b013e31820bf8ac.
Huebner M, le Cessie S, Schmidt CO, Vach W. A contemporary conceptual framework for initial data analysis. Observational Stud. 2018;4:171–92.
Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol. 2015;33(1):90–9 https://doi.org/10.1200/JCO.2014.56.1894.
Xing M, Alzahrani A, Carson K, Shong Y, Kim T, Viola D, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33(1):42–50 https://doi.org/10.1200/JCO.2014.56.8253.
Huzell L, Persson M, Simonsson M, Markkula A, Ingvar C, Rose C, et al. History of oral contraceptive use in breast cancer patients: impact on prognosis and endocrine treatment response. Breast Cancer Res Treat. 2015;149(2):505–15 https://doi.org/10.1007/s10549-014-3252-8.
Thurner E, Krenn-Pilko S, Langsenlehner U, Stojakovic T, Pichler M, Gerger A, et al. The elevated C-reactive protein level is associated with poor prognosis in prostate cancer patients treated with radiotherapy. Eur J Cancer. 2015;51(5):610–9 https://doi.org/10.1016/j.ejca.2015.01.002.
Schirripa M, Cremolini C, Loupakis F, Morvillo M, Bergamo F, Zoratto F, et al. Role of NRAS mutations as prognostic and predictive markers in metastatic colorectal cancer. Int J Cancer. 2014;136(1):83–90 https://doi.org/10.1002/ijc.28955.
Heinze G, Dunkler D. Five myths about variable selection. Transpl Int. 2016;30(1):6–10 https://doi.org/10.1111/tri.12895.
Hayashi N, Niikura N, Masuda N, Takashima S, Nakamura R, Watanabe K, et al. Prognostic factors of HER2-positive breast cancer patients who develop brain metastasis: a multicenter retrospective analysis. Breast Cancer Res Treat. 2014;149(1):277–84 https://doi.org/10.1007/s10549-014-3237-7.
Jerzak K, Cockburn J, Pond G, Pritchard K, Narod S, Dhesy-Thind S, et al. Thyroid hormone receptor α in breast cancer: prognostic and therapeutic implications. Breast Cancer Res Treat. 2014;149(1):293–301 https://doi.org/10.1007/s10549-014-3235-9.
Billingsley C, Cohn D, Mutch D, Stephens J, Suarez A, Goodfellow P. Polymerase ɛ (POLE) mutations in endometrial cancer: clinical outcomes and implications for Lynch syndrome testing. Cancer. 2014;121(3):386–94 https://doi.org/10.1002/cncr.29046.
Huang S, Waldron J, Milosevic M, Shen X, Ringash J, Su J, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer. 2014;121(4):545–55 https://doi.org/10.1002/cncr.29100.
Price T, Beeke C, Ullah S, Padbury R, Maddern G, Roder D, et al. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer. 2014;121(6):830–5 https://doi.org/10.1002/cncr.29129.
González-Vallinas M, Vargas T, Moreno-Rubio J, Molina S, Herranz J, Cejas P, et al. Clinical relevance of the differential expression of the glycosyltransferase gene GCNT3 in colon cancer. Eur J Cancer. 2015;51(1):1–8 https://doi.org/10.1016/j.ejca.2014.10.021.
Hokuto D, Sho M, Yamato I, Yasuda S, Obara S, Nomi T, et al. Clinical impact of herpesvirus entry mediator expression in human hepatocellular carcinoma. Eur J Cancer. 2015;51(2):157–65 https://doi.org/10.1016/j.ejca.2014.11.004.
Keck B, Wach S, Taubert H, Zeiler S, Ott O, Kunath F, et al. Neuropilin-2 and its ligand VEGF-C predict treatment response after transurethral resection and radiochemotherapy in bladder cancer patients. Int J Cancer. 2014;136(2):443–51 https://doi.org/10.1002/ijc.28987.
Rödel F, Wieland U, Fraunholz I, Kitz J, Rave-Fränk M, Wolff H, et al. Human papillomavirus DNA load and p16INK4aexpression predict for local control in patients with anal squamous cell carcinoma treated with chemoradiotherapy. Int J Cancer. 2014;136(2):278–88 https://doi.org/10.1002/ijc.28979.
Ostronoff F, Othus M, Lazenby M, Estey E, Appelbaum F, Evans A, et al. Prognostic significance of NPM1 mutations in the absence of FLT3–internal tandem duplication in older patients with acute myeloid leukemia: aA SWOG and UK National Cancer Research Institute/Medical Research Council Report. J Clin Oncol. 2015;33(10):1157–64 https://doi.org/10.1200/JCO.2014.58.0571.
Altman DG, Gore SM, Gardner MJ, Pocock SJ. Statistical guidelines for contributors to medical journals. Br Med J (Clin Res Ed). 1983;286(6376):1489.
Lang TA, Secic M. How to report statistics in medicine: annotated guidelines for authors, editors and reviewers. Philadelphia: American College of Physicians; 1997.
Lang T, Altman D. Statistical analyses and methods in the published literature: the SAMPL guidelines. Medical Writing. 2016;25:31–6.
Diong J, Butler AA, Gandevia SC, Héroux ME. Poor statistical reporting, inadequate data presentation and spin persist despite editorial advice. PLoS One. 2018;13(8):e0202121.
Brick C, McDowell M, Freeman ALJ. Risk communication in tables versus text: a registered report randomized trial on “fact boxes.” R Soc Open Sci 2020;7(3):190876.
Riley RD, Moons KGM, Hayden JA, Sauerbrei W, Altman DG. Prognostic factor research. In: Riley RD, van der Windt D, Croft P, Moons KGM, editors. Prognosis research in healthcare: concepts, methods, and impact. London, England: Oxford University Press; 2019. p. 107–38.
Simon R, Altman D. Statistical aspects of prognostic factor studies in oncology. Br J Cancer 1994;69(6):979-985. https://doi.org/https://doi.org/10.1038/bjc.1994.192.
Altman D, Lyman G. Methodological challenges in the evaluation of prognostic factors in breast cancer. Prognostic variables in node-negative and node-positive breast cancer. 1998;:379-393. https://doi.org/10.1007/978-1-4615-5195-9_28.
Moher D, Altman D, Schulz K, Simera I, Wager E, editors. Guidelines for reporting health research: A user’s manual: Bmj Publishing Group; 2014.
Altman, DG., McShane, L. M., Sauerbrei, W., Taube, S. E., & Cavenagh M. M. (2014). REMARK (Reporting Recommendations for Tumor MARKer Prognostic Studies). In Moher D, Altman D, Schulz K, Simera I, Wager E, editors. Guidelines for reporting health research: A user’s manual (p. 241-249). John Wiley & Sons, Ltd.
Mallett S, Timmer A, Sauerbrei W, Altman DG. Reporting of prognostic studies of tumour markers: a review of published articles in relation to REMARK guidelines. Br J Cancer. 2010;102(1):173–80.
Altman DG. Systematic reviews in health care: Systematic reviews of evaluations of prognostic variables. BMJ. 2001;323(7306):224–8 https://doi.org/10.1136/bmj.323.7306.224.
Sauerbrei W. Prognostic factors. Confusion caused by bad quality design, analysis and reporting of many studies. Adv Otorhinolaryngol. 2005;62:184–200.
Sauerbrei W, Holländer N, Riley RD, Altman DG. Evidence-based assessment and application of prognostic markers: the long way from single studies to meta-analysis. CommunStat - Theory Methods. 2006;35(7):1333–42 https://doi.org/10.1080/03610920600629666.
Malats N, Bustos A, Nascimento C, Fernandez F, Rivas M, Puente D, et al. P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol. 2005;6(9):678–86 https://doi.org/10.1016/S1470-2045(05)70315-6.
Papadakis ES, Reeves T, Robson NH, Maishman T, Packham G, Cutress RI. BAG-1 as a biomarker in early breast cancer prognosis: a systematic review with meta-analyses. Br J Cancer. 2017;116(12):1585–94 https://doi.org/10.1038/bjc.2017.130.
Sauerbrei W, Haeussler T. Comment on ‘BAG-1 as a biomarker in early breast cancer prognosis: a systematic review with meta-analyses’. Br J Cancer. 2018;118(8):1152–3 https://doi.org/10.1038/s41416-018-0023-z.
Real FX, Malats N. Bladder cancer and apoptosis: matters of life and death. Lancet Oncol. 2007;8(2):91–2.
Kern SE. Why your new cancer biomarker may never work: recurrent patterns and remarkable diversity in biomarker failures. Cancer Res. 2012;72(23):6097–101.
Moher D, Glasziou P, Chalmers I, Nasser M, Bossuyt PMM, Korevaar DA, et al. Increasing value and reducing waste in biomedical research: who’s listening? Lancet. 2016;387(10027):1573–86.
Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement JAMA. 1996;276(8):637–9.
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Standards for reporting of diagnostic accuracy. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for reporting of diagnostic accuracy. Clin Chem. 2003;49(1):1–6.
Elm E von, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007; 18(6):800–804.
EQUATOR Network: Enhancing the QUAlity and Transparency Of health Research. https://www.equator-network.org/. .
Moher D, Schulz K, Simera I, Altman D. Guidance for developers of health research reporting guidelines. PLoS Med. 2010;7(2):e1000217 https://doi.org/10.1371/journal.pmed.1000217.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55–63.
Moons KGM, Altman DG, Reitsma JB, Ioannidis JPA, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73 https://doi.org/10.7326/M14-0698.
Heus P, Damen JAAG, Pajouheshnia R, Scholten RJPM, Reitsma JB, Collins GS, et al. Poor reporting of multivariable prediction model studies: towards a targeted implementation strategy of the TRIPOD statement. BMC Med. 2018;16(1) https://doi.org/10.1186/s12916-018-1099-2.
Perel P, Edwards P, Wentz R, Roberts I. Systematic review of prognostic models in traumatic brain injury. BMC Med Inform Decis Mak. 2006;6(1):38.
Shariat SF, Karakiewicz PI, Margulis V, Kattan MW. Inventory of prostate cancer predictive tools. Curr Opin Urol. 2008;18(3):279–96.
Wyatt JC, Altman DG. Commentary: Prognostic models: clinically useful or quickly forgotten? BMJ. 1995;311(7019):1539–41 https://doi.org/10.1136/bmj.311.7019.1539.
Moons KGM, de Groot JAH, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11(10):e1001744 https://doi.org/10.1371/journal.pmed.1001744.
Bradley A, Van Der Meer R, McKay CJ. A systematic review of methodological quality of model development studies predicting prognostic outcome for resectable pancreatic cancer. BMJ Open. 2019;9(8):e027192 https://doi.org/10.1136/bmjopen-2018-027192.
Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of COVID-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328 https://doi.org/10.1136/bmj.m1328.
Sperrin M, Grant SW, Peek N. Prediction models for diagnosis and prognosis in Covid-19. BMJ. 2020;369:m1464.
Collins GS, Moons KGM. Reporting of artificial intelligence prediction models. Lancet. 2019;393(10181):1577–1579. https://doi.org/10.1016/S0140-6736(19)30037-6.67
Vollmer S, Mateen BA, Bohner G, Király FJ, Ghani R, Jonsson P, et al. Machine learning and artificial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness. BMJ. 2020;368:l6927 https://doi.org/10.1136/bmj.l6927.
Sendak MP, Gao M, Brajer N, Balu S. Presenting machine learning model information to clinical end users with model facts labels. NPJ Digit Med. 2020;3(1):41. https://doi.org/10.1038/s41746-020-0253-3.
Birnbaum DJ, Finetti P, Lopresti A, Gilabert M, Poizat F, Raoul J-L, et al. A 25-gene classifier predicts overall survival in resectable pancreatic cancer. BMC Med. 2017;15(1). https://doi.org/10.1186/s12916-017-0936-z.
Rifai N, Altman DG, Bossuyt P. Reporting bias in diagnostic and prognostic studies: time for action. Clin Chem. 2008;54:1101–3.
Ioannidis JPA. Why most published research findings are false. PLoS Med. 2005;2(8):e124. https://doi.org/10.1371/journal.pmed.0020124.
Andre F, McShane LM, Michiels S, Ransohoff DF, Altman DG, Reis-Filho JS, et al. Biomarker studies: a call for a comprehensive biomarker study registry. Nat Rev Clin Oncol. 2011;8(3):171–6.
Yavchitz A, Ravaud P, Altman DG, Moher D, Hrobjartsson A, Lasserson T, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56–65.
Sekula P, Pressler JB, Sauerbrei W, Goebell PJ, Schmitz-Dräger BJ. Assessment of the extent of unpublished studies in prognostic factor research: a systematic review of p53 immunohistochemistry in bladder cancer as an example. BMJ Open. 2016;6(8):e009972. https://doi.org/10.1136/bmjopen-2015-009972.
Doussau A, Vinarov E, Barsanti-Innes B, Kimmelman J. Comparison between protocols and publications for prognostic and predictive cancer biomarker studies. Clin Trials. 2020;17(1):61–8.
Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med. 2019;170(1):51–8 https://doi.org/10.7326/M18-1376.
Debray TPA, de Jong VMT, Moons KGM, Riley RD. Evidence synthesis in prognosis research. Diagn Progn Res. 2019;3(1):13.
Abo-Zaid G, Sauerbrei W, Riley RD. Individual participant data meta-analysis of prognostic factor studies: state of the art? BMC Med Res Methodol. 2012;12(1):56. https://doi.org/10.1186/1471-2288-12-56.
Inker LA, Grams ME, Levey AS, Coresh J, Cirillo M, Collins JF, et al. Relationship of estimated GFR and albuminuria to concurrent laboratory abnormalities: an individual participant data meta-analysis in a global consortium. Am J Kidney Dis. 2019;73(2):206–17.
Holden S, Kasza J, Winters M, van Middelkoop M, Rathleff MS. Prognostic factors for adolescent knee pain: an individual participant data meta-analysis of 1281 patients. Pain. 2021;162(6):1597–607.
Ventresca M, Schünemann HJ, Macbeth F, Clarke M, Thabane L, Griffiths G, et al. Obtaining and managing data sets for individual participant data meta-analysis: scoping review and practical guide. BMC Med Res Methodol. 2020;20(1):113.
Riley RD, Ridley G, Williams K, Altman DG, Hayden J, de Vet HCW. Prognosis research: toward evidence-based results and a Cochrane methods group. J Clin Epidemiol. 2007;60(8):863–5 author reply 865-6.
Cochrane Prognosis - Cochrane Methods. https://methods.cochrane.org/prognosis/. Accessed 29 July 2021.
Sauerbrei W, Bland M, Evans SJW, Riley RD, Royston P, Schumacher M, et al. Doug Altman: Driving critical appraisal and improvements in the quality of methodological and medical research. Biom J. 2021;63(2):226–46.
Prognosis Research. https://www.prognosisresearch.com/. Accessed 29 July 2021.
Peat G, Riley RD, Croft P, Morley KI, Kyzas PA, Moons KGM, et al. Improving the transparency of prognosis research: the role of reporting, data sharing, registration, and protocols. PLoS Med. 2014;11(7):e1001671.
Riley RD, van der Windt D, Croft P, Moons KGM, editors. Prognosis research in healthcare: concepts, methods, and impact. London, England: Oxford University Press; 2019.
Riley RD, Snell KIE, Moons KGM, Debray TPA. Ten principles to strengthen prognosis research. In: Riley RD, van der Windt D, Croft P, Moons KGM, editors. Prognosis research in healthcare: concepts, methods, and impact. London, England: Oxford University Press; 2019. p. 69–84.
Sauerbrei W, Abrahamowicz M, Altman DG, le Cessie S, Carpenter J, STRATOS initiative. STRengthening analytical thinking for observational studies: the STRATOS initiative. Stat Med. 2014;33(30):5413–32.
Huebner M, Vach W, le Cessie S, Schmidt CO, Lusa L. Hidden analyses: a review of reporting practice and recommendations for more transparent reporting of initial data analyses. BMC Med Res Methodol. 2020;20(1):1–10.
Dwivedi AK, Shukla R. Evidence-based statistical analysis and methods in biomedical research (SAMBR) checklists according to design features. Cancer Rep. 2020;3(4):e1211.
Altman DG. The time has come to register diagnostic and prognostic research. Clin Chem. 2014;60(4):580–2.
Riley RD, Sauerbrei W, Altman DG. Prognostic markers in cancer: the evolution of evidence from single studies to meta-analysis, and beyond. Br J Cancer. 2009;100(8):1219–29.
Tomar T, Alkema NG, Schreuder L, Meersma GJ, de Meyer T, van Criekinge W, et al. Methylome analysis of extreme chemoresponsive patients identifies novel markers of platinum sensitivity in high-grade serous ovarian cancer. BMC Med. 2017;15(1). https://doi.org/10.1186/s12916-017-0870-0.
Van Calster B, Wynants L, Riley RD, van Smeden M, Collins GS. Methodology over metrics: current scientific standards are a disservice to patients and society. J Clin Epidemiol. 2021.
Altman DG. The scandal of poor medical research. BMJ (Clinical Research Edition). 1994;308(6924):283–4.
Acknowledgements
We thank Jannik Braun and Sarah Hag-Yahia for the administrative assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. WS, TH, and JB were partially supported by grant SA580/8-3 from the German Research Foundation (DFG) to WS.
Author information
Authors and Affiliations
Contributions
WS conceived the idea for this paper and started with work on profiles. WS, MH, and TH discussed the design, wrote the protocol, and selected the papers. TH and WS created the first version of all REMARK profiles. MH and JB commented on them, and all authors agreed on the final version. WS wrote the first draft of the paper, and all authors contributed to the manuscript. JB died before we finalized the manuscript. The other authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
All authors have given their consent for the publication of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sauerbrei, W., Haeussler, T., Balmford, J. et al. Structured reporting to improve transparency of analyses in prognostic marker studies. BMC Med 20, 184 (2022). https://doi.org/10.1186/s12916-022-02304-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-022-02304-5