Abstract
Background
The associations of maternal cigarette smoking with congenital anomalies in offspring have been inconsistent. This study aimed to clarify the associations of the timing and intensity of maternal cigarette smoking with 12 subtypes of birth congenital anomalies based on a nationwide large birth cohort in the USA.
Methods
We used nationwide birth certificate data from the US National Vital Statistics System during 2016–2019. Women reported the average daily number of cigarettes they consumed 3 months before pregnancy and in each subsequent trimester during pregnancy. Twelve subtypes of congenital anomalies were identified in medical records. Poisson regression analysis was used to estimate the risk ratios (RRs) with 95% confidence intervals (CIs) for 12 subtypes of congenital anomalies associated with the timing (i.e., before pregnancy, and during three different trimesters of pregnancy) and intensity (i.e., number of cigarettes consumed per day) of maternal cigarette smoking.
Results
Among the 12,144,972 women included, 9.3% smoked before pregnancy and 7.0%, 6.0%, and 5.7% in the first, second, and third trimester, respectively. Maternal smoking before or during pregnancy significantly increased the risk of six subtypes of birth congenital anomalies (i.e., congenital diaphragmatic hernia, gastroschisis, limb reduction defect, cleft lip with or without cleft palate, cleft palate alone, and hypospadias), even as low as 1–5 cigarettes per day. The adjusted RRs (95% CIs) for overall birth congenital anomalies (defined as having any one of the congenital malformations above significantly associated with maternal cigarette smoking) among women who smoked 1–5, 6–10, and ≥ 11 cigarettes per day before pregnancy were 1.31 (1.22–1.41), 1.25 (1.17–1.33), and 1.35 (1.28–1.43), respectively. Corresponding values were 1.23 (1.14–1.33), 1.33 (1.24–1.42), 1.33 (1.23–1.43), respectively, for women who smoked cigarettes in the first trimester; 1.32 (1.21–1.44), 1.36 (1.26–1.47), and 1.38 (1.23–1.54), respectively, for women who smoked cigarettes in the second trimester; and 1.33 (1.22–1.44), 1.35 (1.24–1.47), and 1.35 (1.19–1.52), respectively, for women who smoked cigarettes in the third trimester. Compared with women who kept smoking before and throughout pregnancy, women who never smoked had significantly lower risk of congenital anomalies (RR 0.77, 95% CI 0.73–0.81), but women who smoked before pregnancy and quitted during each trimester of pregnancy had no reduced risk (all P > 0.05).
Conclusions
Maternal smoking before or during pregnancy increased the risk of several birth congenital anomalies, even as low as 1–5 cigarettes per day. Maternal smokers who stopped smoking in the subsequent trimesters of pregnancy were still at an increased risk of birth congenital anomalies. Our findings highlighted that smoking cessation interventions should be implemented before pregnancy.
Similar content being viewed by others
Background
Congenital anomalies, also known as congenital malformations or birth defects, can be defined as structural or functional anomalies that are diagnosed prenatally or after delivery [1]. Globally, approximately 8 million infants (6% of total births) are estimated to be born with a serious congenital anomaly every year [2]. In the USA, congenital anomalies are the leading cause of infant mortality, accounting for 21% of infant deaths in 2017 [3]. The total hospital charges for treating congenital anomalies exceed $2.6 billion each year in the USA [4]. Thus, identifying modifiable risk factors of congenital anomalies and implementing effective primary prevention strategies remain a priority of public health policies in the USA and worldwide [1, 3].
In the USA, nearly 16.0% of women aged 25–44 years were current cigarette smokers in 2015 [5], and at least half of them continued smoking during pregnancy [6, 7]. It means that approximately 360,000 infants born in the USA were exposed to cigarette smoking [8]. Maternal cigarette smoking during pregnancy is associated with many detrimental infant health outcomes, such as delayed intrauterine development, preterm birth, and infant mortality [9,10,11]. However, the associations between maternal cigarette smoking during pregnancy and congenital anomalies remain less clear. Several studies reported that maternal cigarette smoking was significantly associated with an increased risk of certain type of congenital anomalies, such as oral clefts [12, 13] and congenital cardiovascular malformations [13, 14]. In contrast, other studies showed no association between maternal cigarette smoking and any type of congenital anomalies [15, 16]. Moreover, a few studies even reported a decreased risk of certain specific anomalies among women who smoked in pregnancy, such as anomalies on musculoskeletal system [13], and hypospadias [17]. These previous studies [12,13,14,15,16,17] had several limitations such as the inclusion of relatively small number of cases, especially for rare type of congenital anomalies (i.e., being underpowered to detect small or moderate risk effect), the use of case-control design (i.e., being more prone to selection bias), or insufficient adjustment for potential confounders (i.e., being more prone to confounding bias). In terms of smoking intensity, the associations of maternal cigarette smoking and congenital anomalies in offspring were also inconsistent. For example, a case-control study in the USA showed that light smoking (< 10 cigarettes per day) significantly increased the risk of cleft lip with or without cleft palate (odds ratio [OR] 1.50, 95% CI 1.28–1.76) [18]. Another case-control study in the UK reported that smoking 1–10 cigarettes per day during pregnancy marginally increased the risk of this anomaly (OR 1.70, 95% CI 0.90–3.90) [12]. In contrast, a large population-based prospective cohort study in Denmark showed that light smoking (≤ 5 cigarettes per day) was associated with a marginally reduced risk of major malformations (OR 0.96, 95% CI 0.92–1.01) [13]. These inconsistent results can obscure the teratogenic effects of maternal cigarette smoking during pregnancy to public policy makers and weaken tobacco control efforts aiming at preventing congenital anomalies. In addition, the previous studies mentioned above [12,13,14,15,16,17] did not distinguish the effect of different maternal smoking timing (before or during different trimesters of pregnancy) on congenital anomalies, and little is known about whether and when smoking cessation during pregnancy matter for birth congenital anomalies.
Based on a nationwide large birth cohort data in the USA (N = 12,144,972), this study was aimed to examine the associations of the timing (before pregnancy, and during different trimesters of pregnancy) and intensity (number of cigarettes consumed per day) of maternal cigarette smoking with 12 subtypes of birth congenital anomalies.
Methods
Study population
This study used nationwide birth certificate data from the National Vital Statistics System (NVSS) in the USA (2016-2019). The NVSS is a joint program led by the US Centers for Disease Control and Prevention and all US states. It collected data on a wide range of maternal and infant demographic and health information. Standardized questionnaires and registration procedures have been developed and recommended for all states and the District of Columbia in the USA. Two worksheets have been developed since 2003, including the mother’s worksheet and the facility worksheet. The mother`s worksheet compiles mother data around the time of delivery (e.g., maternal demographics, and cigarette smoking before and during pregnancy). The facility worksheet compiles other data appearing in medical records (e.g., risk factors in this pregnancy, and birth congenital anomalies). More details about NVSS data collection can be found at the official website (https:// www.cdc.gov/nchs/nvss/births.htm). The birth certificate data from the NVSS are de-identified and do not include any protected health information. The data are publically available [19] and exempt under the ethical board review of the corresponding author’s institution.
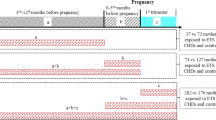
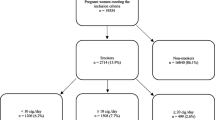
In this study, we used NVSS data from 2016 to 2019 because all US states and the District of Columbia had completely implemented the 2003 version of Standard Certificate of Live Birth to collect birth information since 2016. A total of 15,379,982 live births registered in the NVSS from 2016 to 2019 were preliminarily included in the study. We then excluded women aged < 18 or ≥ 50 years (n = 200,589) in order to only include adult women of reproductive age, women with twin or multiple births (n = 516,374) because they differed from the singletons with congenital anomalies [20], and women with pre-existing hypertension or diabetes (n = 396,324) because these chronic conditions were strong risk factors for birth congenital anomalies [21, 22]. We further excluded women with missing data on maternal cigarette smoking (n = 67,209), birth congenital anomalies (n = 20,693), or on covariates (maternal demographics, maternal pregnancy risk factors, gestational age at delivery, and pregnancy history or prenatal care; n = 2,033,821). This left a total of 12,144,972 live singleton births included for the final data analysis. Figure 1 shows the flow chart of the inclusion/exclusion of the participants.
Maternal cigarette smoking
Information on maternal cigarette smoking was collected using the mother’s worksheet in the hospital around the time of delivery. Mothers reported the average daily number of cigarettes consumed 3 months prior to pregnancy and during the first, second, and third trimesters of pregnancy. In each time period, women who smoked one or more cigarettes per day were considered as smokers, whereas women who smoked “0” cigarette per day were considered as non-smokers. Smokers were further categorized into three smoking intensity subgroups: 1–5, 6–10, and ≥ 11 cigarettes per day. These categories were defined in order to warrant robust statistical power in each subgroup and also were based on previous studies [13].
Congenital anomalies
Information on birth congenital anomalies was obtained from the facility worksheet. Twelve subtypes of congenital anomalies were identified at birth, including anencephaly, meningomyelocele/spina bifida, cyanotic congenital heart disease, congenital diaphragmatic hernia, omphalocele, gastroschisis, limb reduction defect, cleft lip with or without cleft palate, cleft palate alone, Down syndrome, suspected chromosomal disorder, and hypospadias. For hypospadias, data were restricted to male infants. For Down syndrome and other suspected chromosomal disorders, we combined results of the pending and confirmed karyotype. In addition, we defined “overall congenital anomalies” as having any type of congenital malformations above that were significantly associated with maternal cigarette smoking.
Study covariates
Data on maternal demographics, educational level, marital status, and maternal pre-pregnancy weight and height were collected using the mother’s worksheet. Data on maternal risk factors (i.e. eclampsia, gestational hypertension and gestational diabetes), parity, infant sex, gestational age at delivery, and prenatal care visits were obtained from the facility worksheet.
We categorized covariates as follows: maternal age at delivery (18–29, 30–39, or 40–49 years), race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, or other), educational level (less than high school, high school, or more than high school), marital status (married or unmarried), maternal pre-pregnancy body mass index (BMI) (< 18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, 35.0–39.9, or ≥ 40 kg/m2 based on the World Health Organization guidelines [23]), eclampsia (yes or no), gestational hypertension (yes or no), gestational diabetes (yes or no), parity (1, 2, 3, or ≥ 4), infant sex (male or female), and total number of prenatal care visits (0, 1–4, 5–9, or ≥ 10).
Statistical analysis
Baseline characteristics of the participants were presented according to maternal cigarette smoking status before pregnancy and in the first, second, and third pregnancy trimesters. Comparisons between groups were analyzed using χ2 test for categorical variables and Wilcoxon test for continuous variables. We first used Poisson regression analysis to estimate the associations of maternal cigarette smoking status (yes versus no) before or during pregnancy with each of 12 subtypes of congenital anomalies using women who did not smoke cigarettes in the corresponding period as the reference. We adjusted for several covariates, including maternal age at delivery, race/ethnicity, educational level, marital status, maternal pre-pregnancy BMI, eclampsia, gestational hypertension, gestational diabetes, parity, infant sex, gestational age at delivery, and total number of prenatal care visits. These potential confounders were selected based on the literature (i.e., based on their known/hypothetic correlations with both the exposures [i.e., maternal smoking] and outcomes [i.e., congenital anomalies]) as well as the information that was available on the birth certificate. We then estimated the risk ratios (RRs) and 95% confidence intervals (CIs) of birth congenital anomalies (i.e., which showed positive associations in the initial analysis) according to smoking intensity before and during each trimester of pregnancy. We also examined the association of smoking cessation in different trimesters of pregnancy with overall birth congenital anomalies using women who kept smoking before and throughout pregnancy as the reference. We performed four sensitivity analyses to test the stability of our main findings (i.e., the association between the timing of maternal cigarette smoking and six subtypes of congenital anomalies), including the exclusion of women with cesarean section, the exclusion of those with eclampsia, gestational hypertension or gestational diabetes, the exclusion of those with preterm delivery, and the use of imputation analyses (using the fully conditional specification method [24]) to deal with missing data on all variables of interest. All statistical analyses were performed using SAS V.9.4 (SAS Institute Inc., Cary, North Carolina). A two-tailed p < 0.05 was considered statistically significant. The Bonferroni adjustment method was used for multiple comparisons.
Results
Population characteristics
A total of 12,144,972 women with live singleton births were included, with a mean age at delivery of 29 years (interquartile range 25–33 years). 9.3% of the women smoked cigarettes before pregnancy, respectively 7.0%, 6.0%, and 5.7% in the first, second, and third trimesters. In general, women who smoked cigarettes before or during pregnancy tended to be younger and non-Hispanic white, to have a low educational level, to be unmarried, obese, and multiparous (all p < 0.0001). Detailed characteristics of the study population are shown in Table 1.
Associations between the timing of maternal cigarette smoking and birth congenital anomalies
Maternal cigarette smoking before pregnancy or during either trimester was significantly associated with six subtypes of congenital anomalies: congenital diaphragmatic hernia, gastroschisis, limb reduction defect, cleft lip with or without cleft palate, cleft palate alone, and hypospadias. For example, the adjusted RRs (95% CIs) of cleft lip with or without cleft palate of infants born to women who smoked cigarettes before pregnancy, and in the first, second, and third trimesters were 1.28 (1.18–1.38), 1.26 (1.16–1.37), 1.26 (1.15–1.38), and 1.24 (1.13–1.36), respectively (all p < 0.0001) (Table 2). Associations withstood multiple comparison correction, except for congenital diaphragmatic hernia and hypospadias during pregnancy.
Regarding overall congenital anomalies (defined as having any one of the congenital malformations above significantly associated with maternal cigarette smoking), the adjusted RRs (95% CIs) were 1.30 (1.25–1.36) for women who smoked before pregnancy and 1.30 (1.24–1.36), 1.35 (1.27–1.43), and 1.34 (1.26–1.42) for women who smoked in the first, second, and third trimesters, respectively (all p < 0.0001).
Considering smoking cessation during pregnancy, we found that compared with women who kept smoking before and throughout pregnancy, maternal smokers who quitted smoking in the first, second, or third trimester did not have a significantly reduced risk of overall congenital anomalies, with adjusted RRs (95% CI) of 0.99(0.91–1.08), 1.06(0.95–1.19), and 0.93 (0.78–1.10), respectively (Additional file 1: Table S1). In contrast, those who never smoked before and during pregnancy had a significantly lower risk of overall birth congenital anomalies (RR 0.77, 95% CI 0.73–0.81).
Associations between the intensity of maternal cigarette smoking and birth congenital anomalies
Compared with non-smokers, infants born to women who consumed any number of cigarettes per day before or during pregnancy, even only 1–5 cigarettes per day, had an increased risk of four subtypes of congenital anomalies, including gastroschisis, limb reduction defect, cleft lip with or without cleft palate, and cleft palate alone. For example, the adjusted RRs (95% CIs) of cleft lip with or without cleft palate of infants born to women who smoked 1–5, 6–10, and ≥ 11 cigarettes per day before pregnancy were 1.33 (1.16–1.52), 1.19 (1.05–1.35), and 1.32 (1.18–1.47), respectively. The corresponding values were 1.26 (1.10–1.45), 1.21 (1.07–1.38), and 1.33 (1.15–1.53) for women who smoked in the first trimester; 1.22 (1.06–1.41), 1.26 (1.10–1.43), and 1.33 (1.11–1.59) for women who smoked in the second trimester; and 1.20 (1.05–1.38), 1.23 (1.07–1.41), and 1.33 (1.10–1.63) for women who smoked in the third trimester. For congenital diaphragmatic hernia and hypospadias, the significant association was observed for the specific intensity of maternal cigarette smoking in specific trimester (Tables 3, 4, 5, and 6). The statistical significance still existed in most groups even after the Bonferroni correction (the corrected p values ranged from 0.0024 (0.05/(3*7)) to 0.0028 (0.05/(3*6)) when subgroups ranged from 21 (Tables 3 and 4) to 18 (Tables 5 and 6), respectively).
Regarding overall congenital anomalies, the adjusted RRs (95% CIs) for women who smoked 1–5, 6–10, and ≥ 11 cigarettes per day before pregnancy were 1.31 (1.22–1.41), 1.25 (1.17–1.33), and 1.35 (1.28–1.43), respectively. The corresponding values were 1.23 (1.14–1.33), 1.33 (1.24–1.42), and 1.33 (1.23–1.43) for women who smoked cigarettes in the first trimester; 1.32 (1.21–1.44), 1.36 (1.26–1.47), and 1.38 (1.23–1.54) for women who smoked in the second trimester; and 1.33 (1.22–1.44), 1.35 (1.24–1.47), and 1.35 (1.19–1.52) for women who smoked in the third trimester (all p < 0.0001) (Tables 3, 4, 5, and 6).
Sensitivity analyses
Sensitivity analyses yielded similar results to our main analyses, including by excluding women with cesarean section or excluding women with eclampsia, gestational hypertension or diabetes, excluding women with preterm delivery, and based on imputation analyses. All these analyses above showed that maternal cigarette smoking before pregnancy or during each trimester significantly increased the risk of six subtypes of congenital anomalies mentioned before (Additional file 1: Table S2).
Discussion
In this nationwide population-based cohort study of more than 12 million live births in the USA, we found that maternal cigarette smoking, even at a low intensity (1–5 cigarettes per day), before pregnancy or during each trimester of pregnancy significantly increased the risk of six specific subtypes of congenital anomalies (i.e., congenital diaphragmatic hernia, gastroschisis, limb reduction defect, cleft lip with or without cleft palate, cleft palate alone, and hypospadias). Compared with persistent smokers before and throughout pregnancy, a significantly lower risk of congenital anomalies was observed for never smokers, but no reduced risk was observed for women who smoked before pregnancy and quitted during each trimester of pregnancy.
Comparisons with other studies
In the present study, we found an increased risk of overall congenital anomalies in offspring born to mothers who smoked cigarettes during pregnancy, which contrasts with a previous large population-based cohort study using data from the Danish Medical Birth Register, which did not find an overall increased risk of congenital anomalies (OR 0.98, 95% CI 0.95–1.01) [13]. It should be noted that the Danish study included a smaller sample size (n = 838,265) than ours (n = 12,144,972, especially for rare outcomes, for example, the risk estimate for omphalocele in the Danish study was based on 25 exposed cases). The Danish study also did not adjust for potentially important covariates which we have adjusted for in our analysis (e.g., race/ethnicity, maternal pre-pregnancy BMI, infant sex, eclampsia, gestational hypertension, and gestational diabetes). In addition, disparities in definitions of “overall congenital anomalies” may also contribute to the inconsistent results. However, when we used having any of the 12 congenital anomalies to define “overall congenital anomalies,” we still found an increased risk of overall congenital anomalies for maternal smoking (data not shown).
Since different anomalies can have different etiologies, defect-specific analyses may better reflect the association between maternal cigarette smoking and congenital anomalies. A meta-analysis of 6 case-control studies (4209 malformed cases and 10,646 controls) published between 2000 and 2010 revealed that maternal smoking during pregnancy significantly increased the risk of cleft lip with or without cleft palate (OR 1.48, 95% CI 1.36–1.61) [25]. The Danish study [13] mentioned above also showed that maternal smoking in pregnancy was associated with an increased risk of this anomaly (OR 1.36, 95% CI 1.18–1.56), similar to our results. We also observed an increased risk of limb reduction defects, congenital diaphragmatic hernia, and gastroschisis among women who smoked cigarettes, consistent with a meta-analysis by Hackshaw et al. [26] which identified 172 studies (173,687 malformed cases and 11,674,332 controls; conducted between 1959 and 2010; limb reduction defects: OR 1.26, 95% CI 1.15–1.39; hernia: OR 1.40, 95% CI 1.23–1.59; gastroschisis: OR 1.50, 95% CI 1.28–1.76). However, the Danish study [13] reported a marginally reduced risk of gastroschisis (OR 0.89, 95% CI 0.56–1.39; based on only 25 exposed cases) and no association with diaphragmatic hernia (OR 1.00, 95% CI 0.68–1.49; based on only 32 exposed cases). In addition, the meta-analysis by Hackshaw et al. [26] reported a reduced risk for hypospadias (OR 0.90, 95% CI 0.85–0.95), similar with the Danish study [13] which reported a marginally reduced risk of hypospadias (OR 0.94, 95% CI 0.83–1.06), inconsistent with our results (showing a significantly increased risk). Due to the large number of cases in our study, we do not believe the observed increased risk of hypospadias was entirely due to chance although the association needs to be warranted in further studies. Furthermore, in contrast to previous studies [13, 27], we did not detect a significant association between maternal cigarette smoking during pregnancy and cyanotic congenital heart disease. This result may be expected, as congenital heart defects consist of many heterogeneous subtypes that have different genetic and embryological origins. Further research should examine the effects of maternal smoking on specific subtypes of congenital heart defects.
In addition to clarifying the associations of maternal cigarette smoking with birth congenital anomalies, a novel finding of this study was that maternal cigarette smoking either before pregnancy or during each trimester of pregnancy significantly increased the risk of birth congenital anomalies, suggesting that there is no safe period of maternal smoking, which has been seldom reported in the previous studies mentioned above. It is interesting that the associations of smoking with congenital anomalies did not change significantly according to different trimesters. One speculative, possible explanation would be that the detrimental effects of maternal smoking on congenital anomalies mainly originate from an earlier period in pregnancy: morphogenesis is influenced mainly during the first days of embryogenesis, whereas later days of pregnancy involve mostly about growth of the embryo. The observed similar association between maternal smoking in the second and third trimesters of pregnancy and congenital anomalies might be due to the fact that the majority of women who smoked in early pregnancy continued to smoke cigarettes throughout pregnancy.
Another important finding of this study was that smoking exposure within three months before pregnancy, but with cessation during the pregnancy, had the similar risk of birth congenital anomalies compared with persistent smokers before and during pregnancy. One possible explanation was the detrimental action of maternal smoking on some genes—genetic or epigenetic—of the ovules (i.e., the potentially critical periods for preventing congenital anomalies seemed to be pre-conception and/or during the first days of pregnancy) [28]. Another speculative explanation was the known longer half-life of nicotine [29] and the nicotine withdrawal effect [30]. In contrast, women who never smoked before and during pregnancy were less likely to have birth congenital anomalies compared to persistent smokers throughout pregnancy. We believe that these findings have important public health implications. Many women who smoke cigarettes believe that it is acceptable to smoke before pregnancy or in the first trimester [31]. Besides, when a woman finds herself pregnant and begins prenatal care, many fetal organs may have already been formed, and smoking cessation might be too late to prevent congenital anomalies. Therefore, there is an urgent need to strengthen pre-pregnancy health services to help minimize the risk of congenital anomalies.
The third important finding of our study was that mothers who smoked only few cigarettes (1–5 cigarettes per day) had an increased risk of congenital anomalies compared with non-smokers, suggesting there is no safe level of cigarette smoking for pregnant women. Similarly, Chung, et al. [18] used the 1996 US Natality database (2207 cases and 4414 controls) found an increased risk for cleft lip with or without cleft palate among light smokers (1–10 cigarettes per day; OR 1.50, 95% CI 1.28–1.76). Another large study used Swedish register data (n = 1,413,811; 1983–1996) showed a marginally positive association between light smokers (< 10 cigarettes per day) and any malformation (OR 1.02, 95% CI 0.98–1.05) [32]. However, the two studies used smoking 1–9 cigarettes per day to define light smoking. The Danish study [13] used smoking 1–5 cigarettes per day to define light smoking and showed that infants born to mothers who smoked a low dose of cigarettes per day were at a marginally reduced risk of major malformations (OR 0.96, 95% CI 0.92–1.01). These previous studies also did not distinguish the effect of intensity of maternal smoking in different periods (before or during different trimesters of pregnancy) on congenital anomalies, while our study showed the similar effect highlighting maternal smoking even at a low intensity during any period increased the risk of congenital anomalies. It should be noted that our study did not find clear dose-response associations between maternal smoking before or during the pregnancy and birth congenital anomalies, similar to a previous study on maternal smoking and congenital heart defects by Alverson et al. (2525 malformations and 3435 controls) [14]. Another older study showed no dose-response association between maternal smoking and cleft lip with or without cleft palate [33]. The possible explanations included the competing risks (e.g., abortion and stillbirth) caused by high doses of consumed cigarettes [34], which might bias the relationship with congenital anomalies toward null. In addition, other unmeasured confounding factors might have also biased our findings.
Finally, it should be mentioned that magnitudes of the positive association between maternal smoking and congenital anomalies in our study were somewhat higher than those in previous studies mentioned above. We speculate that this may be due to variability in exposure assessment (i.e., cigarette smoking) and case ascertainment (i.e., congenital anomalies). In addition, many previous studies were based on relatively small number of cases on congenital anomalies and with adjustment for a limited number of potential confounders. Further large well-designed prospective cohort studies are needed to warrant the observed association and to examine the possible explanations.
Strengths and limitations
The main strengths of this study include the population-based cohort design, the large sample size of over 12 million live births, the detailed examination of the associations between the timing and intensity of maternal cigarette smoking and many congenital anomalies subtypes considered (including rare subtypes), the adjustment for several (but not all possible) covariates, and the sensitivity analyses used to confirm our findings. However, this study also has several limitations. First, there were limitations to use birth certificate data for assessing the association with birth defects. Data on pregnancy complications, and comorbid conditions tended to be underreported on the birth certificates [35]. However, a previous study used data from the West Virginia (1990–2009) to assess the effects of misclassification bias on reported congenital anomalies based on birth certificate data, and the results showed that specified birth defects collected using checked-box format with definite criteria on the 2003 version birth certificate showed consistent patterns over time [36]. In addition, several other studies have been performed to assess the validity of birth certificate data for effect estimation [22, 37] and the results suggest that birth certificate data may be useful for exploratory studies assessing the association between birth congenital anomalies and some risk factors. Second, maternal cigarette smoking was self-reported and recall bias might have occurred. However, a previous study showed that self-reported number of cigarettes were highly correlated with objectively measured cotinine during pregnancy [38]. Moreover, pregnant women were unwilling to report smoking if they had illness conditions around the time of delivery in the hospital. This also could cause some bias or misclassification. Third, information on non-daily cigarette smoking (< 1 cigarette per day) before or during pregnancy was unavailable on the birth certificate. Fourth, for “maternal pre-pregnancy smoking,” the NVSS only collected information on smoking within 3 months before pregnancy. Thus, we could not differentiate women who never smoked from those who did within 3 months before pregnancy. We then also could not compare the risk of congenital anomalies for smoking cessation before pregnancy vs. persist smoking, and the former period is the potentially critical period for preventing congenital anomalies. Fifth, the NVSS only collected data on congenital anomalies identified at birth. However, some types of congenital anomalies could not be identified at that time, and usually appear in childhood or even adulthood. This may cause outcome misclassification (i.e., the infants who did not have congenital anomalies at birth but diagnosed in later life were treated as not having congenital anomalies in this study). Furthermore, we only included live births and did not account for congenital anomalies in aborted fetuses and stillbirths, which may lead to selection bias (i.e., newborns with malformations among maternal smokers may be underrepresented in a study of live births). Finally, although we have adjusted for a range of potential confounders, we could not completely rule out the possibility of residual confounding or confounding from unmeasured confounders. For example, one important potential confounder that we did not adjust for was second-hand smoke exposure, which has been shown to be nearly as deleterious as active smoking to the developing fetus [39]. Another important potential confounder was alcohol consumption as drinking was associated with both smoking and embryo malformations [40]. In addition, we only adjusted for maternal race/ethnicity without considering that of the infant. It is possible that the infant may have a different racial make-up than the mother, especially if one race/ethnicity was more susceptible to the effects of smoking but only race/ethnicity of mother is considered, this could cause some bias or misclassification.
Conclusions
In conclusion, our findings showed that maternal cigarette smoking, even at a low intensity (1–5 cigarettes per day), before pregnancy or during either trimester of pregnancy significantly increased the risk of birth congenital anomalies. Women who smoked before pregnancy and quitted during each trimester of pregnancy still had an increased risk of congenital anomalies in offspring than women who did not smoke before and throughout pregnancy. Therefore, smoking cessation interventions should be implemented before pregnancy. Early initiation of pre-pregnancy counseling is of paramount importance which should include an explanation of the adverse effects of cigarette smoking on both mothers and their children including congenital anomalies and suggest on not to start smoking (for never smokers) or to stop smoking completely (for current smokers) both before and during pregnancy. The current study also provided strong evidence to strengthen the recommendation proposed by the US public health service (i.e., 5A’s: ask, advise, assess, assist, and arrange) for health care providers to help maternal smokers to quit smoking [41]. Interventions should also emphasize the detrimental effects of even light smoking for pregnant women.
Availability of data and materials
The birth certificate data from the National Vital Statistics System are available at the official website (https:// www.cdc.gov/nchs/nvss/births.htm).
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- NVSS:
-
National Vital Statistics System
- OR:
-
Odds ratios
- RR:
-
Risk ratios
References
World Health Organization. Congenital anomalies fact sheet. Geneva, WHO, 2020.http://www.who.int/news-room/fact-sheets/detail/congenital-anomalies
Christianson A, Howson CP, Modell B. March of Dimes global report on birth defects: the hidden toll of dying and disabled children. Research report March of Dimes Birth Defects Foundation. USA: White Plains; 2005.
Ely DM, Driscoll AK. Infant mortality in the United States, 2017: data from the period linked birth/infant death file. Natl Vital Stat Rep. 2019;68(10):1–20.
National Center on Birth Defects and Developmental Disabilities (NCBDDD). Fiscal year 2013 annual report. Atlanta, Georgia: National Center on Birth Defects and Developmental Disabilities; 2011.
Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults - United States, 2005-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1205–11. https://doi.org/10.15585/mmwr.mm6544a2.
Tong VT, Dietz PM, Morrow B, D'Angelo DV, Farr SL, Rockhill KM, et al. Trends in smoking before, during, and after pregnancy--Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000-2010. MMWR Surveill Summ. 2013;62(6):1–19.
Lange S, Probst C, Rehm J, Popova S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health. 2018;6(7):e769–e76. https://doi.org/10.1016/s2214-109x(18)30223-7.
Scherman A, Tolosa JE, McEvoy C. Smoking cessation in pregnancy: a continuing challenge in the United States. Ther Adv Drug Saf. 2018;9(8):457–74. https://doi.org/10.1177/2042098618775366.
Akinyemi JO, Adedini SA, Wandera SO, Odimegwu CO. Independent and combined effects of maternal smoking and solid fuel on infant and child mortality in sub-Saharan Africa. Trop Med Int Health. 2016;21(12):1572–82. https://doi.org/10.1111/tmi.12779.
Liu B, Xu G, Sun Y, Qiu X, Ryckman KK, Yu Y, et al. Maternal cigarette smoking before and during pregnancy and the risk of preterm birth: a dose-response analysis of 25 million mother-infant pairs. PLoS Med. 2020;17(8):e1003158. https://doi.org/10.1371/journal.pmed.1003158.
Marufu TC, Ahankari A, Coleman T, Lewis S. Maternal smoking and the risk of still birth: systematic review and meta-analysis. BMC Public Health. 2015;15:239. https://doi.org/10.1186/s12889-015-1552-5.
Little J, Cardy A, Arslan MT, Gilmour M, Mossey PA. Smoking and orofacial clefts: a United Kingdom-based case-control study. Cleft Palate Craniofac J. 2004;41(4):381–6. https://doi.org/10.1597/02-142.1.
Leite M, Albieri V, Kjaer SK, Jensen A. Maternal smoking in pregnancy and risk for congenital malformations: results of a Danish register-based cohort study. Acta Obstet Gynecol Scand. 2014;93(8):825–34. https://doi.org/10.1111/aogs.12433.
Alverson CJ, Strickland MJ, Gilboa SM, Correa A. Maternal smoking and congenital heart defects in the Baltimore-Washington Infant Study. Pediatrics. 2011;127(3):e647–53. https://doi.org/10.1542/peds.2010-1399.
Morales-Suárez-Varela MM, Bille C, Christensen K, Olsen J. Smoking habits, nicotine use, and congenital malformations. Obstet Gynecol. 2006;107(1):51–7. https://doi.org/10.1097/01.AOG.0000194079.66834.d5.
Malloy MH, Kleinman JC, Bakewell JM, Schramm WF, Land GH. Maternal smoking during pregnancy: no association with congenital malformations in Missouri 1980-83. Am J Public Health. 1989;79(9):1243–6. https://doi.org/10.2105/ajph.79.9.1243.
Källén K. Role of maternal smoking and maternal reproductive history in the etiology of hypospadias in the offspring. Teratology. 2002;66(4):185–91. https://doi.org/10.1002/tera.10092.
Chung KC, Kowalski CP, Kim HM, Buchman SR. Maternal cigarette smoking during pregnancy and the risk of having a child with cleft lip/palate. Plast Reconstr Surg. 2000;105(2):485–91. https://doi.org/10.1097/00006534-200002000-00001.
Centers for Disease Control and Prevention (CDC). National Vital Statistics System. https://www.cdc.gov/nchs/data_access/vitalstatsonline.htm
Best KE, Rankin J. Increased risk of congenital heart disease in twins in the North of England between 1998 and 2010. Heart. 2015;101(22):1807–12. https://doi.org/10.1136/heartjnl-2015-307826.
Bateman BT, Huybrechts KF, Fischer MA, Seely EW, Ecker JL, Oberg AS, et al. Chronic hypertension in pregnancy and the risk of congenital malformations: a cohort study. Am J Obstet Gynecol. 2015;212(3):337.e1–14. https://doi.org/10.1016/j.ajog.2014.09.031.
Wu Y, Liu B, Sun Y, Du Y, Santillan MK, Santillan DA, et al. Association of maternal prepregnancy diabetes and gestational diabetes mellitus with congenital anomalies of the newborn. Diabetes Care. 2020;43(12):2983–90. https://doi.org/10.2337/dc20-0261.
World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation (WHO Technical Report Series 894). Geneva: World Health Organization; 2000.http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en
van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–42. https://doi.org/10.1177/0962280206074463.
Molina-Solana R, Yáñez-Vico RM, Iglesias-Linares A, Mendoza-Mendoza A, Solano-Reina E. Current concepts on the effect of environmental factors on cleft lip and palate. Int J Oral Maxillofac Surg. 2013;42(2):177–84. https://doi.org/10.1016/j.ijom.2012.10.008.
Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. 2011;17(5):589–604. https://doi.org/10.1093/humupd/dmr022.
Zhang D, Cui H, Zhang L, Huang Y, Zhu J, Li X. Is maternal smoking during pregnancy associated with an increased risk of congenital heart defects among offspring? A systematic review and meta-analysis of observational studies. J Matern Fetal Neonatal Med. 2017;30(6):645–57. https://doi.org/10.1080/14767058.2016.1183640.
Mai Z, Lei M, Yu B, Du H, Liu J. The effects of cigarette smoke extract on ovulation, oocyte morphology and ovarian gene expression in mice. PLoS One. 2014;9(4):e95945. https://doi.org/10.1371/journal.pone.0095945.
Dempsey D, Jacob P 3rd, Benowitz NL. Nicotine metabolism and elimination kinetics in newborns. Clin Pharmacol Ther. 2000;67(5):458–65. https://doi.org/10.1067/mcp.2000.106129.
Maritz GS, Mutemwa M, Kayigire AX. Tomato juice protects the lungs of the offspring of female rats exposed to nicotine during gestation and lactation. Pediatr Pulmonol. 2011;46(10):976–86. https://doi.org/10.1002/ppul.21462.
Levis DM, Stone-Wiggins B, O'Hegarty M, Tong VT, Polen KN, Cassell CH, et al. Women’s perspectives on smoking and pregnancy and graphic warning labels. Am J Health Behav. 2014;38(5):755–64. https://doi.org/10.5993/ajhb.38.5.13.
Källén K. Multiple malformations and maternal smoking. Paediatr Perinat Epidemiol. 2000;14(3):227–33. https://doi.org/10.1046/j.1365-3016.2000.00269.x.
Lieff S, Olshan AF, Werler M, Strauss RP, Smith J, Mitchell A. Maternal cigarette smoking during pregnancy and risk of oral clefts in newborns. Am J Epidemiol. 1999;150(7):683–94. https://doi.org/10.1093/oxfordjournals.aje.a010071.
Pineles BL, Park E, Samet JM. Systematic review and meta-analysis of miscarriage and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol. 2014;179(7):807–23. https://doi.org/10.1093/aje/kwt334.
Gregory ECW, Martin JA, Argov EL, Osterman MJK. Assessing the quality of medical and health data from the 2003 birth certificate revision: results from New York city. Natl Vital Stat Rep. 2019;68(8):1–20.
Li J, Robbins S, Lamm SH. The influence of misclassification bias on the reported rates of congenital anomalies on the birth certificates for West Virginia--a consequence of an open-ended query. Birth Defects Res A Clin Mol Teratol. 2013;97(3):140–51. https://doi.org/10.1002/bdra.23119.
Honein MA, Paulozzi LJ, Watkins ML. Maternal smoking and birth defects: validity of birth certificate data for effect estimation. Public Health Rep. 2001;116(4):327–35. https://doi.org/10.1093/phr/116.4.327.
Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatr Perinat Epidemiol. 2005;19(5):368–76. https://doi.org/10.1111/j.1365-3016.2005.00660.x.
Yildiz S, Sezer S, Boyar H, Cece H, Ziylan SZ, Vural M, et al. Impact of passive smoking on uterine, umbilical, and fetal middle cerebral artery blood flows. Jpn J Radiol. 2011;29(10):718–24. https://doi.org/10.1007/s11604-011-0622-6.
Grewal J, Carmichael SL, Ma C, Lammer EJ, Shaw GM. Maternal periconceptional smoking and alcohol consumption and risk for select congenital anomalies. Birth Defects Res A Clin Mol Teratol. 2008;82(7):519–26. https://doi.org/10.1002/bdra.20461.
Anderson JE, Jorenby DE, Scott WJ, Fiore MC. Treating tobacco use and dependence: an evidence-based clinical practice guideline for tobacco cessation. Chest. 2002;121(3):932-41. https://doi.org/10.1378/chest.121.3.932.
Acknowledgements
We thank the US Centers for Disease Control and Prevention for making the data freely accessible.
Funding
BX was supported by Youth Team of Humanistic and Social Science of Shandong University (20820IFYT1902). The funders had no role in the study design or implementation; data collection, management, analysis, or interpretation; manuscript preparation, review, or approval; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
BX conceptualized the study. HW and LY analyzed the data. LLY drafted the first draft of manuscript. BX, MZ, YG, and PB critically revised the manuscript for key intellectual content. All authors approved the final version of the manuscript. BX is the guarantor. The corresponding author attests that all the listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The birth certificate data from the National Vital Statistics System are de-identified and do not include any protected health information. The data are publically available and exempt under the ethical board review of the corresponding author’s institution.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary results. Table S1. Associations of maternal smoking cessation during pregnancy with overall birth congenital anomalies. Table S2. Sensitivity analyses of associations between the timing of maternal cigarette smoking and birth congenital anomalies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, L., Wang, H., Yang, L. et al. Maternal cigarette smoking before or during pregnancy increases the risk of birth congenital anomalies: a population-based retrospective cohort study of 12 million mother-infant pairs. BMC Med 20, 4 (2022). https://doi.org/10.1186/s12916-021-02196-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-021-02196-x