Abstract
Background
The rapid process of research and development and lack of follow-up time post-vaccination aroused great public concern about the safety profile of COVID-19 vaccine candidates. To provide comprehensive overview of the safety profile of COVID-19 vaccines by using meta-analysis technique.
Methods
English-language articles and results posted on PubMed, Embase, Web of Science, PMC, official regulatory websites, and post-authorization safety surveillance data were searched through June 12, 2021. Publications disclosing safety data of COVID-19 candidate vaccines in humans were included. A meta-analysis of proportions was performed to estimate the pooled incidence and the pooled rate ratio (RR) of safety outcomes of COVID-19 vaccines using different platforms.
Results
A total of 87 publications with safety data from clinical trials and post-authorization studies of 19 COVID-19 vaccines on 6 different platforms were included. The pooled rates of local and systemic reactions were significantly lower among inactivated vaccines (23.7%, 21.0%), protein subunit vaccines (33.0%, 22.3%), and DNA vaccines (39.5%, 29.3%), compared to RNA vaccines (89.4%, 83.3%), non-replicating vector vaccines (55.9%, 66.3%), and virus-like particle vaccines (100.0%, 78.9%). Solicited injection-site pain was the most common local reactions, and fatigue and headache were the most common systemic reactions. The frequency of vaccine-related serious adverse events was low (< 0.1%) and balanced between treatment groups. Vaccine platforms and age groups of vaccine recipients accounted for much of the heterogeneity in safety profiles between COVID-19 vaccines. Reporting rates of adverse events from post-authorization observational studies were similar to results from clinical trials. Crude reporting rates of adverse events from post-authorization safety monitoring (passive surveillance) were lower than in clinical trials and varied between countries.
Conclusions
Available evidence indicates that eligible COVID-19 vaccines have an acceptable short-term safety profile. Additional studies and long-term population-level surveillance are strongly encouraged to further define the safety profile of COVID-19 vaccines.
Similar content being viewed by others
Introduction
The first coronavirus disease 2019 (COVID-19) case was reported in December 2019 [1]. As of June 15, 2021, more than 175 million COVID-19 cases, including over 3.8 million deaths, were reported in 221 countries and territories [2]. In response to the COVID-19 pandemic, 102 candidate vaccines on 10 platforms are in clinical development, and 15 vaccines have already been licensed or approved for emergency use [3].
These platforms can be classified either as traditional approaches that have previously resulted in licensed vaccines (e.g., inactivated, recombinant proteins, vectored vaccines), or as approaches that have never before been used for a licensed vaccine (e.g., RNA and DNA vaccines) [4]. Since no vaccine against coronaviruses had ever been licensed for use in humans before [4], the rapid process of research and development and limited follow-up time post-vaccination aroused great public concern about the safety profile of COVID-19 vaccine candidates, especially for new platforms such as RNA vaccines. Common reasons given for not intending to receive these vaccines included “concern about the safety of the vaccine in its development” and “potential side effects” [5]. As mass vaccination has progressed, more occurrences of adverse events following immunization (AEFI) have been reported, especially the rare AEFIs. Demonstrating and summarizing vaccine safety from clinical trials and post-authorization surveillance is critical for public confidence, and for enabling timely, evidence-based policy decisions for population-level use [6].
Current evidence about the safety of COVID-19 vaccines relies mainly on data from phase 1–3 randomized controlled trials and vaccine safety surveillance system in several countries. We found three reviews of the safety of COVID-19 vaccines [7,8,9], which combined study experimental groups, and did not examine the heterogeneity between vaccine platforms and participant age groups. Here, we conduct a rapid review and meta-analysis to summarize the safety data of COVID-19 vaccine candidates. We aim to comprehensively evaluate the rate of solicited, unsolicited, and serious AEFI in each clinical trial and to estimate the relative risk of AEFI by vaccine platform and participant age group. We also collected post-authorization surveillance data from around the world to look for uncommon and delayed onset reactions. This overview of the safety profile of COVID-19 vaccines will support responses to potential safety issues and inform decision-makers evaluating vaccination strategies around the globe.
Methods
Data sources and searches
We conducted a rapid review, adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses standards whenever possible. For the published results of clinical trials, we searched PubMed, Embase, and Web of Science for peer-reviewed articles, and PMC for preprints. We also used various combinations of the search terms “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)”, “coronavirus”, “vaccines”, “safety”, “adverse event”, and “side effect” to identify relevant regulatory documents disclosing experimental and surveillance data. In addition, we searched official websites and reports using terms for “COVID-19 vaccine safety monitor/monitoring/surveillance” and the names of countries with COVID-19 vaccine programs to identify their available safety surveillance data. Searches were conducted as of June 12, 2021. Details of the search strategy are presented in Additional file 1: Table S1.
Study selection
Three researchers (Q.W., X.C., X.B.) assessed eligible studies, conducted data extraction, and cross-checked. We looked for clinical trials and post-authorization reports that examined safety data of COVID-19 candidate vaccines, and included manuscripts published in peer-reviewed journals, preprints, and unpublished data disclosed by regulatory agencies. No restrictions were placed on publication date. We excluded study protocols, media news, commentaries, reviews, case reports, reports of non-human clinical trials, reports among specific populations (such as pregnant and lactating women, cancer patients, and other immunosuppressed persons), and abstracts of congress meetings or conference proceedings. We also excluded interim reports of clinical trials that did not clearly show safety data of specific COVID-19 candidate vaccines selected for further use, and reports on vaccines no longer under clinical evaluation. Post-authorization observational studies with sample sizes less than one thousand were excluded as well.
Data extraction and quality assessment
Information extracted from qualified studies included basic clinical trial details (e.g., study design, study location, phase, arms), characteristics of subjects (e.g., age group, proportion of subjects with underlying conditions, proportion of subjects seropositive at baseline), vaccine formulations (e.g., antigen content, adjuvant, injection route, vaccination schedule), the number of subjects in the safety dataset, and the rate of AEFI during the follow-up period. If data for the same subjects were presented in multiple publications, these data were only counted once. Due to phase 1 and 2 trials often including multiple differing experimental groups, we focused exclusively on safety data from experimental groups in phase 3 clinical trials. Any discrepancies were resolved by consensus or in consultation with a third researcher. Two researchers (Q.W., X.C.) assessed the methodological quality of studies using the Cochrane risk of bias tool [10]. Disagreements were resolved by consensus. Certainty of evidence was assessed by researchers according to the grading recommendations assessment, development and evaluation (GRADE) framework [11, 12].
Data synthesis and analysis
For the safety profile of COVID-19 vaccines in clinical trials, the primary outcomes were the proportion of vaccine recipients experiencing at least one AEFI and the rates of selected AEFI of COVID-19 candidate vaccines versus placebos. We specified severe versus mild-to-moderate AEFI in our extraction and analyzed these categories separately. For post-authorization safety data, we examined rates of AEFIs, serious adverse events (SAE), and adverse events of special interest (AESI).
We performed meta-analyses of proportions to estimate the pooled rate of safety outcomes of eligible COVID-19 vaccines (i.e., those both with reports of phase 1–3 trials and still under ongoing clinical evaluation) using different platforms. In addition, we estimated the pooled rate ratio (RR) using the rate of safety outcomes of COVID-19 vaccines in vaccinated groups divided by those in control groups in each study. We synthesized evidence for the following events: local reactions (e.g., injection-site pain, injection-site induration, tenderness, swelling), systemic reactions (e.g., nausea, vomiting, fever, rash, myalgia, arthralgia, headache, fatigue, malaise, diarrhea, cough), unsolicited AEFI by system organ class (SOC), AESI, serious AEFI, medically attended events, and study withdrawal of subjects as a result of AEFI and death. Definitions of the study outcomes and the grading scale of selected AEFI were provided in Additional file 1: Table S2-S3.
We explored the reasons for variations among eligible vaccines and examined whether rate of AEFI varied by vaccine platform, age group of participants and serostatus of participants against SARS-CoV-2 at baseline. For the purposes of stratifying safety data by age group, we defined younger adults as under 65 years of age and elderly as over 65 years of age. If the age group of the clinical trial was not completely consistent with our study, the safety data of the closest age stratification was extracted. We classified all participants in the Ad5 nCoV trials as younger adults, since no stratified analyses by age were performed and the proportion of the participants under 55 years old was reported as 86% [13, 14].
Based on a random-effect meta-analysis model, we used the inverse variance method to estimate pooled rate by platform, and the Clopper-Pearson method to calculate 95% confidence intervals [15, 16]. Heterogeneity tests (chi-squared test) with Higgins’ I2 statistics were used to determine the extent of variation between vaccines. Multivariate meta-regression models were used to determine the relative contribution of vaccine platform and age of participants to the rate of AEFI. All meta-analyses were performed using per-protocol data. Small study effects (potentially caused by publication bias) were assessed using funnel plots, and formally tested through the rank correlation test for those meta-analyses including more than 10 studies. All statistical analyses were done using R (version 4.0.2), using the “meta” package to conduct the meta-analysis. For all statistical tests, two tailed P-value less than 0.05 were considered statistically significant.
Results
Study characteristics
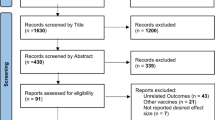
Our search identified a total of 7231 records after removal of duplicates (Fig. 1). After initial title/abstract screening, 157 articles were assessed for eligibility via full-text review. For the safety data among general population, 43 articles reporting on 19 vaccines of 6 different platforms [14, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] and 10 documents released by WHO (World Health Organization) [55,56,57,58,59], US Food and Drug Administration (FDA) [60,61,62] and UK Medicines & Healthcare products Regulatory Agency (MHRA) [63, 64] from clinical trials were included. A total of 123,540 study participants receiving COVID-19 vaccines and 97,944 participants receiving placebos were included in safety set of clinical trials. Post-authorization safety profiles were assessed through 3 reports released by the European Medicines Agency (EMA) [65,66,67], 20 reports including large-scale monitoring data [68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87], 11 observational studies [88,89,90,91,92,93,94,95,96,97,98], and 26 reports from countries’ national surveillance systems.
The main characteristics of included vaccines and relevant clinical trials are reported in Table 1 and Additional file 1: Table S4. The methodological quality of the included studies is reported in Additional file 1: Table S5-S6. Interim and/or final reports of phase 3 clinical trials were available for 8 vaccines: BNT162b2 (RNA vaccine manufactured by Pfizer and BioNTech), mRNA-1273 (RNA vaccine manufactured by Moderna), ChAdOx1-nCoV (non-replicating vector vaccine manufactured by Oxford and AstraZeneca), Gam-COVID-Vac (non-replicating vector vaccine manufactured by Gamaleya Research Institute), Ad26.COV2.S (non-replicating vector vaccine manufactured by Janssen Vaccines & Prevention B.V.), CoronaVac (inactivated vaccine manufactured by SinoVac), BBIBP-CorV and WBIP (inactivated vaccine manufactured by Sinopharm) (Table 1). AEFIs were mainly graded according to the latest scales issued by the US FDA and the China State Food and Drug Administration (CFDA), which are very similar except for a difference of 0.3–0.5 °C in the definition of fever (Table 1 and Additional file 1: Table S3). The funnel plots for safety outcomes including local reaction, systemic reaction, and medically attended events did not appear to be skewed, and the corresponding rank correlation test did not identify asymmetry (Additional file 1: Figure S1).
Local and systemic reactions in clinical trials
The pooled rates of local and systemic reactions, respectively, were significantly lower among inactivated vaccines (23.7%, 21.0%), protein subunit vaccine (33.0%, 22.3%), and DNA vaccines (39.5%, 29.3%) than the 3 other types of COVID-19 vaccines (RNA vaccines, 89.4%, 83.3%; non-replicating vector vaccines, 55.9%, 66.3%; virus-like particle vaccines, 100%, 78.9%) (Figs. 2 and 3). Among all vaccines, solicited injection-site pain and tenderness were the most common local reactions, and fatigue and headache were the most common systemic reactions (Additional file 1: Table S7). Compared to controls, the highest risk of local reactions (RR 4.5, 95% Cl 3.4–5.9) was observed for protein subunit vaccines (Table 2), and a higher risk of medically attended events (RR 1.7, 95% Cl 1.3–2.2) was observed for RNA vaccines (Table 2).
Unsolicited AEFI, serious AEFI, and AESI in phase 3 clinical trials
For RNA and non-replicating vector vaccines, most unsolicited AEFI and highest risk of unsolicited AEFI by SOC within 28 days post-vaccination were general disorders and administration site conditions, and the rate of common AEFI by SOC was significantly different among vaccines (Additional file 1: Figure S2-S3). The most common serious AEFI by SOC was infections and infestations, while the rate of identified serious AEFIs was similar in the overall vaccine and placebo groups (Additional file 1: Table S8).
For vaccine-related serious AEFI, there was no difference between vaccine and placebo groups (Table 2 and Additional file 1: Table S9). For adverse events of special interest (AESI), approximately 1% and 0.6% of participants vaccinated with RNA vaccines reported hypersensitivity and lymphadenopathy, respectively, and potential risk of hypersensitivity and lymphadenopathy was observed in RNA vaccines compared to control groups (Additional file 1: Table S10). It was worth noting that a total of 7 cases of Bell’s palsy were identified among 36,805 RNA vaccine recipients, indicating a numerical imbalance compared to placebo (Additional file 1: Table S10). There was no imbalance in the number of reported SAEs or grade 3 and over adverse events between vaccine and placebo groups for CoronaVac, BBIBP-CorV, and WBIP.
Age subgroup analysis based on data from clinical trials
The rate of the most common solicited symptoms was significantly higher among younger adults compared to the elderly (Additional file 1: Table S11). RNA vaccines had significantly higher rate of most common solicited reactions (e.g., injection-site pain, fatigue, headache) among younger adults compared to the other 5 platforms, regardless of the grades of adverse reactions (except overall injection-site pain which was also quite high for virus-like particle vaccines) (Additional file 1: Figures S4-S6). Meanwhile, the highest risk of these common systemic reactions (including fever) was observed in RNA vaccine recipients in this age group, compared to controls (Additional file 1: Table S12). While the highest rate of fever was shown in virus-like particle vaccines (Additional file 1: Figure S7). Differences between vaccine platforms and age groups of vaccine recipients accounted for much of the heterogeneity in safety profiles between COVID-19 vaccines (Additional file 1: Table S13). In addition, the rate of AEFI after CoronaVac was less frequent in children and adolescents than in younger adults, whereas the reverse was found with BNT162b2 (Additional file 1: Table S7).
Post-authorization observational studies
The most common AEFIs observed in post-authorization observational studies were local injection pain, fatigue, and headache (Additional file 1: Table S14). Adverse events were more frequent in females and subjects with a history of SARS-CoV-2 infection, and decreased with age (Additional file 1: Table S14). Several studies explored COVID-19 vaccination safety signals, including anaphylaxis, cerebral venous sinus thrombosis (CVST), thrombocytopenia, myocarditis, and pericarditis.
Post-authorization national safety surveillance
Nationwide safety surveillance data for COVID-19 vaccines (mainly BNT162b2, mRNA-1273, ChAdOx1, and nCoV-19) were reported in 26 countries (Additional file 1: Table S15). Most of this reporting was based on passive surveillance and thus not necessarily indicative of true rates or causal relationships with vaccination. Crude reporting rates of common AEFI and SAE varied between countries and were lower than that in clinical trials (Table 3, Additional file 1: Table S16). National rates of anaphylaxis ranged from 2.5 to 15.8 per million doses after mRNA COVID-19 vaccination and were estimated at 0.8 per million doses after Sinopharm vaccination and < 0.5 per million doses after Janssen vaccination (Additional file 1: Table S16).
Discussion
The pooled rates of local and systemic reactions were significantly different between vaccine platforms. Inactivated vaccines, protein subunit vaccines, and DNA vaccines had lower rates of local and systemic reactions compared to RNA vaccines, non-replicating vector vaccines, and virus-like particle vaccines. The safety profiles of BNT162b2, mRNA-1273, ChAdOx1-nCoV, Ad26.COV2.S, and CoronaVac were relatively benign in the elderly, and both the frequency and the intensity of local and systemic reactions decreased with age. The rates of SAE, including non-fatal serious AEFI and death, were similar in vaccine and placebo groups in clinical trials. Reporting rates of common AEFI after mass public vaccination were lower than in clinical trials. Several unexpected rare adverse events, which resulted in severe outcomes, have been noted in post-authorization surveillance.
Differences in safety profiles of vaccines must be considered in the context of efficacy. Both RNA vaccines (BNT162b2 and mRNA-1273) reported 95% [28] and 94% [99] vaccine efficacy, respectively (symptomatic PCR-confirmed cases were the primary clinical trial outcomes). This is substantially higher than the reported efficacy of other vaccine platforms. The efficacy of inactivated vaccines was reported as 78.1% for BBIBP-CorV [55] and 50.7% for CoronaVac [21]. Efficacy of Ad26.COV2.S against moderate to severe critical Covid-19 with onset at least 14 days after administration was 66.9% [37]. Overall efficacy of ChAdOx1-nCoV in preventing symptomatic COVID-19 across both the low dose and standard dose groups was reported as 70.4% [43]. The efficacy of Gam-COVID-Vac, another non-replicating vector vaccine, was 91.6% [45]. Based on the current evidence, RNA vaccines have both higher rates of adverse reactions and higher efficacy. Due to the relative mild and transient nature of most of these reactions, RNA vaccines should be considered an excellent option to protect against COVID-19, especially in the absence of other viable candidates with similar efficacy. In addition to safety and efficacy, vaccine candidates must also be assessed in the context of the risk of disease, to determine whether each vaccine supports a favorable benefit-risk ratio or not. Such a determination is undoubtedly more important than comparing safety and efficacy between vaccine candidates as long as vaccine supply is limited and disease is prevalent.
Direct comparisons between efficacy data should also be interpreted with caution due to the inconsistency of environmental risk, endpoints, and statistical methods between studies. Current efficacy data show that all authorized vaccines exceed the 50% threshold set by WHO [100], indicating they prevent substantial disease, especially severe cases. Authorized COVID-19 vaccines can prevent a large proportion of symptomatic cases, hospitalizations, severe diseases, and death [101, 102]. Mass vaccination efforts can prevent disease, save lives, reduce pressure on the medical system, and hopefully eventually relieve the need for many of the non-pharmaceutical interventions currently used to contain the epidemic, reopen economies, and allow a return to normalcy worldwide.
As of May 9, 2021, about 0.6 billion people around the world had been vaccinated with at least one dose of COVID-19 vaccines, accounting for about 7.8% of the world’s population [103]. This mass vaccination should allow for the identification of more uncommon and rare AEFI. According to the Vaccine Adverse Event Reporting System (VAERS) and V-safe system of the US Centers for Disease Control and Prevention (CDC) [104], the rates of non-serious AEFI after public administration of BNT162b2 and mRNA-1273 were similar to the clinical trials. Anaphylaxis, a severe, life-threatening allergic reaction, typically occurs at a rate of approximately 1 case per million doses for most vaccines [105]; the rates of anaphylaxis associated with BNT162b2 and mRNA-1273 appear to be approximately 4.7 times and 2.5 times higher than this, respectively, although no cases progressed to serious long-term outcomes thanks to their prompt treatment [106]. Variations in the incidence of anaphylaxis between countries are to be expected, as the numbers vaccinated in most countries to date are relatively small compared with the USA, and the reporting rates of AEFI from passive surveillance are biased. A causal link of thrombosis and thrombocytopenia with adenoviral vector vaccines (ChAdOx1 nCoV-19 and Ad26.COV2.S) was noted after mass public vaccination, including several deaths and severe outcomes [107,108,109,110]. While rare side effects should not derail vaccination efforts [111], a thorough risk-benefit analysis is required. Several studies have explored the safety profile of two mRNA vaccines (BNT162b2 and mRNA-1273) in HIV-positive populations [112, 113], immunosuppressive patients [114, 115], and pregnant women [116], revealing no evidence of unexpected serious adverse events. Further evaluation of the benefit-risk profile is warranted in these specific populations.
According to the Chinese government [117], 333 million doses have been administrated as of May 10, 2021 (mainly with BBIBP-CorV and CoronaVac), and the rate of overall AEFI was close to the previous inactivated vaccines given routinely, while the rate of allergic reactions and other non-fatal serious AEFI was about 2 cases per million doses [21]. No major safety concerns have been identified so far. Safety data on Russian vaccines need to be disclosed further so that safety signals can be identified and appropriate risk minimization measures quickly implemented.
The safety profiles of COVID-19 vaccines are still incomplete, even for those currently in use. The safety and efficacy of COVID-19 vaccines in certain subpopulations, such as children and adolescents, pregnant woman, and people with multiple underlying conditions, have not yet been fully studied. Although crude reporting rates of AEFIs from post-authorization safety monitoring have so far been lower than in clinical trials, adverse reactions that are uncommon or have delayed onset require extended post-authorization study to detect. Investigation of safety signals, a lack of epidemiological tools for active surveillance, obstacles at the national regulatory authority level, and a lack of information sharing between countries are still major challenges for most countries. Pharmacovigilance mechanisms must be put in place, with all the necessary training, especially in low- and middle-income countries [118]. Further study will strengthen and expand upon our knowledge in these areas and enable the refinement of vaccine recommendations and injury compensation programs. Safety issues noted in mass vaccination may have a deleterious impact on the global vaccine supply and the already fragile confidence in vaccines. The benefits of vaccines still outweigh the risks at present. Government agencies and vaccine developers should continue to take action to encourage vaccination and reduce public vaccine hesitancy.
Our analysis has several limitations. Firstly, we only included data reported at the study level, due to limited access to individual-level data. Secondly, there are factors we did not include in the meta-analysis, such as seropositivity against SARS-CoV-2 at baseline and underlying conditions, so the potential effects of such heterogeneity were not quantitatively assessed. Thirdly, in the clinical trials for BNT162b2 and ChAdOx1-nCoV, age groups were divided at 55 years of age, which differed from our subgroup analysis of age divided at 65 years of age. Finally, although we included currently available post-authorization safety monitoring data, such monitoring programs are still in their infancy and often rely on a mix of active and passive surveillance.
Conclusions
In conclusion, the available evidence indicates that eligible COVID-19 vaccines have an acceptable short-term safety profile. Additional studies and long-term population-level surveillance are strongly encouraged to further augment the safety profile of COVID-19 vaccines. This should include essential active vaccine safety surveillance systems, enhanced monitoring of early COVID-19 vaccine recipients and passive surveillance, standardized reporting and pharmacovigilance mechanisms, platforms in hospitals to evaluate the vaccine-specific antibody correlates, and cross-reactivity to other strains. All reports of suspected adverse reactions should be investigated and warning signals rapidly evaluated, to allow implementation of appropriate risk minimization measures and update the benefit/risk ratio of vaccination.
Availability of data and materials
The datasets used and analyzed during the current study are available in appendix.
Abbreviations
- AEFI:
-
Adverse events following immunization
- AESI:
-
Adverse events of special interest
- CDC:
-
Centers for Disease Control and Prevention
- CFDA:
-
China State Food and Drug Administration
- COVID-19:
-
Coronavirus diseases 2019
- CVST:
-
Cerebral venous sinus thrombosis
- EMA:
-
European Medicines Agency
- GRADE:
-
Grading Recommendations Assessment, Development and Evaluation
- RR:
-
Rate ratio
- SAE:
-
Serious adverse events
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SOC:
-
System organ class
- UK MHRA:
-
UK Medicines & Healthcare products Regulatory Agency
- US FDA:
-
US Food and Drug Administration
- VAERS:
-
Vaccine Adverse Event Reporting System
- WHO:
-
World Health Organization
References
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. https://doi.org/10.1056/NEJMoa2001017.
World Health Organization situation report. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed 8 May 2021.
Draft landscape and tracker of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed 11 May 2021.
Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–27. https://doi.org/10.1038/s41586-020-2798-3.
Dodd RH, Pickles K, Nickel B, Cvejic E, Ayre J, Batcup C, et al. Concerns and motivations about COVID-19 vaccination. Lancet Infect Dis. 2021;21(2):161–63.
Lee GM, Romero JR, Bell BP. Postapproval vaccine safety surveillance for COVID-19 vaccines in the US. JAMA. 2020;324(19):1937–8. https://doi.org/10.1001/jama.2020.19692.
Yuan P, Ai P, Liu Y, Ai Z, Wang Y, Cao W, Xia X, Zheng JC. Safety, Tolerability, and immunogenicity of COVID-19 vaccines: a systematic review and meta-analysis. medRxiv. 2020.11.03.20224998. https://doi.org/10.1101/2020.11.03.20224998.
Hernandez AF, Calina D, Poulas K, Docea AO, Tsatsakis AM. Safety of COVID-19 vaccines administered in the EU: should we be concerned? Toxicol Rep. 2021;8:871–9. https://doi.org/10.1016/j.toxrep.2021.04.003.
Yan ZP, Yang M, Lai CL. COVID-19 vaccines: a review of the safety and efficacy of current clinical trials. Pharmaceuticals (Basel). 2021;14(5):406.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(2):d5928. https://doi.org/10.1136/bmj.d5928.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. https://doi.org/10.1136/bmj.39489.470347.AD.
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4–13.
Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–54. https://doi.org/10.1016/S0140-6736(20)31208-3.
Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–88.
Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–13. https://doi.org/10.1093/biomet/26.4.404.
Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Statistics in medicine. 1998;17(8):857–72. https://doi.org/10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E.
Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infectious Dis. 2021;21(1):39–51.
Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324(10):951–60. https://doi.org/10.1001/jama.2020.15543.
Bihua Han YS, Changgui Li, Wanqi Yang, Qingxia Ma, Zhiwei Jiang, et al. Safety, tolerability and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a randomised, double-blind, and placebo-controlled, phase 1/2 clinical trial. Available at SSRN: https://ssrn.com/abstract=3820545 or https://doi.org/10.2139/ssrn.3820545.
Bueno SM, Abarca K, González PA, Gálvez NMS, Soto JA, Duarte LF, Schultz BM, Pacheco GA, González LA, Vázquez Y, et al. Interim report: safety and immunogenicity of an inactivated vaccine against SARS-CoV-2 in healthy chilean adults in a phase 3 clinical trial. medRxiv. https://doi.org/10.1101/2021.03.31.21254494.
Ricardo Palacios APB. Camila Santos Nascimento Albuquerque, Elizabeth González Patiño, Joane do Prado Santos, Mônica Tilli Reis Pessoa Conde et al: Efficacy and safety of a COVID-19 inactivated vaccine in healthcare professionals in Brazil: The PROFISCOV study Ssrn; 2021.
Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803–12. https://doi.org/10.1016/S1473-3099(20)30987-7.
Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–92. https://doi.org/10.1016/S1473-3099(20)30843-4.
Che Y, Liu X, Pu Y, Zhou M, Zhao Z, Jiang R, et al. Randomized, double-blinded and placebo-controlled phase II trial of an inactivated SARS-CoV-2 vaccine in healthy adults. Clin Infect Dis. 2020;ciaa1703. https://doi.org/10.1093/cid/ciaa1703.
Pu J, Yu Q, Yin Z, Zhang Y, Li X, Yin Q, et al. The safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in Chinese adults aged 18-59 years: a phase I randomized, double-blinded, controlled trial. Vaccine. 2021;39(20):2746–54. https://doi.org/10.1016/j.vaccine.2021.04.006.
Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21(7):950–61. https://doi.org/10.1016/S1473-3099(21)00070-0.
Pan HX, Liu JK, Huang BY, Li GF, Chang XY, Liu YF, et al. Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy adults: randomized, double-blind, and placebo-controlled phase 1 and phase 2 clinical trials. Chinese Med J. 2021;134(11):1289–98. https://doi.org/10.1097/CM9.0000000000001573.
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. https://doi.org/10.1056/NEJMoa2034577.
Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv. 2020;2020.12.09.20245175. https://doi.org/10.1101/2020.12.09.20245175.
Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–50. https://doi.org/10.1056/NEJMoa2027906.
Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–38. https://doi.org/10.1056/NEJMoa2028436.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. https://doi.org/10.1056/NEJMoa2035389.
Chu L, McPhee R, Huang W, Bennett H, Pajon R, Nestorova B, et al. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39(20):2791–9. https://doi.org/10.1016/j.vaccine.2021.02.007.
Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. New Engl J Med. 2020;383(20):1920–31.
Kremsner P, Mann P, Bosch J, Fendel R, Gabor JJ, Kreidenweiss A, et al. Phase 1 assessment of the safety and immunogenicity of an mRNA- lipid nanoparticle vaccine candidate against SARS-CoV-2 in human volunteers. medRxiv. 2020;2020.11.09.20228551. https://doi.org/10.1101/2020.11.09.20228551.
Zhu F-C, Li Y-H, Guan X-H, Hou L-H, Wang W-J, Li J-X, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet (London). 2020;395(10240):1845–54.
Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–201. https://doi.org/10.1056/NEJMoa2101544.
Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384(19):1824–35. https://doi.org/10.1056/NEJMoa2034201.
Barrett JR, Belij-Rammerstorfer S, Dold C, Ewer KJ, Folegatti PM, Gilbride C, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27(2):279–88.
Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):466.
Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384(20):1885–98. https://doi.org/10.1056/NEJMoa2102214.
Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–93.
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. https://doi.org/10.1016/S0140-6736(20)32661-1.
Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–91. https://doi.org/10.1016/S0140-6736(21)00432-3.
Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–81.
Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Shcheblyakov DV, Dzharullaeva AS, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396(10255):887–97.
Ward BJ, Gobeil P, Séguin A, Atkins J, Boulay I, Charbonneau P-Y, Couture M, D’Aoust M-A, Dhaliwall J, Finkle C, et al. Phase 1 trial of a candidate recombinant virus-like particle vaccine for Covid-19 disease produced in plants. medRxiv. 2020;2020.11.04.20226282. https://doi.org/10.1101/2020.11.04.20226282.
Ryzhikov AB, Ryzhikov EA, Bogryantseva MP, Usova SV, Danilenko ED, Nechaeva EA, et al. A single blind, placebo-controlled randomized study of the safety, reactogenicity and immunogenicity of the “Epivaccorona” vaccine for the prevention of Covid-19, in volunteers aged 18-60 years (phase I-Ii). Infektsiya I Immunitet. 2021;11(2):283–96.
Mammen MP, Tebas P, Agnes J, Giffear M, Kraynyak KA, Blackwood E, Amante D, Reuschel EL, Purwar M, Christensen-Quick A, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of a randomized, blinded, placebo-controlled, phase 2 clinical trial in adults at high risk of viral exposure. medRxiv. 2021;2021.05.07.21256652. https://doi.org/10.1101/2021.05.07.21256652.
Tebas P, Yang S, Boyer JD, Reuschel EL, Patel A, Christensen-Quick A, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2021;31:100689. https://doi.org/10.1016/j.eclinm.2020.100689.
Formica N, Mallory R, Albert G, Robinson M, Plested JS, Cho I, et al. Evaluation of a SARS-CoV-2 vaccine NVX-CoV2373 in younger and older adults. medRxiv. 2021;2021.02.26.21252482. https://doi.org/10.1101/2021.02.26.21252482.
Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383(24):2320–32. https://doi.org/10.1056/NEJMoa2026920.
Richmond P, Hatchuel L, Dong M, Ma B, Hu B, Smolenov I, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397(10275):682–94.
Yang S, Li Y, Dai L, Wang J, He P, Li C, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;S1473-3099(21)00127-4. https://doi.org/10.1016/S1473-3099(21)00127-4.
World Health Organization. Evidence Assessment: Sinopharm/BBIBP COVID-19 vaccine. https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/2_sage29apr2021_critical-evidence_sinopharm.pdf. Accessed 15 May 2021.
World Health Organization. Evidence Assessment: Sinovac/CoronaVac COVID-19 vaccine. https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/5_sage29apr2021_critical-evidence_sinovac.pdf?sfvrsn=2488098d_5. Accessed 15 May 2021.
World Health Organization. Evidence Assessment: ChAdOx1-S [recombinant] vaccine (AZD1222) vaccine against COVID-19 developed by Oxford University and Astra Zeneca. https://cdn.who.int/media/docs/default-source/immunization/sage/2021/february/3---sage_8-feb_evidence-assessment_azd1222_-final.pdf?sfvrsn=ce0bcfb1_8. Accessed 15 May 2021.
World Health Organization. Evidence Assessment: Pfizer-BioNTech COVID-19 vaccine. https://www.who.int/docs/default-source/immunization/sage/2021/january/4-evidence-assessment5-jan-2021-final.pdf?sfvrsn=cf627b70_9. Accessed 15 May 2021.
World Health Organization. Evidence Assessment: mRNA-1273 COVID-19 vaccine. https://cdn.who.int/media/docs/default-source/immunization/sage/2021/january/3-evidence-assessment_covid19_moderna_revised_final.pdf?sfvrsn=61ab4400_8. Accessed 15 May 2021.
Vaccines and Related Biological Products Advisory Committee Meeting. FDA Briefing Document-Pfizer-BioNTech COVID-19 Vaccine. https://www.fda.gov/media/144245/download. Accessed 1 Mar 2021.
Vaccines and Related Biological Products Advisory Committee Meeting. FDA Briefing Document-Moderna COVID-19 Vaccine. https://www.fda.gov/media/144434/download. Accessed 1 Mar 2021.
Vaccines and Related Biological Products Advisory Committee Meeting. FDA Briefing Document-Janssen Ad26.COV2.S Vaccine. https://www.fda.gov/media/146217/download. Accessed 1 Mar 2021.
Medicine & Healthcare products, Regulatory Agency, UK. Public Assessment Report for COVID-19 Vaccine AstraZeneca. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/963928/UKPAR_COVID_19_Vaccine_AstraZeneca_23.02.2021.pdf. Accessed 20 Jan 2021.
Medicine & Healthcare products, Regulatory Agency, UK. Public Assessment Report for COVID-19 Vaccine Pfizer/BioNTech. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/997584/COVID-19_mRNA_Vaccine_BNT162b2__UKPAR___PFIZER_BIONTECH_ext_of_indication_11.6.2021.pdf. Accessed 20 Jan 2021.
European Medicines Agency. COVID-19 vaccine safety update-COMIRNATY. https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-comirnaty-29-march-2021_en.pdf. Accessed 9 Apr 2021.
European Medicines Agency. COVID-19 vaccine safety update-VAXZEVRIA. https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-vaxzevria-previously-covid-19-vaccine-astrazeneca-29-march-2021_en.pdf. Accessed 9 Apr 2021.
European Medicines Agency. COVID-19 vaccine safety update - COVID-19 VACCINE MODERNA. https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-spikevax-previously-covid-19-vaccine-moderna-29-march-2021_en.pdf. Accessed 9 Apr 2021.
Bardenheier BH, Gravenstein S, Blackman C, Gutman R, Sarkar IN, Feifer RA et, al. Adverse events following mRNA SARS-CoV-2 vaccination among U.S. nursing home residents. Vaccine 2021;39(29):3844–51. https://doi.org/10.1016/j.vaccine.2021.05.088.
Bikdeli B, Chatterjee S, Arora S, Monreal M, Jimenez D, Krumholz HM, et al. Cerebral venous sinus thrombosis in the US population, after adenovirus-based SARS-CoV-2 vaccination, and after COVID-19. J Am Coll Cardiol. 2021;S0735-1097(21)05194-9. https://doi.org/10.1016/j.jacc.2021.06.001.
Chen G, Li X, Sun M, Zhou Y, Yin M, Zhao B, et al. COVID-19 mRNA vaccines are generally safe in the short term: a vaccine vigilance real-world study says. Front Immunol. 2021;12:669010. https://doi.org/10.3389/fimmu.2021.669010.
de Simone G, Stranges S, Gentile I. Incidence of cerebral venous thrombosis and covid-19 vaccination. Eur Heart J Cardiovasc Pharmacother. 2021;pvab036. https://doi.org/10.1093/ehjcvp/pvab036.
Formeister EJ, Chien W, Agrawal Y, Carey JP, Stewart CM, Sun DQ. Preliminary analysis of association between COVID-19 vaccination and sudden hearing loss ising US Centers for Disease Control and Prevention vaccine adverse events reporting system data. JAMA Otolaryngol Head Neck Surg. 2021;147(7):674–6. https://doi.org/10.1001/jamaoto.2021.0869.
Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortality Wkly Rep. 2021;70(8):283–8. https://doi.org/10.15585/mmwr.mm7008e3.
Gras-Champel V, Liabeuf S, Baud M, Albucher J-F, Benkebil M, Boulay C, et al. Atypical thrombosis associated with VaxZevria (AstraZeneca) vaccine: data from the French Network of Regional Pharmacovigilance Centres. Therapie. 2021;S0040-5957(21)00130-X. https://doi.org/10.1016/j.therap.2021.05.007.
Gringeri M, Mosini G, Battini V, Cammarata G, Guarnieri G, Carnovale C, et al. Preliminary evidence on the safety profile of BNT162b2 (Comirnaty): new insights from data analysis in EudraVigilance and adverse reaction reports from an Italian health facility. Hum Vaccines Immunother. 2021:1–3. https://doi.org/10.1080/21645515.2021.1917236.
Hause AM, Gee J, Johnson T, Jazwa A, Marquez P, Miller E, et al. Anxiety-related adverse event clusters after Janssen COVID-19 vaccination - five U.S. mass vaccination sites, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):685–8. https://doi.org/10.15585/mmwr.mm7018e3.
McMurry R, Lenehan P, Awasthi S, Silvert E, Puranik A, Pawlowski C, et al. Real-time analysis of a mass vaccination effort confirms the safety of FDA-authorized mRNA vaccines for COVID-19 from Moderna and Pfizer/BioNtech. medRxiv. 2021;2021.02.20.21252134. https://doi.org/10.1101/2021.02.20.21252134.
Pawlowski C, Rincón-Hekking J, Awasthi S, Pandey V, Lenehan P, Venkatakrishnan AJ, Bade S, O’Horo JC, Virk A, Swift MD, et al. Cerebral venous sinus thrombosis (CVST) is not significantly linked to COVID-19 vaccines or non-COVID vaccines in a large multi-state US health system. medRxiv. 2021;2021.04.20.21255806
Pottegard A, Lund LC, Karlstad O, Dahl J, Andersen M, Hallas J, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. https://doi.org/10.1136/bmj.n1114.
Renoud L, Khouri C, Revol B, Lepelley M, Perez J, Roustit M, et al. Association of facial paralysis with mRNA COVID-19 vaccines: a disproportionality analysis using the World Health Organization Pharmacovigilance Database. JAMA Intern Med. 2021;e212219. https://doi.org/10.1001/jamainternmed.2021.2219.
Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, et al. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine - United States, March-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680–4. https://doi.org/10.15585/mmwr.mm7018e2.
Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14-23, 2020. MMWR. 2021;70(2):46–51.
Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine — United States, December 21, 2020–January 10, 2021. MMWR. 2021;21(3):1326–31. https://doi.org/10.1111/ajt.16517.
Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325(8):780–1.
Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–2.
Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–7. https://doi.org/10.1038/s41591-021-01408-4.
Vanijcharoenkarn K, Lee FE, Martin L, Shih J, Sexton ME, Kuruvilla ME. Immediate reactions following the first dose of the SARS-CoV2 mRNA vaccines do not preclude second dose administration. Clin Infect Dis. 2021;ciab448. https://doi.org/10.1093/cid/ciab448.
Zhang MX, Zhang TT, Shi GF, Cheng FM, Zheng YM, Tung TH, et al. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021;1–8. https://doi.org/10.1080/14760584.2021.1925112.
Wang G, Zhu L, Zhu Y, Ye Q, Yu X, Fu M, et al. Safety survey by clinical pharmacists on COVID-19 vaccination from a single center in China. Hum Vaccines Immunother. 2021;1–5. https://doi.org/10.1080/21645515.2021.1913964.
Song JY, Cheong HJ, Kim SR, Lee SE, Kim SH, Noh JY, et al. Early safety monitoring of COVID-19 vaccines in healthcare workers. J Korean Med Sci. 2021;36(15):e110. https://doi.org/10.3346/jkms.2021.36.e110.
Montalti M, Soldà G, Di Valerio Z, Salussolia A, Lenzi J, Forcellini M, et al. ROCCA study protocol and interim analysis on safety of Sputnik V vaccine (Gam-COVID-Vac) in the Republic of San Marino: an observational study using active surveillance. medRxiv. 2021;2021.05.03.21256509. https://doi.org/10.1101/2021.05.03.21256509.
Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939-949. https://doi.org/10.1016/S1473-3099(21)00224-3.
Konu YR, Gbeasor-Komlanvi FA, Yerima M, Sadio A, Tchankoni MK, Zida-Compaore WIC, Nayo-Apetsianyi J, Afanvi KA, Agoro S, Salou M, et al. Prevalence of severe adverse events in health professionals after receiving the first dose of the COVID-19 vaccination in Togo, March 2021. medRxiv. 2021;2021.04.20.21254863.
Kim SH, Wi YM, Yun SY, Ryu JS, Shin JM, Lee EH, et al. Adverse events in healthcare morkers after the first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccination: a single center experience. J Korean Med Sci. 2021;36(14):e107.
Kataria S, Sharma P, Deswal V, Kumar K, Singh M, Alam S, et al. A real world evaluation of the safety and immunogenicity of the Covishield vaccine, ChAdOx1 nCoV- 19 corona virus vaccine (recombinant) in health care workers (HCW) in National Capital Region (NCR) of India: a preliminary report. medRxiv. 2021;2021.04.14.21255452.
d'Arminio Monforte A, Tavelli A, Perrone PM, Za A, Razzini K, Tomasoni D, et al. Association between previous infection with SARS CoV-2 and the risk of self-reported symptoms after mRNA BNT162b2 vaccination: data from 3,078 health care workers. EClinicalMed. 2021;36:100914. https://doi.org/10.1016/j.eclinm.2021.100914.
Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325(21):2201–2.
Bae S, Lee YW, Lim SY, Lee JH, Lim JS, Lee S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36(17):e115. https://doi.org/10.3346/jkms.2021.36.e115.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. https://doi.org/10.1056/NEJMoa2035389.
World Health Organization. WHO target product profiles for COVID-19 vaccines. https://www.who.int/publications/m/item/whotarget-product-profiles-for-covid-19-vaccines. Accessed 11 May 2021
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–23. https://doi.org/10.1056/NEJMoa2101765.
William Daniel MN, John Warner, Daniel K. Podolsky: Early evidence of the effect of SARS-CoV-2 vaccine at one medical center. N Engl J Med. 2021;384(20):1962–3. https://doi.org/10.1056/NEJMc2102153.
WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed 13 May 2021.
Zhang; JGPMJSGMCRLTMNNSMTCLMNLB: First month of COVID-19 vaccine safety monitoring — United States, December 14, 2020–January 13, 2021. Morb Mortal Wkly Rep. 2021;70(8):283–88. https://doi.org/10.15585/mmwr.mm7008e3.
McNeil MM, DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018;141(2):463–72.
Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–2. https://doi.org/10.1001/jama.2021.1967.
Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–101. https://doi.org/10.1056/NEJMoa2104840.
Sadoff J, Davis K, Douoguih M. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination - response from the manufacturer. N Engl J Med. 2021;384(20):1965–6. https://doi.org/10.1056/NEJMc2106075.
Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–30.
See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448–56. https://doi.org/10.1001/jama.2021.7517.
Hunter PR. Thrombosis after covid-19 vaccination these rare events must not derail vaccination efforts. BMJ. 2021;373:n958.
Itzchak L, Anat W, Vladyslav L, Asaf B, Victoria I, Liraz O, et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in People Living with HIV-1. Available at SSRN: https://ssrn.com/abstract=3829650 or https://doi.org/10.2139/ssrn.3829650.
Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV. 2021;S2352-3018(21)00103-X. https://doi.org/10.1016/S2352-3018(21)00103-X.
Cohen D, Krauthammer SH, Wolf I, Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [18F]FDG PET-CT and relevance to study interpretation. Eur J Nuclear Med Mol Imaging. 2021;48(6):1854–63. https://doi.org/10.1007/s00259-021-05314-2.
Boyarsky BJ, Ou MT, Greenberg RS, Teles AT, Werbel WA, Avery RK, et al. Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021;105(5):e56–7. https://doi.org/10.1097/TP.0000000000003654.
Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–82. https://doi.org/10.1056/NEJMoa2104983.
The State Council of the People's Republic of China. http://www.gov.cn/xinwen/2021-05/11/content_5606725.htm. Accessed 13 May 2021.
Petousis-Harris H. Assessing the safety of COVID-19 vaccines: a primer. Drug Saf. 2020;43(12):1205–10. https://doi.org/10.1007/s40264-020-01002-6.
Acknowledgements
We thank Wei Wang, Xinhua Chen, and Xiaowei Deng from the Fudan University for statistical expertise.
Funding
The study was supported by grants from the National Science Fund for Distinguished Young Scholars (No. 81525023) and the National Institute for Health Research (NIHR) (grant no. 16/137/109) using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the UK Department of Health and Social Care. No other relationships or activities that could appear to have influenced the submitted work. The sponsors have no role in the study design; the collection, analysis, or interpretation of data; the writing of the report; or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
H.Y. conceived, designed, and supervised the study. Q.W, X.C., K.D., X.B., and T.Z. did the literature search, data extraction, and data collection. Q.W., M.D., X.C., and K.D. analyzed the data and prepared the tables and figures. Q.W. prepared the first draft of the manuscript. M.D., D.S., and H.Y. commented on the data and its interpretation and revised the content critically. All authors contributed to review and revision and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
H.Y. has received research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, and Shanghai Roche Pharmaceutical Company. M.D. has received research support from Walgreen Company and Merck. D.S. has received consulting or grant funding from Merck and Janssen. None of those research funding is related to development of COVID-19 vaccines. All other authors report no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Search strategy. Table S2. Definitions of outcomes. Table S3. Grading scale for selected clinical abnormalities. Table S4. Brief description of included COVID-19 candidate vaccines and platforms. Table S5. Methodological characteristics of included studies of clinical trials: risk of bias on specific items. Table S6. Methodological characteristics of included studies of post-marketing studies: methodological index for non-randomized studies (MINORS) score. Table S7. Raw data of common AEFIs in the total safety set for candidate vaccines in clinical trials among general population (n/N, %). Table S8. Serious adverse events of COVID-19 vaccines by system organ class in phase 3 clinical trials (n/N, %). Table S9. Serious safety outcomes of vaccines in phase 3 clinical trials. Table S10. Summary of unbalanced AESIs between intervention and control groups in phase 3 clinical trials of mRNA vaccines. Table S11. Age group comparison of most common adverse reactions and fever within 7 days post-vaccination between younger adults and elderly (n/N, %). Table S12. Meta-analyses for comparing the rates of most common AEFI of COVID-19 candidate vaccines versus placebo or control vaccine by platform among younger adults (18-65 years old). Table S13. Multivariate meta-regression determining factors accounting for the heterogeneity of safety profile. Table S14. Summary of post-authorization active surveillance studies among general population. Table S15. Sources of nationwide safety surveillance data. Table S16 Summary of COVID-19 vaccine safety surveillance data. Figure S1. Funnel plots to assess publication bias. Figure S2. Forest plot of estimated results from meta-analysis of unsolicited adverse events by common system organ class (SOC). Figure S3. Comparing rates of unsolicited adverse events by common system organ class (SOC) of COVID-19 vaccines versus placebos. Figure S4. Forest plot of estimated results from meta-analysis of local injection pain in adults from clinical trials. Figure S5. Forest plot of estimated results from meta-analysis of fatigue in adults from clinical trials. Figure S6. Forest plot of estimated results from meta-analysis of headache in adults from clinical trials. Figure S7. Forest plot of estimated results from meta-analysis of fever in adults from clinical trials.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, Q., Dudley, M.Z., Chen, X. et al. Evaluation of the safety profile of COVID-19 vaccines: a rapid review. BMC Med 19, 173 (2021). https://doi.org/10.1186/s12916-021-02059-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-021-02059-5