Abstract
Background
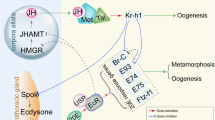
The zinc-finger transcription factor Krüppel-homolog 1 (Kr-h1) exerts a dual regulatory role during insect development by preventing precocious larval/nymphal metamorphosis and in stimulating aspects of adult reproduction such as vitellogenesis. However, how Kr-h1 functions both as a transcriptional repressor in juvenile metamorphosis and an activator in adult reproduction remains elusive. Here, we use the insect Locusta migratoria to dissect the molecular mechanism by which Kr-h1 functions as activator and repressor at these distinct developmental stages.
Results
We report that the kinase PKCα triggers Kr-h1 phosphorylation at the amino acid residue Ser154, a step essential for its dual functions. During juvenile stage, phosphorylated Kr-h1 recruits a corepressor, C-terminal binding protein (CtBP). The complex of phosphorylated Kr-h1 and CtBP represses the transcription of Ecdysone induced protein 93F (E93) and consequently prevents the juvenile-to-adult transition. In adult insects, phosphorylated Kr-h1 recruits a coactivator, CREB-binding protein (CBP), and promotes vitellogenesis by inducing the expression of Ribosomal protein L36. Furthermore, Kr-h1 phosphorylation with the concomitant inhibition of E93 transcription is evolutionarily conserved across insect orders.
Conclusion
Our results suggest that Kr-h1 phosphorylation is indispensable for the recruitment of transcriptional cofactors, and for its anti-metamorphic and vitellogenic actions in insects. Our data shed new light on the understanding of Kr-h1 regulation and function in JH-regulated insect metamorphosis and reproduction.
Similar content being viewed by others
Background
Juvenile hormone (JH), an arthropod-specific sesquiterpenoid secreted by the corpora allata, plays a central role in insect metamorphosis and reproduction. In juvenile stages, JH maintains the larval/nymphal status by suppressing the metamorphic action of the steroid hormone 20-hydroxyecdysone (20E) [1,2,3,4,5]. In adult insects, JH stimulates aspects of reproduction including post-emergence development, vitellogenesis, and oogenesis [6, 7]. Krüppel-homolog 1 (Kr-h1) is a primary JH early-inducible gene coding for a zinc-finger transcription factor that mediates both anti-metamorphic and vitellogenic actions of JH [8,9,10,11]. Kr-h1 prevents immature larvae from precocious larval-pupal metamorphosis by inhibiting the transcription of pupa-specifier gene Broad-complex (Br-C) in holometabolous insects [12,13,14]. Kr-h1 also prevents precocious nymphal-adult or pupal-adult transition by inhibiting the expression of Ecdysone induced protein 93F (E93), an adult-specifier gene in both hemimetabolous and holometabolous insects [14,15,16,17] in the context of the MEKRE93 pathway, the general regulatory axis of insect metamorphosis [8, 15]. In addition, Kr-h1 suppresses 20E biosynthesis by inhibiting the expression of steroidogenic enzyme gene Spok in prothoracic glands of the fruit fly Drosophila melanogaster and thus prevents precocious larval-pupal transformation [18]. Stimulation of female reproduction by Kr-h1 is reported in a variety of insect species [9, 10, 19]. RNAi-mediated knockdown of Kr-h1 resulted in blocked vitellogenesis and impaired egg development in the migratory locust Locusta migratoria, the rice borer Chilo suppressalis, the oriental fruit fly Bactrocera dorsalis, the cotton bollworm Helicoverpa armigera, and the brown planthopper Nilaparvata lugens [20,21,22,23,24]. In the mosquito Aedes aegypti, Kr-h1 regulates the developmental phase in preparation for competence acquisition for blood feeding, as well as subsequent vitellogenesis and egg development [25,26,27]. In the common bed bug Cimex lectularius, depletion of Kr-h1 in adult females caused severely reduced egg hatchability [28].
Kr-h1 is transcriptionally activated by the JH-receptor complex comprising Methoprene-tolerant (Met) and Taiman, two members of the bHLH-PAS transcription factor family [15, 29,30,31,32,33]. Met also dimerizes with Cycle, which upregulates Kr-h1 transcription in JH-mediated previtellogenic development of Ae. aegypti [25]. In the beetle Tribolium castaneum, JH represses the expression of Histone deacetylase 1 (HDAC1), leading to increased levels of histone acetylation and consequently promoting Kr-h1 transcription [34, 35]. Beside transcriptional regulation, Kr-h1 is post-transcriptionally regulated by microRNAs [19, 36]. In the cockroach Blattella germanica, miR-2 eliminates Kr-h1 transcripts at final instar nymphs, which crucially contributes to the onset of metamorphosis [37]. In L. migratoria, Kr-h1 is downregulated by let-7 and miR-278, whereas JH suppresses the expression of these two miRNAs. This regulatory loop ensures a proper level of Kr-h1 essential for preventing precocious metamorphosis in nymphs and stimulating JH-dependent vitellogenesis in adults [38,39,40,41,42,43,44].
In an effort to elucidate how Kr-h1 functions in repressing precocious nymph metamorphosis and stimulating adult reproduction in L. migratoria, we investigated Kr-h1 phosphorylation and its involvement in transcriptional repression and activation. The migratory locust L. migratoria is a destructive insect pest worldwide as well as a representative of evolutionarily basal insects with hemimetabolous development and JH-dependent vitellogenesis. We found that PKCα triggers Kr-h1 phosphorylation. Phosphorylated Kr-h1 recruited C-terminal binding protein (CtBP), consequently inhibiting E93 expression and nymphal-adult metamorphosis. Phosphorylated Kr-h1 interacted with CREB-binding protein (CBP), which stimulated the transcription of Ribosomal protein L36 (RL36) and reproduction. We also provide evidence that the essential role of phosphorylated Kr-h1 in recruiting CtBP and repressing E93 expression is evolutionarily conserved in other representative insects including the silkworm Bombyx mori, the beetle T. castaneum and the fruit fly D. melanogaster.
Results
Kr-h1 is phosphorylated by PKCα at Ser154
We initially predicted the phosphorylation of L. migratoria Kr-h1 (GenBank: KJ425482) computationally by DISPHOS (V1.3) software [45]. Three serine residues, Ser154, Ser371, and Ser554 were suggested as potential phosphorylation sites, with Ser154 at the highest score (Additional file 1: Fig. S1A). To validate Kr-h1 phosphorylation, we performed immunoprecipitation using a commercial anti-phospho-(Ser) antibody and a polyclonal anti-Kr-h1 antibody [38]. Phosphorylated Kr-h1 (p-Kr-h1) was detected in protein extracts from both nymphs and adults (Additional file 1: Fig. S1B). We generated an anti-phospho-Kr-h1 (Ser154) antibody (Additional file 1: Fig. S1C). Its specificity was verified by western blot using proteins extracted from adult female fat bodies subjected to Kr-h1 knockdown as well as those treated with phosphatase λpp (Fig. 1A). The specificity of anti-phospho-Kr-h1 (Ser154) antibody was also verified by western blot using the recombinant Flag-tagged proteins of wildtype Kr-h1 and mutated Kr-h1S154A(Ser154 to Ala154) expressed in Drosophila S2 cells treated with methoprene as well as the bacterially expressed GST-tagged peptides of Kr-h1(aa1-290) and Kr-h1S154A(aa1-290) incubated with PKCα (Additional file 1: Fig. S1D). We next investigated the kinase triggering Kr-h1 phosphorylation at Ser154. The motif KAFSVK at amino acid residues 151-156 of L. migratoria Kr-h1 (Additional file 1: Fig. S1A) is a conserved motif recognized by PKC [46,47,48], presumably PKCα and PKCη as predicted by a GPS algorithm [49]. As evaluated by western blots, application of the PKC inhibitor NPC15437 in nymphs and adult females reduced p-Kr-h1 levels (Fig. 1B). Depletion of PKCα (GenBank: MT081310) in nymphs and adult females caused significant reduction of p-Kr-h1 but not total Kr-h1 abundance (Fig. 1B and Additional file 1: Fig. S2A). In contrast, PKCη (GenBank: MT081311) knockdown had no obvious effect on Kr-h1 phosphorylation (Additional file 1: Fig. S2B). These results imply that PKCα is likely to mediate Kr-h1 phosphorylation at Ser154. To confirm the action of PKCα on Kr-h1 phosphorylation, we synthesized wildtype Kr-h1(aa125-159) and mutated Kr-h1S154A(aa125-159) peptides, followed by incubating them separately with PKCα for LC-MS/MS analysis. As illustrated in Fig. 1C, Kr-h1(aa125-159) peptide without PKCα treatment had a molecular mass of 4,254 Da. However, incubation of Kr-h1(aa125-159) peptide with PKCα yielded a molecular mass of 4,334 Da (Fig. 1D), exhibiting an 80 Da shift compared to Kr-h1(aa125-159) peptide without PKCα treatment. When mutated Kr-h1S154A(aa125-159) peptide was incubated with PKCα, a molecular mass of 4238 Da was detected, same as that observed with Kr-h1S154A(aa125-159) peptide alone (Additional file 1: Fig. S2C). To further define PKCα-mediated Kr-h1 phosphorylation at Ser154, we carried out Pro-Q Diamond Phosphoprotein Gel Staining with purified bacterially expressed GST-tagged peptides of Kr-h1(aa1-290), Kr-h1S154A(aa1-290), Kr-h1(aa89-312), Kr-h1S154A(aa89-312), and Kr-h1(aa291-591) incubated with PKCα. As shown in Fig. 1E, the specific phosphorylation bands were observed with wildtype Kr-h1(aa1-290) and Kr-h1(aa89-312) peptides, but not mutated Kr-h1S154A(aa1-290) or Kr-h1S154A(aa89-312). No phosphorylation band was observed with the truncated Kr-h1(aa291-591) (Fig. 1E), indicating that PKCα-mediated Kr-h1 phosphorylation is unlikely to occur at Ser371 or Ser554.
Phosphorylation of Kr-h1 by PKCα at Ser154. A Left panel: Kr-h1 RNAi efficiency in the fat body of 3-day-old adult females. *P<0.05. n=8. Right panel: Verification of phospho-Kr-h1 (Ser154) antibody specificity by using protein extracts from the fat body of 3-day-old adult females subjected to Kr-h1 knockdown and phosphatase λpp treatment. B Left panel: PKCα RNAi efficiency in the whole body of penultimate 4th instar nymphs and the fat body of 3-day-old adult females. **P<0.01. n=8. Right panel: Relative levels of Kr-h1 and phosphorylated Kr-h1 (p-Kr-h1) in the whole body of 4th instar nymphs and the fat body of 3-day-old adult females treated by NPC15437 (NPC) vs. DMSO solvent control (Cont.) and dsPKCα vs. dsGFP control. C-D LC-MS/MS analysis of wildtype Kr-h1(aa125-159) peptide (C) and Kr-h1(aa125-159) preincubated with PKCα (D). m/z indicates the mass to charge ratio. E Upper panel: Pro-Q Diamond Phosphoprotein Gel Stain of purified bacterially-expressed GST-tagged peptides of Kr-h1(aa1-290), Kr-h1S154A(aa1-290), Kr-h1(aa89-312), Kr-h1S154A(aa89-312), and Kr-h1(aa291-591) preincubated with or without PKCα. Lower panel: Coomassie brilliant blue staining was used as the loading controls
Kr-h1 expression and phosphorylation are in response to JH
To explore the dynamics of p-Kr-h1 before the onset of locust metamorphosis, we conducted western blot using proteins extracted from the penultimate 4th and final 5th instar nymphs. As shown in Fig. 2A, p-Kr-h1 levels were high in mid and late 4th instar nymphs but markedly declined in 5th instar nymphs. The decreased levels of p-Kr-h1 at final nymphal instar appeared to correlate with the decline of JH titer in this phase [50], suggesting a possible effect of JH on Kr-h1 phosphorylation. It should be noted that Kr-h1 is expressed in response to JH [21, 38]. The abundance of total Kr-h1 also decreased in 5th instar nymphs (Fig. 2A). To evaluate the responsiveness of Kr-h1 phosphorylation to JH in juvenile stage, western blot was performed using protein extracts from mid-5th instar nymphs as well as those further treated with methoprene for 5-60 min. Application of methoprene caused increase of both Kr-h1 and p-Kr-h1 levels, and longer exposure to methoprene tended to have a relatively more pronounced effect on Kr-h1 expression and phosphorylation (Fig. 2B). Notably, p-Kr-h1 levels increased more rapidly than total Kr-h1 after 15-min exposure to methoprene (Fig. 2B and Additional file 1: Fig. S3), implying a role of JH in stimulating Kr-h1 phosphorylation. Dose-response experiments demonstrated that higher doses of methoprene induced higher levels of Kr-h1 and p-Kr-h1 (Fig. 2C). The data suggest that JH promotes Kr-h1 expression and phosphorylation in nymphs, and the high levels of Kr-h1 phosphorylation are generally observed with more abundant Kr-h1 proteins.
Responsiveness of Kr-h1 phosphorylation to JH. A Abundance of Kr-h1 and p-Kr-h1 in the whole body of penultimate 4th and final 5th instar nymphs. E, M and L indicate the early (day 1), mid (day 2 for 4th, and day 3 for 5th), and late (day 4 for 4th, and day 5 for 5th) stages, respectively. B Relative levels of Kr-h1 and p-Kr-h1 in mid-5th instar nymphs and in those further treated with methoprene at 100 μg per locust for 5–60 min. C Relative abundance of Kr-h1 and p-Kr-h1 in mid-5th instar nymphs (5M) and those further treated with methoprene at 10–100 μg per locust for 8 h. D Developmental dynamics of Kr-h1 and p-Kr-h1 in the fat body of adult females at 0–6 days post adult emergence. E Relative levels of Kr-h1 and p-Kr-h1 in the fat body of newly-emerged adult females (A0) as well as those further treated with methoprene at 100 μg per locust for 5–60 min. F Relative levels of Kr-h1 and p-Kr-h1 in the fat body of newly-emerged adult females (A0) as well as those further treated with methoprene at 10–100 μg per locust for 8 h. A2, 2-day-old adult female as a control
We next studied the temporal abundance of p-Kr-h1 after adult ecdysis using protein extracts from the fat body of adult females at 0–6 days post adult emergence (PAE). Compared to that on the day of adult emergence, p-Kr-h1 levels increased at 1–4 days PAE and remained high on days 5–6, resembling that of total Kr-h1 (Fig. 2D). As JH is undetectable in the hemolymph at adult emergence but sharply increases thereafter [51], the enhanced levels of Kr-h1 and p-Kr-h1 appeared to positively correlate with elevated hemolymph JH titer. To elucidate the responsiveness of Kr-h1 phosphorylation to JH in adult locusts, western blot analysis was carried out using protein extracts isolated from the fat body of newly emerged adult females and those further treated with methoprene. As observed in nymphs, methoprene-induced Kr-h1 expression and phosphorylation were also seen in adults (Fig. 2E, F). Likewise, p-Kr-h1 abundance increased more rapidly than total Kr-h1 in the fat body of adult females treated with methoprene for 15 min (Fig. 2E and Additional file 1: S3). Taken together, our data suggest that JH-induced Kr-h1 expression is accompanied by increased levels of Kr-h1 phosphorylation in both nymphal and adult locusts.
Kr-h1 phosphorylation is required for its anti-metamorphic action
Previous studies have documented that E93 controls metamorphic nymphal-adult or pupal-adult transition [14,15,16]. Kr-h1 represses E93 transcription [15] by binding to the promoter sequence bearing the core Kr-h1 binding site (KBS) [17]. As expected, depletion of E93 (GenBank: MT081312) in the final instar nymph of locusts resulted in supernumerary nymphs and delayed adult morphogenesis (Additional file 1: Fig. S4). Knockdown of Kr-h1 in penultimate instar nymphs caused 4.5-fold increase of E93 transcripts (Fig. 3A). Application of a PKC inhibitor, NPC15437, or knockdown of PKCα led to significant increase of E93 mRNA levels (Fig. 3A), suggesting the possible requirement of Kr-h1 phosphorylation for repressing E93 transcription. Analysis of upstream 3-kb sequence revealed a conserved KBS in the proximal promoter region (nt -617 to -612) of L. migratoria E93 gene (Additional file 1: Fig. S5A). We then carried out dual luciferase reporter assays by co-transfection of pGL4.10-4×E93-623 to -606 with pAc5.1/Flag-Kr-h1, pAc5.1/Flag-Kr-h1S154A, pAc5.1/Flag-Kr-h1S154D or pAc5.1/Flag empty control into Drosophila S2 cells treated with methoprene. Western blot demonstrated that methoprene treatment stimulated Flag-Kr-h1 phosphorylation (Additional file 1: Fig. S5B). Overexpression of Flag-Kr-h1 plus methoprene treatment caused about 58% reduction of E93 reporter activity compared to the empty vector control (Fig. 3B). The capacity of Kr-h1 to inhibit E93 reporter activity was blocked by overexpression of Flag-Kr-h1S154A, a mutated p-Kr-h1 (Fig. 3B). In contrast, overexpression of p-Kr-h1 wildtype variant, Flag-Kr-h1S154D, restored the inhibitory constraints of Kr-h1 on E93 reporter activity (Fig. 3B). As illustrated in Additional file 1: Fig. S5B, Flag-Kr-h1S154D but not Flag-Kr-h1S154A was recognized by the anti-phospho-Kr-h1 (Ser154) antibody. Knowing that p-Kr-h1 had an essential role in suppressing E93 reporter activity, we next performed in vivo ChIP analysis using anti-phospho-Kr-h1 (Ser154) antibody and nuclear extracts from mid-4th and 5th instar nymphs. The antibodies against Kr-h1 and IgG were used as the positive and negative controls, respectively. As shown in Fig. 3C, p-Kr-h1 was remarkably enriched with E93 promoter region covering the KBS motif in penultimate 4th instar nymphs in which JH, Kr-h1, and p-Kr-h1 were in high levels. Conversely, a marginal precipitation of p-Kr-h1 was observed at final 5th nymphal instar when JH, Kr-h1, and p-Kr-h1 levels were low (Fig. 3C). NPC15437 treatment or PKCα knockdown restrained p-Kr-h1 enrichment with KBS-containing E93 promoter region in 4th instar nymphs (Fig. 3D). Moreover, methoprene treatment of 5th instar nymphs caused noticeable increase of p-Kr-h1 enrichment (Fig. 3E). Collectively, these results suggest an essential role of Kr-h1 phosphorylation in transcriptional repression of E93 in the nymphs of L. migratoria.
Requirement of Kr-h1 phosphorylation in inhibiting E93 transcription. A Relative levels of E93 mRNA in mid-4th instar nymphs treated with dsKr-h1 vs. dsGFP control, NPC15437 (NPC) vs. DMSO solvent control (Cont.), and dsPKCα vs. dsGFP control. **P<0.01 and ***P<0.001. n=8. B Luciferase reporter assays using S2 cells co-transfected with pGL4.10-4×E93-623 to -606 plus pAc5.1/Flag-Kr-h1, pAc5.1/Flag-Kr-h1S154A, pAc5.1/Flag-Kr-h1S154D, or pAc5.1/Flag empty control with or without 10 μM methoprene treatment. Co-transfection of pGL4.10-4×E93-623 to -606 and pAc5.1/Flag empty control without methoprene treatment was used as the control. Means labeled with different letters indicate significant difference at P<0.05. n=4. C ChIP assays showing relative precipitation of E93 promoter region with the KBS motif (RPEP-KBS) in mid-4th (4M) and 5th (5M) instar nymphs. D RPEP-KBS in 4M nymphs treated with NPC15437 (NPC) vs. DMSO solvent control (Cont.) and dsPKCα vs. dsGFP control. E RPEP-KBS in 5M nymphs treated with 50 μg methoprene vs. acetone solvent control. In C–E, p-Kr-h1, phospho-Kr-h1 (Ser154) antibody; Kr-h1, Kr-h1 antibody; and IgG, non-specific rabbit IgG control. Means labeled with different letters indicate significant difference at P<0.05. n=4
Kr-h1 phosphorylation is required for its role in stimulating reproduction
Kr-h1 has a dual role in preventing precocious nymphal/larval metamorphosis and in promoting adult reproduction. In L. migratoria, Ribosomal protein L36 (RL36) (GenBank: MT081313) was previously found to express in response to the JH-Met-Kr-h1 pathway [52]. RL36 is a component of the 60S subunit of ribosomes involved in ribosome biogenesis and protein translation as well as extra-ribosomal functions in various cellular processes [53]. Knocking down RL36 resulted in blocked ovarian growth and arrested oocyte maturation (Additional file 1: Fig. S6). As shown in Fig. 4A, Kr-h1 knockdown caused 54% reduction of RL36 mRNA levels. Similarly, NPC15437 treatment and PKCα knockdown resulted in 41% and 58% decrease of RL36 transcripts, respectively (Fig. 4A), suggesting a possible role of p-Kr-h1 in RL36 expression. For luciferase reporter assay, RL36 promoter region (nt -1647 to -1632) comprising a KBS motif (Additional file 1: Fig. S5A) was cloned into pGL4.10 vector. Co-transfection of pAc5.1/Flag-Kr-h1 and pGL4.10-4×RL36-1647 to -1632 in S2 cells treated with methoprene brought about 2-fold induction of RL36 reporter activity compared to the empty vector control (Fig. 4B). When pAc5.1/Flag-Kr-h1S154A was co-transfected with pGL4.10-4×RL36-1647 to -1632, no significant induction of RL36 reporter activity was observed (Fig. 4B). However, the induction of RL36 reporter activity was restored by overexpression of Flag-Kr-h1S154D (Fig. 4B). The data indicate an essential role of Kr-h1 phosphorylation in RL36 transcription. We next performed ChIP assays to quantify in vivo binding of p-Kr-h1 to KBS-containing promoter region of RL36 in the fat body of adult females. Compared to the day of adult emergence, p-Kr-h1 was more enriched with the KBS-containing promoter sequence of RL36 on day 3, and even more on day 6 (Fig. 4C). However, NPC15437 treatment and PKCα knockdown in 6-day-old adult females resulted in significant reduction of p-Kr-h1 enrichment with RL36 promoter (Fig. 4D). Furthermore, application of methoprene to newly emerged adult females led to significantly enhanced precipitation of p-Kr-h1 in RL36 promoter region (Fig. 4E). These results together indicate a pivotal role of Kr-h1 phosphorylation in induction of RL36 transcription during female reproduction.
Requirement of Kr-h1 phosphorylation in induction of RL36 transcription. A Relative levels of RL36 transcript in the fat body of 3-day-old adult females treated with dsKr-h1 vs. dsGFP control, NPC15437 (NPC) vs. DMSO solvent control (Cont.), and dsPKCα vs. dsGFP control. *P<0.05 and **P<0.01. n=8. B Luciferase reporter assays using S2 cells co-transfected with pGL4.10-4×RL36-1647 to -1632 plus pAc5.1/Flag-Kr-h1, pAc5.1/Flag-Kr-h1S154A, pAc5.1/Flag-Kr-h1S154D, or pAc5.1/Flag empty control. Methoprene was applied at 10 μM. Co-transfection of pGL4.10-4×RL36-1647 to -1632 and pAc5.1/Flag empty vector without methoprene treatment was used as the control. Means labeled with different letters indicate significant difference at P<0.05. n=4. C ChIP assays showing relative precipitation of RL36 promoter region with the KBS motif (RPRP-KBS) in the fat body of adult females on day 0 (A0), day 3 (A3), and day 6 (A6). D RPRP-KBS in the fat body of 3-day-old adult females treated with NPC15437 (NPC) vs. DMSO solvent control (Cont.) and dsPKCα vs. dsGFP control. E RPRP-KBS in the fat body of 3-day-old adult females treated with 50 μg methoprene vs. acetone solvent control. In C–E, p-Kr-h1, phospho-Kr-h1 (Ser154) antibody; Kr-h1, Kr-h1 antibody; and IgG, non-specific rabbit IgG control. Means labeled with different letters indicate significant difference at P<0.05. n=4
Phosphorylated Kr-h1 recruits distinct cofactors in anti-metamorphic and vitellogenic actions
Kr-h1 is known to act as a repressor and an activator in transcriptional response to JH [8, 11, 26, 27]. We performed ChIP analysis using the Kr-h1 antibody followed by LC-MS/MS as well as yeast two-hybrid assay to identify the co-factors of Kr-h1 in repressing nymphal metamorphosis and promoting adult reproduction. C-terminal binding protein (CtBP) is a highly conserved transcriptional corepressor involved in insect development and reproduction [54,55,56]. In L. migratoria, CtBP (GenBank: MT081314) expression was high in nymphs, but significantly decreased in adults (Additional file 1: Fig. S7A). Knockdown of CtBP caused significantly increased levels of E93 transcript in penultimate 4th instar nymphs (Additional file 1: Fig. S7B), suggesting a crucial role of CtBP in repressing E93 expression. To assess the p-Kr-h1 and CtBP interaction as well as the effect on E93 transcription, Co-IP and luciferase reporter assays were performed using S2 cells co-transfected with recombinant pAc5.1/Flag-CtBP along with pAc5.1/Flag-Kr-h1, pAc5.1/Flag-Kr-h1S154A, or pAc5.1/Flag-Kr-h1S154D plus pGL4.10-4×E93-623 to -606. Immunoprecipitation with anti-Kr-h1 antibody followed by western blot with anti-Flag antibody demonstrated that methoprene-exposed Flag-Kr-h1 and Flag-Kr-h1S154D but not Flag-Kr-h1S154A interacted with Flag-CtBP (Fig. 5A). Dual luciferase reporter assays showed that E93 reporter activity was reduced by 47% and 50%, respectively when Flag-CtBP was co-expressed with Flag-Kr-h1 or Flag-Kr-h1S154D (Fig. 5B). In contrast, co-expression of Flag-CtBP and Flag-Kr-h1S154A had no significant inhibitory effect on E93 reporter activity (Fig. 5B). The data suggest that phosphorylated Kr-h1 recruits a repressor, CtBP in transcriptional repression of E93 gene for anti-metamorphic action in nymphal locusts.
Essential role of Kr-h1 phosphorylation in the interaction with transcriptional cofactors. A Upper panel: immunoprecipitation (IP) and western blot (WB) showing the interaction of Flag-Kr-h1, Flag-Kr-h1S154A, or Flag-Kr-h1S154D with Flag-CtBP. Middle and lower panels: the expression of above recombinant proteins in S2 cells. α-Kr-h1, Kr-h1 antibody; α-Flag, Flag antibody. WT, wildtype; MT, mutant. B Luciferase reporter assays after co-transfection of pGL4.10-4×E93-623 to -606 and pAc5.1/Flag-CtBP plus pAc5.1/Flag-Kr-h1, pAc5.1/Flag-Kr-h1S14A, or pAc5.1/Flag-Kr-h1S154D into S2 cells. Co-transfection of pGL4.10-4×E93-623 to -606 and pAc5.1/Flag-Kr-h1 was used as the control. Methoprene was applied at 10 μM. Means labeled with different letters indicate significant difference at P<0.05. n=4. C Upper panel: IP and WB showing interaction of Flag-Kr-h1, Flag-Kr-h1S154A or Flag-Kr-h1S154D with Flag-CBP. Mid and lower panels: the expression of above recombinant proteins in S2 cells. α-Kr-h1, Kr-h1 antibody; α-Flag, Flag antibody. WT, wildtype; MT, mutant. D Luciferase reporter assays after co-transfection of pGL4.10-4×RL36-1647 to -1632 and pAc5.1/Flag-CBP plus pAc5.1/Flag-Kr-h1, pAc5.1/Flag-Kr-h1S154A or pAc5.1/Flag-Kr-h1S154D into S2 cells. Co-transfection of pGL4.10-4×RL36-1647 to -1632 and pAc5.1/Flag-Kr-h1 was used as the control. Methoprene was applied at 10 μM. Means labeled with different letters indicate significant difference at P<0.05. n=4
CREB-binding protein (CBP), a transcriptional coactivator with histone acetyltransferase activity, has been demonstrated to play an important role in JH action [34, 57, 58]. In L. migratoria, CBP (GenBank: MT081315) mRNA levels significantly increased after adult ecdysis (Additional file 1: Fig. S7C). Depletion of CBP caused 49% reduction of RL36 mRNA levels in the fat body of 3-day-old adult females (Additional file 1: Fig. S7D), suggesting that CBP is likely to participate in Kr-h1 regulation of RL36 transcription. Co-IP assays showed that Flag-CBP dimerized with methoprene-treated Kr-h1 and Kr-h1S154D, but not Kr-h1S154A (Fig. 5C). In dual luciferase reporter assays, co-transfection of pAc5.1/Flag-CBP and pAc5.1/Flag-Kr-h1S154D caused 1.7-fold increase of RL36 reporter activity, mimicking that observed with co-expression of Flag-CBP and Flag-Kr-h1 (Fig. 5D). Conversely, no significantly enhanced RL36 reporter activity was observed with co-expression of Flag-CBP and Flag-Kr-h1S154A (Fig. 5D). Taken together, these results imply that phosphorylated Kr-h1 recruits a coactivator, CBP for induction of RL36 transcription that is involved in locust vitellogenesis and egg maturation.
Kr-h1 phosphorylation is evolutionarily conserved
We next investigated the evolutionary conservation of Kr-h1 phosphorylation across insect orders. Protein sequence alignment indicated that this phosphorylation residue is conserved in Kr-h1 orthologues of other 22 insect species with available cDNA sequences in the NCBI database (Additional file 1: Fig. S8A). We selected the Kr-h1 orthologues of holometabolous species B. mori, T. castaneum, and D. melanogaster for further study. Ser154 of L. migratoria Kr-h1 is homologous to Ser76 of B. mori Kr-h1 (BmKr-h1), Ser124 of T. castaneum Kr-h1 (TcKr-h1), and Ser255 of D. melanogaster Kr-h1 (DmKr-h1). Amino acids at the flanking regions of these serine residues occur in a highly conserved context (Additional file 1: Fig. S8A). The phosphorylated forms of Kr-h1 orthologues in B. mori, T. castaneum and D. melanogaster were recognized by anti-phospho-Kr-h1 (Ser154) antibody (Fig. 6A). The results indicate the conservation of Kr-h1 phosphorylation across insect orders, including hemimetabolous and holometabolous species. The regulatory sequences containing the core KBS motif were previously identified in the promoters of B. mori, T. castaneum, and D. melanogaster E93 corresponding genes [17] (Additional file 1: Fig. S8B). Thus, we performed dual luciferase reporter assays to characterize the inhibitory effect of BmKr-h1, TcKr-h1, and DmKr-h1 phosphorylation on transcription of respective E93 genes. Compared to the empty vector control, overexpression of methoprene-treated BmKr-h1 and BmKr-h1S76D led to 67% and 73% reduction of BmE93 reporter activity, whereas overexpression of BmKr-h1S76A had no inhibitory effect (Fig. 6B). With respect to TcKr-h1 phosphorylation, methoprene-exposed TcKr-h1 and TcKr-h1S124D caused 91% and 71% reduction, respectively of TcE93 reporter activity (Fig. 6C). No inhibitory effect of TcKr-h1S124A on TcE93 reporter activity was observed (Fig. 6C). In the case of DmKr-h1 phosphorylation, methoprene-treated DmKr-h1 and DmKr-h1S255D brought about 85% and 81% reduction, respectively, of DmE93 reporter activity (Fig. 6D). Overexpression of DmKr-h1S255A led to 44% reduction of DmE93 reporter activity. Nevertheless, the transcriptional activity of DmKr-h1S255A was significantly lower than that of methoprene-exposed DmKr-h1 and DmKr-h1S255D (Fig. 6D). Collectively, these results indicate that Kr-h1 phosphorylation and its indispensable role in regulating E93 expression are evolutionarily conserved in B. mori, T. castaneum, and D. melanogaster.
Conservation of Kr-h1 phosphorylation in other insects. A Western blot showing Kr-h1 phosphorylation in penultimate instar larvae of Bombyx mori, Tribolium castaneum, and Drosophila melanogaster, respectively. B Luciferase reporter assays after co-transfection of pGL4.10-4×BmE93-2844 to -2827 with pAc5.1/Flag-BmKr-h1, pAc5.1/Flag-BmKr-h1S76A, pAc5.1/Flag-BmKr-h1S76D or pAc5.1/Flag vector control into S2 cells. Methoprene was applied at 10 μM. C Luciferase reporter assays using S2 cells co-transfected with pGL4.10-4×TcE93 -50 to -33 with pAc5.1/Flag-TcKr-h1, pAc5.1/Flag-TcKr-h1S124A, pAc5.1/Flag-TcKr-h1S124D, or pAc5.1/Flag. D Luciferase reporter assays using S2 cells co-transfected with pGL4.10-4×DmE93-2095 to -2078 with pAc5.1/Flag-DmKr-h1, pAc5.1/Flag-DmKr-h1S255A, pAc5.1/Flag-DmKr-h1S255D or pAc5.1/Flag. Means labeled with different letters indicate significant difference at P<0.05. n=3
Discussion
As a primary JH early-response gene, Kr-h1 plays an essential role in mediating JH action in repressing metamorphosis in juveniles and stimulating reproduction in adults [8,9,10,11]. Previous studies have established that Kr-h1 is transcriptionally activated by the JH-receptor complex [15, 29,30,31]. In addition, Kr-h1 is reported to be post-transcriptionally regulated by miRNAs, including miR-2, let-7, and miR-278, in different species [37, 38]. Furthermore, Kr-h1 transcription is regulated by HDAC1-mediated histone deacetylation, suggesting an epigenetic modification in JH action [34, 35]. Thus, Kr-h1 phosphorylation represents an interesting question for comprehensively deciphering the molecular basis of JH action and Kr-h1 function. By approaches of site-directed mutagenesis, phosphoprotein gel staining, LC-MS/MS, RNAi, western blot, and ChIP, we found in this study that Kr-h1 was phosphorylated by PKCα at Ser154 and that Kr-h1 phosphorylation levels increased along with JH-induced Kr-h1 expression. We observed more rapid increase of Kr-h1 phosphorylation than total Kr-h1 protein after 15-min exposure to methoprene in locusts. JH-induced phosphorylation was also seen with the recombinant Flag-Kr-h1 protein expressed in S2 cells. It has been previously reported that JH promotes Met phosphorylation by CaMKII and PKC and thus enhances the transcriptional activity of Met in Ae. aegypti [41, 43]. Moreover, JH triggers Akt-mediated serine/arginine-rich (pre-mRNA) splicing factor (SRSF) phosphorylation that induces Taiman alternative splicing and promotes Ae. aegypti vitellogenesis [40]. Additionally, it has been shown that JH induces Met phosphorylation and consequently increases the dimerization of Met and Tai in H. armigera [59]. Recently, a functional phosphorylation site (Ser694) located outside of multiple zinc-finger domains was identified in Ae. aegypti Kr-h1 (AaKr-h1). JH treatment caused dephosphorylation of AaKr-h1 at Ser694. Dephosphorylation mimic mutants (AaKr-h1S694V and AaKr-h1S694C) showed significantly higher transcriptional activity than wildtype AaKr-h1 [60]. Our present study provides evidence on Kr-h1 phosphorylation at a serine residue in the zinc-finger domains and extends the view of post-translational modification of key players in the JH pathway. In a previous report, we demonstrated that JH activates the GPCR/RTK-PLC-IP3R signaling pathway that triggers PKC-mediated phosphorylation of Na+/K+-ATPase involved in patency induction and Vg transportation in vitellogenic female locusts [39]. We speculate that JH-activated GPCR/RTK-PLC-IP3R signaling cascade might induce PKCα-triggered Kr-h1 phosphorylation.
Kr-h1 is capable of activating or repressing transcription of genes in response to JH bound to its receptor Met [8, 11, 26, 27]. Our cell culture-based luciferase reporter assay and in vivo ChIP analysis demonstrated that Kr-h1 phosphorylation at Ser154 is essential for the transcriptional regulation of E93 and RL36, two representatives of Kr-h1 target genes. Such a phosphorylation was required for Kr-h1 to interact with the corepressor CtBP in inhibiting E93 transcription and with the coactivator CBP in inducing RL36 transcription. The p-Kr-h1 wildtype variant Flag-Kr-h1S154D had similar capability to p-Kr-h1 in binding cofactors and exerting transcriptional activity. However, the Kr-h1S154A mutant was unable to recruit the cofactors, consequently abolishing the repression of E93 transcription and the induction of RL36 transcription. These results together address the importance of Kr-h1 phosphorylation in mediating anti-metamorphic and vitellogenic effects of JH.
The Kr-h1 sequence contains eight C2H2 zinc-finger domains. In addition to potentially recognizing a variety of DNA sequences, the zinc-fingers act as a hub for protein-protein interaction [61, 62]. The Ser154 residue is localized at the 3rd zinc-finger domain of Kr-h1. Phosphorylation modification is likely to induce a conformational change that is optimal for Kr-h1 to recruit cofactors. In the present study, CtBP and CBP were found to bind with phosphorylated Kr-h1 in repressing E93 transcription and activating RL36 transcription, respectively. Nevertheless, phosphorylated Kr-h1 could also interact with other cofactors in transcriptional activation or repression of target genes. In Ae. aegypti, Kr-h1 acts synergistically with Hairy, thereby mediating the action of Met in gene repression during previtellogenic development of adult females [27, 63]. A study in N. lugens has demonstrated that Hairy directly interacts with the N-terminus zinc-finger domains of Kr-h1 in modulating gene transcription [64]. Dual functions of transcriptional activation and repression are widely observed with transcription factors [65,66,67,68,69,70]. In mammals, Krüppel-like factor 4 promotes the transcription of cyclin B1 via interacting with CBP, but downregulates cyclin B1 transcription by recruiting HDAC3 [66].
We have additionally shown Kr-h1 phosphorylation in other insects belonging to divergent orders, including the lepidopteran B. mori, the coleopteran T. castaneum, and the dipteran D. melanogaster. The requirement of phosphorylation for Kr-h1 action on suppressing E93 transcription was found to be also conserved. The findings provide a clear indication that Kr-h1 phosphorylation and its indispensable role in regulating target gene expression are evolutionarily conserved across distant insect orders. These observations further highlight the significance of Kr-h1 phosphorylation in eliciting transcriptional activity. Previously, JH-dependent Ae. aegypti Kr-h1 dephosphorylation at Ser694 has been demonstrated to enhance the transcriptional activity [60]. The phosphoserine residue Ser694 is conserved in some holometabolous insects but not in L. migratoria. The Ser154 of L. migratoria Kr-h1 is homologous to Ser206 of Ae. aegypti Kr-h1. Thus, Kr-h1 orthologues likely bear multiple phosphorylation sites with differential responses to JH. While evolutionarily conserved Kr-h1 phosphorylation sites occur in divergent insect species, the lineage- and species-specific Kr-h1 phosphorylation residues may exist in some insects. It is of interest to address these questions in future research.
Conclusions
Kr-h1 functions both as a transcriptional repressor in preventing precocious larval/nymphal metamorphosis and a transcriptional activator in stimulating adult reproduction in insects. PKCα phosphorylated Kr-h1 at a serine residue localized in the 3rd zinc-finger domain. While Kr-h1 phosphorylation levels increased along with JH-induced total Kr-h1 expression, more rapid increase of Kr-h1 phosphorylation than total Kr-h1 was observed in locusts treated with methoprene. JH-induced Kr-h1 phosphorylation was also seen in methoprene-exposed S2 cells. Phosphorylated Kr-h1 recruited CtBP in nymphs, which inhibited E93 expression and metamorphosis. Phosphorylated Kr-h1 recruited CBP in adults, consequently stimulating RL36 transcription and vitellogenesis. Kr-h1 phosphorylation and its essential role in recruiting CtBP and repressing E93 expression are evolutionarily conserved in L. migratoria, B. mori, T. castaneum, and D. melanogaster. Thus, our present study fills a knowledge gap of phosphorylation modification of Kr-h1, an intermediate regulator in the JH/Met-response gene expression hierarchy.
Methods
Insects and treatments
The gregarious phase of L. migratoria was maintained as previously reported [71]. s-(+)-methoprene (Santa Cruz Biotech) was topically applied at 10–100 μg/5 μl acetone per locust for 8 h or 100 μg/5μl acetone per locust for 5-60 min. NPC15437 (Abcam) was intra-abdominally injected at 0.25 μg/5 μl DMSO per locust.
LC-MS/MS analysis
Synthesized Kr-h1(aa125-159) and Kr-h1S154A(aa125-159) peptides (BiotechPark) were separately incubated with PKCα (SignalChem) in reaction buffer containing 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 20 mM MgCl2, 1 mM DTT, and 1 mM ATP at 30°C for 30 min. After termination with 1/10 volume 1% formaldehyde and centrifugation at 8000×g for 10 min, the supernatant was desalted by C18Zip-Tip (Millipore), reduced by 10 mM DTT at 56°C for 1 h, and alkylated by 20 mM iodoacetamide (IAA) at room temperature in dark for 1 h. Extracted peptides were then lyophilized and resuspended in 0.1% formic acid, followed by LC-MS/MS analysis.
Pro-Q Diamond Phosphoprotein Gel Stain
cDNA fragments for Kr-h1(aa1-290), Kr-h1S154A(aa1-290), Kr-h1(aa89-312), Kr-h1S154A(aa89-312), and Kr-h1(aa291-591) were separately cloned into pGEX-4t-1 vector (GE Healthcare) for overexpression of recombinant GST-tagged proteins in Escherichia coli Rosetta competent cells (Transgen). Cells were lysed by sonication in lysis buffer with 50 mM Tris-HCl pH 7.5 plus 0.1% Triton X-100 and cleared by centrifugation at 8000×g for 30 min at 4°C. GST-fusion proteins were purified by GST resin (Thermo Fisher Scientific) and incubated with PKCα (SignalChem), followed by SDS-PAGE and Pro-Q Diamond Phosphoprotein Gel Stain (Invitrogen).
Eukaryotic cell culture and protein expression
Protein coding sequences of Kr-h1 (nt 1-1776), CtBP (nt 1-1332), CBP (nt 1-1728), BmKr-h1 (nt 1-1086), TcKr-h1 (nt 1-1407), and DmKr-h1 (nt 1-2376) were separately cloned into pAc5.1/Flag vectors (Invitrogen). Site-directed mutagenesis for Kr-h1S154A, Kr-h1S154D, BmKr-h1S76A, BmKr-h1S76D, TcKr-h1S124A, TcKr-h1S124D, DmKr-h1S255A, and DmKr-h1S255D was performed using Q5 Site-Directed Mutagenesis Kit (NEB). S2 cells were transfected with the recombinant vectors using Lipofectamine 3000 (Thermo). Primers used for recombinant vector construction and site-directed mutagenesis are provided in Table S1 (Additional file 1) and Table S2 (Additional file 1), respectively.
Western blot and immunoprecipitation
Protein extracts from insects and S2 cells were isolated in ice-cold lysis buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1% Nonidet P-40, 1% Triton-X 100, 0.5% sodium deoxycholate, 1 mM PMSF plus protease, and phosphatase inhibitors (Roche). Lysates were cleared by centrifugation, subjected to 8% SDS-PAGE, and transferred to PVDF membrane (Millipore). Western blots were conducted using antibodies against Kr-h1 [38], phospho-Kr-h1 (Ser154) (Jingjie PTM-Biolab), VgA [39] and Flag (MBL), corresponding HRP-conjugated secondary antibody (CWBIO), and a Superstar ECL Plus Ready-to-use Kit (BOSTER). β-actin antibody [39] was used as a reference control. Band intensity was quantified by ImageJ. For immunoprecipitation, precleared lysates were incubated with anti-Kr-h1 antibody for 60 min at 4°C. The immunocomplexes were then captured with protein-A agarose (Sigma-Aldrich) at 4°C overnight and eluted in Laemmli sample buffer, followed by western blots with anti-phospho-(Ser) (Blue Light Biotech) or anti-Flag antibody. For phosphatase treatment, protein extracts were preincubated with λpp (New England Biolabs) for 1 h at 30°C.
RNA isolation and qRT-PCR
Total RNAs were extracted from insects and S2 cells using TRIzol reagent (Invitrogen), and first-strand cDNAs were reverse transcribed using FastQuant RT kit with gDNase (Tiangen). qRT-PCR was performed using a RealMasterMix SYBR Green kit (Tiangen) with a LightCycler 96 system (Roche), initiated at 95°C for 15 min, and followed by 40 cycles of 95°C for 10 s, 58°C for 20 s, and 72°C for 30 s. Relative expression levels were calculated using 2−ΔΔCt method, normalized by ribosomal protein 49 (Rp49). Primers for qRT-PCR are listed in Table S3 (Additional file 1).
RNA interference and tissue imaging
cDNA templates were amplified by PCR, cloned into pGEM-T vector (Tiangen), and confirmed by sequencing. dsRNAs were synthesized by in vitro transcription with T7 RiboMAX Express RNAi System (Promega). Locusts were intra-abdominally injected with 15 μg dsRNA, and boosted once on day 5. Phenotypes were photographed by Canon EOS550D camera and Leica M205C stereomicroscope. Primers used for RNAi are given in Table S3 (Additional file 1).
Dual luciferase reporter assay
E93 and RL36 promoter regions bearing the KBS motif including 4×E93-623 to -606, 4×RL36-1647 to -1632, 4×BmE93-2844 to -2827, 4×TcE93-50 to -33, and 4×DmE93-2095 to -2078 were separately ligated into pGL4.10 vector (Promega) and confirmed by sequencing. S2 cells were co-transfected with these constructs along with recombinant vectors expressing wildtype or mutated Kr-h1 of L. migratoria, B. mori, T. castaneum, and D. melanogaster. Methoprene was applied at 10 μM 48 h post transfection and for 6 h. The luciferase activity was measured using a Dual-Luciferase Reporter Assay System and a GloMAX 96 Microplate Luminometer (Promega).
Chromatin immunoprecipitation
ChIP assays were performed using an EZ-Magna ChIP A/G Kit (Millipore). Briefly, fat bodies collected from nymph and adult females were fixed with 1% formaldehyde to crosslink chromatin for 10 min at 37°C. After addition of 125 mM glycine, chromatin was sonicated to shear into 200-1000 bp DNA fragments. The complexes were then immunoprecipitated with antibody against Kr-h1, phospho-Kr-h1 (Ser154) or IgG, followed by qPCR. Primers used for ChIP are listed in Table S3 (Additional file 1).
Statistical analysis
Statistical analyses were performed by Student’s t-test or one-way analysis of variance (ANOVA) with Tukey’s post hoc test using the SPSS22.0 software. Significant difference was considered at P < 0.05. Values were reported as mean ± S.E.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files. The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Riddiford LM. Cellular and molecular actions of juvenile hormone I. General considerations and premetamorphic actions. Adv In Insect Phys. 1994;24:213–74. https://doi.org/10.1016/S0065-2806(08)60084-3.
Truman JW, Riddiford LM. The evolution of insect metamorphosis: a developmental and endocrine view. Philos Trans R Soc Lond B Biol Sci. 2019;374(1783):20190070. https://doi.org/10.1098/rstb.2019.0070.
Belles X. The innovation of the final moult and the origin of insect metamorphosis. Philos Trans R Soc Lond B Biol Sci. 2019;374(1783):20180415. https://doi.org/10.1098/rstb.2018.0415.
Jindra M. Where did the pupa come from? The timing of juvenile hormone signalling supports homology between stages of hemimetabolous and holometabolous insects. Philos Trans R Soc Lond B Biol Sci. 2019;374(1783):20190064. https://doi.org/10.1098/rstb.2019.0064.
Belles X. Insect metamorphosis. From natural history to regulation of development and evolution. London: Academic Press; 2020.
Wyatt GR, Davey KG. Cellular and molecular actions of juvenile hormone II. Roles of juvenile hormone in adult insects. Adv In Insect Phys. 1996;26:1–155. https://doi.org/10.1016/S0065-2806(08)60030-2.
Raikhel AS, Brown MR, Belles X. Hormonal control of reproductive processes. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive molecular insect science, vol. 3. Boston: Elsevier; 2005. p. 433–91. https://doi.org/10.1016/B0-44-451924-6/00040-5.
Belles X. Krüppel homolog 1 and E93: The doorkeeper and the key to insect metamorphosis. Arch Insect Biochem Physiol. 2020;103(3):e21609. https://doi.org/10.1002/arch.21609.
Roy S, Saha TT, Zou Z, Raikhel AS. Regulatory pathways controlling female insect reproduction. Annu Rev Entomol. 2018;63(1):489–511. https://doi.org/10.1146/annurev-ento-020117-043258.
Santos CG, Humann FC, Hartfelder K. Juvenile hormone signaling in insect oogenesis. Curr Opin Insect Sci. 2019;31:43–8. https://doi.org/10.1016/j.cois.2018.07.010.
Truman JW. The evolution of insect metamorphosis. Curr Biol. 2019;29(23):R1252–r1268. https://doi.org/10.1016/j.cub.2019.10.009.
Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58(1):181–204. https://doi.org/10.1146/annurev-ento-120811-153700.
Kayukawa T, Nagamine K, Ito Y, Nishita Y, Ishikawa Y, Shinoda T. Krüppel homolog 1 inhibits insect metamorphosis via direct transcriptional repression of Broad-Complex, a pupal specifier gene. J Biol Chem. 2016;291(4):1751–62. https://doi.org/10.1074/jbc.M115.686121.
Urena E, Chafino S, Manjon C, Franch-Marro X, Martin D. The occurrence of the holometabolous pupal stage requires the interaction between E93, Krüppel-Homolog 1 and Broad-Complex. PLoS Genet. 2016;12(5):e1006020. https://doi.org/10.1371/journal.pgen.1006020.
Belles X, Santos CG. The MEKRE93 (Methoprene tolerant-Krüppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect Biochem Mol Biol. 2014;52:60–8. https://doi.org/10.1016/j.ibmb.2014.06.009.
Gujar H, Palli SR. Krüppel homolog 1 and E93 mediate Juvenile hormone regulation of metamorphosis in the common bed bug, Cimex lectularius. Sci Rep. 2016;6(1):26092. https://doi.org/10.1038/srep26092.
Kayukawa T, Jouraku A, Ito Y, Shinoda T. Molecular mechanism underlying juvenile hormone-mediated repression of precocious larval-adult metamorphosis. Proc Natl Acad Sci U S A. 2017;114(5):1057–62. https://doi.org/10.1073/pnas.1615423114.
Zhang T, Song W, Li Z, Qian W, Wei L, Yang Y, et al. Krüppel homolog 1 represses insect ecdysone biosynthesis by directly inhibiting the transcription of steroidogenic enzymes. Proc Natl Acad Sci U S A. 2018;115(15):3960–5. https://doi.org/10.1073/pnas.1800435115.
Song J, Zhou S. Post-transcriptional regulation of insect metamorphosis and oogenesis. Cell Mol Life Sci. 2019;77(10):1893–909. https://doi.org/10.1007/s00018-019-03361-5.
Jiang J, Xu Y, Lin X. Role of Broad-Complex (Br) and Krüppel homolog 1 (Kr-h1) in the ovary development of Nilaparvata lugens. Front Physiol. 2017;8:1013. https://doi.org/10.3389/fphys.2017.01013.
Song J, Wu Z, Wang Z, Deng S, Zhou S. Krüppel-homolog 1 mediates juvenile hormone action to promote vitellogenesis and oocyte maturation in the migratory locust. Insect Biochem Mol Biol. 2014;52:94–101. https://doi.org/10.1016/j.ibmb.2014.07.001.
Tang Y, He H, Qu X, Cai Y, Ding W, Qiu L, et al. RNA interference-mediated knockdown of the transcription factor Krüppel homologue 1 suppresses vitellogenesis in Chilo suppressalis. Insect Mol Biol. 2020;29(2):183–92. https://doi.org/10.1111/imb.12617.
Yue Y, Yang RL, Wang WP, Zhou QH, Chen EH, Yuan GR, et al. Involvement of Met and Kr-h1 in JH-mediated reproduction of female Bactrocera dorsalis (Hendel). Front Physiol. 2018;9:482. https://doi.org/10.3389/fphys.2018.00482.
Zhang WN, Ma L, Liu C, Chen L, Xiao HJ, Liang GM. Dissecting the role of Krüppel homolog 1 in the metamorphosis and female reproduction of the cotton bollworm, Helicoverpa armigera. Insect Mol Biol. 2018;27(4):492–504. https://doi.org/10.1111/imb.12389.
Shin SW, Zou Z, Saha TT, Raikhel AS. bHLH-PAS heterodimer of methoprene-tolerant and Cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proc Natl Acad Sci U S A. 2012;109(41):16576–81. https://doi.org/10.1073/pnas.1214209109.
Ojani R, Fu X, Ahmed T, Liu P, Zhu J. Krüppel homologue 1 acts as a repressor and an activator in the transcriptional response to juvenile hormone in adult mosquitoes. Insect Mol Biol. 2018;27(2):268–78. https://doi.org/10.1111/imb.12370.
Saha TT, Roy S, Pei G, Dou W, Zou Z, Raikhel AS. Synergistic action of the transcription factors Krüppel homolog 1 and Hairy in juvenile hormone/Methoprene-tolerant-mediated gene-repression in the mosquito Aedes aegypti. PLoS Genet. 2019;15(10):e1008443. https://doi.org/10.1371/journal.pgen.1008443.
Gujar H, Palli SR. Juvenile hormone regulation of female reproduction in the common bed bug, Cimex lectularius. Sci Rep. 2016;6(1):35546. https://doi.org/10.1038/srep35546.
Cui Y, Sui Y, Xu J, Zhu F, Palli SR. Juvenile hormone regulates Aedes aegypti Krüppel homolog 1 through a conserved E box motif. Insect Biochem Mol Biol. 2014;52:23–32. https://doi.org/10.1016/j.ibmb.2014.05.009.
Kayukawa T, Minakuchi C, Namiki T, Togawa T, Yoshiyama M, Kamimura M, et al. Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci U S A. 2012;109(29):11729–34. https://doi.org/10.1073/pnas.1204951109.
Wang Z, Yang L, Song J, Kang L, Zhou S. An isoform of Taiman that contains a PRD-repeat motif is indispensable for transducing the vitellogenic juvenile hormone signal in Locusta migratoria. Insect Biochem Mol Biol. 2017;82:31–40. https://doi.org/10.1016/j.ibmb.2017.01.009.
Lozano J, Kayukawa T, Shinoda T, Belles X. A role for Taiman in insect metamorphosis. PLoS Genet. 2014;10(10):e1004769. https://doi.org/10.1371/journal.pgen.1004769.
Jindra M, Tumova S, Milacek M. A decade with the juvenile hormone receptor. In: Bittova L. Adv In Insect Phys: A decade with the juvenile hormone receptor; 2021.
George S, Gaddelapati SC, Palli SR. Histone deacetylase 1 suppresses Krüppel homolog 1 gene expression and influences juvenile hormone action in Tribolium castaneum. Proc Natl Acad Sci U S A. 2019;116(36):17759–64. https://doi.org/10.1073/pnas.1909554116.
Roy A, Palli SR. Epigenetic modifications acetylation and deacetylation play important roles in juvenile hormone action. BMC Genomics. 2018;19(1):934. https://doi.org/10.1186/s12864-018-5323-4.
Belles X. MicroRNAs and the evolution of insect metamorphosis. Annu Rev Entomol. 2017;62(1):111–25. https://doi.org/10.1146/annurev-ento-031616-034925.
Lozano J, Montanez R, Belles X. MiR-2 family regulates insect metamorphosis by controlling the juvenile hormone signaling pathway. Proc Natl Acad Sci U S A. 2015;112(12):3740–5. https://doi.org/10.1073/pnas.1418522112.
Song J, Li W, Zhao H, Gao L, Fan Y, Zhou S. The microRNAs let-7 and miR-278 regulate insect metamorphosis and oogenesis by targeting the juvenile hormone early-response gene Krüppel-homolog 1. Development. 2018;145:dev170670.
Jing YP, An H, Zhang S, Wang N, Zhou S. Protein kinase C mediates juvenile hormone-dependent phosphorylation of Na(+)/K(+)-ATPase to induce ovarian follicular patency for yolk protein uptake. J Biol Chem. 2018;293(52):20112–22. https://doi.org/10.1074/jbc.RA118.005692.
Liu P, Fu X, Zhu J. Juvenile hormone-regulated alternative splicing of the taiman gene primes the ecdysteroid response in adult mosquitoes. Proc Natl Acad Sci U S A. 2018;115(33):E7738–47. https://doi.org/10.1073/pnas.1808146115.
Liu P, Peng HJ, Zhu J. Juvenile hormone-activated phospholipase C pathway enhances transcriptional activation by the methoprene-tolerant protein. Proc Natl Acad Sci U S A. 2015;112(15):E1871–9. https://doi.org/10.1073/pnas.1423204112.
Liu W, Zhang FX, Cai MJ, Zhao WL, Li XR, Wang JX, et al. The hormone-dependent function of Hsp90 in the crosstalk between 20-hydroxyecdysone and juvenile hormone signaling pathways in insects is determined by differential phosphorylation and protein interactions. Biochim Biophys Acta. 1830;2013(11):5184–92. https://doi.org/10.1016/j.bbagen.2013.06.037.
Ojani R, Liu P, Fu X, Zhu J. Protein kinase C modulates transcriptional activation by the juvenile hormone receptor methoprene-tolerant. Insect Biochem Mol Biol. 2016;70:44–52. https://doi.org/10.1016/j.ibmb.2015.12.001.
Cai MJ, Liu W, Pei XY, Li XR, He HJ, Wang JX, et al. Juvenile hormone prevents 20-hydroxyecdysone-induced metamorphosis by regulating the phosphorylation of a newly identified broad protein. J Biol Chem. 2014;289(38):26630–41. https://doi.org/10.1074/jbc.M114.581876.
Iakoucheva LM, Radivojac P, Brown CJ, O'Connor TR, Sikes JG, Obradovic Z, et al. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004;32(3):1037–49. https://doi.org/10.1093/nar/gkh253.
Airas JM, Betz H, El Far O. PKC phosphorylation of a conserved serine residue in the C-terminus of group III metabotropic glutamate receptors inhibits calmodulin binding. FEBS Lett. 2001;494(1-2):60–3. https://doi.org/10.1016/S0014-5793(01)02311-0.
Liu QR, Zhang PW, Lin Z, Li QF, Woods AS, Troncoso J, et al. GBPI, a novel gastrointestinal- and brain-specific PP1-inhibitory protein, is activated by PKC and inactivated by PKA. Biochem J. 2004;377(1):171–81. https://doi.org/10.1042/bj20030128.
Boratko A, Csortos C. PKC mediated phosphorylation of TIMAP regulates PP1c activity and endothelial barrier function. Biochim Biophys Acta Mol Cell Res. 1864;2017(2):431–9. https://doi.org/10.1016/j.bbamcr.2016.12.001.
Xue Y, Ren J, Gao X, Jin C, Wen L, Yao X. GPS 2.0, a tool to predict kinase-specific phosphorylation sites in hierarchy. Mol Cell Proteomics. 2008;7(9):1598–608. https://doi.org/10.1074/mcp.M700574-MCP200.
Truman JW, Riddiford LM. The origins of insect metamorphosis. Nature. 1999;401(6752):447–52. https://doi.org/10.1038/46737.
Guo W, Wu Z, Yang L, Cai Z, Zhao L, Zhou S. Juvenile hormone-dependent Kazal-type serine protease inhibitor Greglin safeguards insect vitellogenesis and egg production. FASEB J. 2019;33(1):917–27. https://doi.org/10.1096/fj.201801068R.
Guo W, Wu Z, Song J, Jiang F, Wang Z, Deng S, et al. Juvenile hormone-receptor complex acts on mcm4 and mcm7 to promote polyploidy and vitellogenesis in the migratory locust. PLoS Genet. 2014;10(10):e1004702. https://doi.org/10.1371/journal.pgen.1004702.
de la Cruz J, Karbstein K, Woolford JL Jr. Functions of ribosomal proteins in assembly of eukaryotic ribosomes in vivo. Annu Rev Biochem. 2015;84(1):93–129. https://doi.org/10.1146/annurev-biochem-060614-033917.
Mannervik M. Control of Drosophila embryo patterning by transcriptional co-regulators. Exp Cell Res. 2014;321(1):47–57. https://doi.org/10.1016/j.yexcr.2013.10.010.
Qiu J, He Y, Zhang J, Kang K, Li T, Zhang W. Discovery and functional identification of fecundity-related genes in the brown planthopper by large-scale RNA interference. Insect Mol Biol. 2016;25(6):724–33. https://doi.org/10.1111/imb.12257.
Eusebio N, Tavares L, Pereira PS. CtBP represses Dpp-dependent Mad activation during Drosophila eye development. Dev Biol. 2018;442(1):188–98. https://doi.org/10.1016/j.ydbio.2018.07.018.
Xu J, Roy A, Palli SR. CREB-binding protein plays key roles in juvenile hormone action in the red flour beetle, Tribolium Castaneum. Sci Rep. 2018;8(1):1426. https://doi.org/10.1038/s41598-018-19667-6.
Fernandez-Nicolas A, Belles X. CREB-binding protein contributes to the regulation of endocrine and developmental pathways in insect hemimetabolan pre-metamorphosis. Biochim Biophys Acta. 1860;2016(3):508–15. https://doi.org/10.1016/j.bbagen.2015.12.008.
Li YX, Wang D, Zhao WL, Zhang JY, Kang XL, Li YL, et al. Juvenile hormone induces methoprene-tolerant 1 phosphorylation to increase interaction with Taiman in Helicoverpa armigera. Insect Biochem Mol Biol. 2021;130:103519. https://doi.org/10.1016/j.ibmb.2021.103519.
Kim K, Albishi NM, Palli SR. Identification of juvenile hormone-induced posttranslational modifications of methoprene tolerant and Krüppel homolog 1 in the yellow fever mosquito, Aedes aegypti. J Proteomics. 2021;242:104257. https://doi.org/10.1016/j.jprot.2021.104257.
Brayer KJ, Segal DJ. Keep your fingers off my DNA: protein-protein interactions mediated by C2H2 zinc finger domains. Cell Biochem Biophys. 2008;50(3):111–31. https://doi.org/10.1007/s12013-008-9008-5.
Gamsjaeger R, Liew CK, Loughlin FE, Crossley M, Mackay JP. Sticky fingers: zinc-fingers as protein-recognition motifs. Trends Biochem Sci. 2007;32(2):63–70. https://doi.org/10.1016/j.tibs.2006.12.007.
Saha TT, Shin SW, Dou W, Roy S, Zhao B, Hou Y, et al. Hairy and Groucho mediate the action of juvenile hormone receptor Methoprene-tolerant in gene repression. Proc Natl Acad Sci U S A. 2016;113(6):E735–43. https://doi.org/10.1073/pnas.1523838113.
Mao Y, Li Y, Gao H, Lin X. Krüppel homologue 1 interacts directly with Hairy and regulates ecdysis in the brown planthopper. Insect Mol Biol. 2020;29(3):293–300. https://doi.org/10.1111/imb.12635.
Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320(5880):1224–9. https://doi.org/10.1126/science.1153252.
Evans PM, Zhang W, Chen X, Yang J, Bhakat KK, Liu C. Krüppel-like factor 4 is acetylated by p300 and regulates gene transcription via modulation of histone acetylation. J Biol Chem. 2007;282(47):33994–4002. https://doi.org/10.1074/jbc.M701847200.
Martinez-Montanes F, Rienzo A, Poveda-Huertes D, Pascual-Ahuir A, Proft M. Activator and repressor functions of the Mot3 transcription factor in the osmostress response of Saccharomyces cerevisiae. Eukaryot Cell. 2013;12(5):636–47. https://doi.org/10.1128/EC.00037-13.
Oswald F, Kovall RA. CSL-associated corepressor and coactivator complexes. Adv Exp Med Biol. 2018;1066:279–95. https://doi.org/10.1007/978-3-319-89512-3_14.
Richier B, Michard-Vanhee C, Lamouroux A, Papin C, Rouyer F. The clockwork orange Drosophila protein functions as both an activator and a repressor of clock gene expression. J Biol Rhythms. 2008;23(2):103–16. https://doi.org/10.1177/0748730407313817.
Tetel MJ, Auger AP, Charlier TD. Who’s in charge? Nuclear receptor coactivator and corepressor function in brain and behavior. Front Neuroendocrinol. 2009;30(3):328–42. https://doi.org/10.1016/j.yfrne.2009.04.008.
Wu Z, Guo W, Yang L, He Q, Zhou S. Juvenile hormone promotes locust fat body cell polyploidization and vitellogenesis by activating the transcription of Cdk6 and E2f1. Insect Biochem Mol Biol. 2018;102:1–10. https://doi.org/10.1016/j.ibmb.2018.09.002.
Acknowledgements
We thank Drs. Lynn Riddiford and Marek Jindra for critical reading of this manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) Grants 31630070 and 31702062.
Author information
Authors and Affiliations
Contributions
Z.W. and S.Z. designed the research; Z.W., L.Y., and H.L. performed the research; Z.W. and S.Z. analyzed the data; and S.Z. and Z.W. wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Identification of Kr-h1 phosphorylation site. Figure S2. Identification of kinase triggering Kr-h1 phosphorylation. Figure S3. Effect of 15-min exposure of methoprene on Kr-h1 phosphorylation. Figure S4. Effect of E93 knockdown on locust metamorphosis. Figure S5. Responsiveness of Kr-h1 phosphorylation to JH. Figure S6. Effect of RL36 knockdown on locust reproduction. Figure S7. Effect of CtBP or CBP knockdown on E93 or RL36 expression. Figure S8. Alignment of the 3rd zinc-finger domain of Kr-h1 and the partial promoter sequences of E93 with KBS motifs. Table S1. Primers used for cloning and gene expression. Table S2. Primers used for site-directed mutagenesis. Table S3. Primers used for qRT-PCR, RNAi and ChIP.
Additional file 3.
Original Western blot data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, Z., Yang, L., Li, H. et al. Krüppel-homolog 1 exerts anti-metamorphic and vitellogenic functions in insects via phosphorylation-mediated recruitment of specific cofactors. BMC Biol 19, 222 (2021). https://doi.org/10.1186/s12915-021-01157-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12915-021-01157-3