Abstract
Background
Informal family caregivers constitute an important and increasingly demanding role in the cancer healthcare system. This is especially true for caregivers of patients with primary malignant brain tumors based on the rapid progression of disease, including physical and cognitive debilitation. Informal social network resources such as friends and family can provide social support to caregivers, which lowers caregiver burden and improves overall quality of life. However, barriers to obtaining needed social support exist for caregivers. To address this need, our team developed and is assessing a multi-component caregiver support intervention that uses a blend of technology and personal contact to improve caregiver social support.
Methods
We are currently conducting a prospective, longitudinal 2-group randomized controlled trial which compares caregivers who receive the intervention to a wait-list control group. Only caregivers directly receive the intervention, but the patient-caregiver dyads are enrolled so we can assess outcomes in both. The 8-week intervention consists of two components: (1) The electronic Social Network Assessment Program, a web-based tool to visualize existing social support resources and provide a tailored list of additional resources; and (2) Caregiver Navigation, including weekly phone sessions with a Caregiver Navigator to address caregiver social support needs. Outcomes are assessed by questionnaires completed by the caregiver (baseline, 4-week, 8-week) and the cancer patient (baseline, and 8-week). At 8 weeks, caregivers in the wait-list condition may opt into the intervention. Our primary outcome is caregiver well-being; we also explore patient well-being and caregiver and patient health care utilization.
Discussion
This protocol describes a study testing a novel social support intervention that pairs a web-based social network visualization tool and resource list (eSNAP) with personalized caregiver navigation. This intervention is responsive to a family-centered model of care and calls for clinical and research priorities focused on informal caregiving research.
Trial registration
clinicaltrials.gov, Registration number: NCT04268979; Date of registration: February 10, 2020, retrospectively registered.
Similar content being viewed by others
Background
Caregiving is an important public health priority [1, 2] and the role of informal family caregivers in the health care system is expanding [3]. Family caregivers are defined by the American Cancer Society as individuals who help or support a person with cancer and are not paid to do so; often, this role is taken on by family members or close friends (“chosen family”; [4]). These caregivers relieve demands on the formal health care system by performing care tasks and help patients remain at home [5, 6]. However, informal caregiving can be burdensome [7,8,9] and adversely affect caregiver well-being [10,11,12], which is associated with higher patient distress, increased risk/rate of hospitalization, and excess mortality [13,14,15].
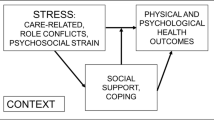
As described in the Stress Process Model of Caregiving [16], social support and other means of coping can impact the relationship between caregiving burden and well-being. Caregivers whose informal social network (friends, family, acquaintances) provides adequate social support experience lower levels of burden, better health, and improved quality of life [17,18,19,20,21]. This support can also positively impact patients, whose psychosocial outcomes are highly interdependent with caregivers’ [22,23,24]. Caregivers with resources to cope, conferred through social support, are also better able to keep patients at home, thus decreasing costly hospitalizations [13, 25,26,27]. However, caregivers experience barriers to obtaining support, including intrapersonal barriers, such as difficulty identifying available support in the moment, interpersonal barriers, such as difficulty asking for help, and systemic barriers, such as a lack of availability of formal, comprehensive, proactive caregiver support programs [20, 28,29,30]. As a result, caregivers may not receive any support or may receive support too late to benefit [31, 32].
Caregivers of patients with a primary malignant brain tumor face unique demands and increased burden [33,34,35] based on rapid disease progression, significant physical debilitation, cognitive decline, as well as personality and behavior changes associated with the disease [33, 36,37,38,39]. However, despite their potentially greater need, few support interventions have been specifically designed for and assessed in caregivers of patients with primary brain tumor [40].
To overcome barriers in caregivers’ social support utilization, our team has developed a caregiver support intervention with input from caregivers of patients with primary malignant brain tumor. The 8-week intervention blends technology with personal contact, and consists of two components. The first component is the electronic Social Network Assessment Program (eSNAP), a web-based tool to visualize existing social support resources and provide a tailored list of additional resources [30, 41]. As seen in Fig. 1, eSNAP quickly collects and organizes social support information entered by caregivers to visualize the size, quality, and function of support networks. Visualizations can help caregivers catalogue support resources and present them in a new way, which may make them more salient and remind caregivers of their availability. The second component of the intervention is assistance from a Caregiver Navigator. The Caregiver Navigator assesses social support via eSNAP and provides navigation via brief, weekly telephone sessions. Navigation sessions are designed to help caregivers identify and problem-solve barriers to finding support resources to meet their needs, including help with identifying and leveraging support within their own informal social network (i.e. from family/friends). These sessions are also intended to enroll or direct caregivers to formal services within the institution or community (e.g. social work, mentorship).
Methods/design
Study aims
The primary aim of this randomized controlled trial (RCT) is to evaluate the efficacy of the eSNAP + Caregiver Navigator intervention on caregiver wellbeing. We hypothesize caregivers of patients with primary malignant brain tumor who receive the intervention will report less perceived burden, operationalized as scores on the Zarit Burden Scale Short Form [42], and lower levels of distress, operationalized as scores on the Patient Health Questionnaire-8 (PHQ-8) [43] and the General Anxiety Disorder-7 (GAD-7) [44], when compared with waitlist controls at the Week 8 follow-up. Although the intervention is caregiver-focused, given the interdependent relationship between caregiver and patient, we anticipate our intervention will also indirectly impact patient well-being and health care utilization outcomes. Thus, our second aim is to evaluate the efficacy of eSNAP + Navigator on patient well-being and patient and caregiver health care utilization. We hypothesize that patients whose caregivers receive the intervention will report less distress, operationalized as scores on the PHQ-8 and GAD-7, lower numbers of patient unplanned outpatient appointments, ER visits, and hospitalizations, and increased caregiver use of social work or other support services, when compared with waitlist controls at the Week 8 follow-up.
Study design
Our study uses a prospective, longitudinal 2-group RCT design, comparing participants who receive the intervention to a wait-list control group (Fig. 2). The COVID-19 pandemic has impacted the ability of study team members to recruit potential participants, due to limits on non-essential staff and visitors allowed in clinic for in-person recruitment as well as lower clinic volumes. However, the key study activities are conducted online (data collection, eSNAP) and by phone (Caregiver Navigation), and as such, the protocol has not significantly been changed by the pandemic.
Participants
Participant inclusion criteria include: 1) age 21+ years, 2) English-speaking, and 3) able to complete questionnaires (including by proxy). Caregivers must self-identify as being a primary caregiver—a family member, friend, or other unpaid person—who provides at least some care at home for a patient with primary malignant brain tumor. Patients must be diagnosed with a new or recurrent primary malignant brain tumor within the last 6 months, be undergoing active treatment, and have a prognosis of at least 6 months. Both patients and caregivers must consent to study participation.
Recruitment
Participants are recruited through the Moffitt Cancer Center Neuro-Oncology Clinic. Patients and accompanying caregivers are approached during scheduled clinic visits and/or mailed study information around the time of a clinic visit. Flyers also are available in clinic for oncologists and other staff to distribute. Study staff meet in person or by phone with caregivers and patients who express interest in the study to provide additional information and answer any questions before obtaining verbal informed consent, which is documented by research staff.
Procedures
Caregiver and patient dyads are recruited within 6 months of patient diagnosis. At enrollment, participants complete baseline questionnaires electronically using REDCap (paper versions will be available upon request). Caregiver questionnaires include demographics, burden, social support, and health care utilization measures. Patient questionnaires include measures of distress. Upon completion of questionnaires, caregiver participants are randomized by study staff to either the intervention (use of eSNAP + Caregiver Navigator) or waitlist control condition. Participants are randomized by study staff to either the intervention (use of eSNAP + Caregiver Navigator) or wait list control conditions. Participants are randomized using an automated random assignment tool in REDCap (Research Electronic Data Capture; [45, 46]), a secure data collection and management software. To ensure temporal balance between conditions, a randomized block design (blocks of 8) is used and participants are stratified by caregiver sex. Condition is not blinded.
Waitlist control
Participants randomly assigned to the waitlist control condition complete questionnaires during the 8-week study period. After the 8 weeks, they are invited to access the intervention as described below, including completion of questionnaires, eSNAP, and 8 weeks of Caregiver Navigator sessions. A waitlist control condition was selected to capture usual care conditions and to avoid potential confounding of the additional social support that often accompanies an attention-control condition. Waitlisted participants, as in usual care, may engage their own support networks and seek or get referrals to formal support services from health care providers as needed. However, waitlisted participants receive no social network visualization and there is no existing process in place to proactively and systematically direct caregivers to engage informal or formal support resources.
eSNAP and caregiver navigation
Caregivers randomly assigned to the intervention condition receive access to eSNAP and Caregiver Navigation sessions. First, caregivers are enrolled in eSNAP. Caregivers are assigned a user name and password and instructed on how to complete their support visualization in eSNAP using a personal device. In addition to a visualization of existing support, eSNAP makes tailored suggestions for additional resources available within the cancer center or community. Study staff are available to oversee the eSNAP process and answer questions. If participants are not able to complete eSNAP in one attempt, they may save their input and return to it later. Intervention participants are able to access and edit their eSNAP visualization or access resources lists at any time (with reminders given weekly during navigation). To assist enrolled caregivers without internet access at home, tablets are available to study participants to use while at Moffitt at any point during the study period.
Second, caregivers receive sessions from a Caregiver Navigator. Two Caregiver Navigators with non-clinical background were provided education on the neuro-oncology patient and caregiver experience, including reading and didactic sessions, shadowing, and informant interviews. Navigators reviewed available support resources collected by the study team and conducted a scan for additional resources in the cancer center and community. Training was also provided on key navigation skills, including assessment, problem-solving, and motivational interviewing, as well as basic research skills and ethics. Upon the first contact, the Caregiver Navigator conducts an intake assessment the caregiver’s social support and burden based on the eSNAP support visualization and through telephone consultation. Based on this assessment, the Caregiver Navigator develops a caregiver-specific plan to address caregiver support needs over the additional 8 navigator sessions (See Table 1). The Caregiver Navigator may provide social support directly (e.g., emotional, informational) or may assist caregivers in obtaining support resources from their network or from formal resources through motivational interviewing or problem solving.
Navigation calls are anticipated to be approximately 30 min weekly over 8 weeks. Caregivers are also able to contact navigators outside of session if needed. Each session begins with a brief assessment and manualized topics are planned, but sessions vary depending on caregiver needs. The navigator is flexible given dynamic changes that occur with each patient’s functioning, treatment, and prognosis, and caregiver’s needs and resources to handle changes. A major goal of the Caregiver Navigator is to provide caregivers with tools to identify and capitalize on their existing support resources and integrate them with available formal services, including Moffitt social work. This integration helps to transition caregivers from the Caregiver Navigator at the end of the study period. However, this transition may be delayed if caregivers are in crisis at the end of 8 weeks.
A log is made of all Caregiver Navigator contacts, including date/time, caregiver needs and barriers to obtaining support, and the actions taken by the navigator to meet needs or overcome barriers [47]. This log will also be valuable in determining the “active ingredient” or most common/most effective Caregiver Navigator activities. Caregiver Navigator sessions may be recorded (with participant permission) for training or fidelity purposes.
All caregiver participants are asked to complete follow-up questionnaires by emailed or mailed surveys at 4 weeks (F1) and at 8 weeks (F2). Patients complete follow-up questionnaires only at their 8 week clinic visit (F2) to reduce burden. In the intervention condition, caregiver questionnaire reminders are paired with a suggestion to review and update their eSNAP visualization, along with a link to do so, prior to questionnaire completion. Every effort is made to obtain questionnaire data within 2 weeks of each scheduled time point, including reminder emails or phone calls or meeting participants in clinic during patient appointments.
At the conclusion of the 8-week study period, participants are debriefed by phone or in person by trained study staff. At this time, waitlisted participants are invited to receive the intervention, completing questionnaires at 4 and 8 weeks to provide some exploratory data on the effect of timing on the intervention. Caregivers who received the intervention will be asked to provide feedback about what they liked and what could be improved.
Measures
Patient and caregiver participants complete basic demographic and health information questionnaires (including patient function) at baseline. Caregivers complete psychosocial questionnaires at baseline, 4 weeks, and 8 weeks, including measures of burden, distress, and their own and the patients’ health care utilization. Patients complete assessments of distress at baseline and 8 weeks. All data is collected, stored, and managed in secure REDCap databases.
Caregiver burden is assessed using the Zarit Burden Interview Short Form [42], a 12-item widely-used measure of burden. Items are summed and higher scores reflect more burden. The scale has been validated in populations of advanced cancer caregivers and has very good internal consistency and discriminative ability [48]. The scale has also been used to identify changes over time [49].
Distress is assessed for both patients and caregivers using the Patient Health Questionnaire-8 (PHQ-8) [43] and the General Anxiety Disorder-7 (GAD-7) [44]. The PHQ-8 is an 8-item commonly-used measure of depression based on the Diagnostic and Statistical Manual, 4th edition (DSM-IV) criteria for depressive disorders, and has been shown to have good reliability and validity [43]. The measure has previously been used in studies of cancer patients and caregivers [50]. Each item is rated from 0 to 3, then all 8 items are summed to create a total score ranging from 0 to 24, with higher scores indicating worse depression symptoms; established cutoffs exist [43]. The PHQ-8 is similar to the PHQ-9 (minus a question relevant to suicidality), which has been shown to be sensitive to change [51]. The GAD-7 is a 7 item commonly-used measure of anxiety based on the DSM-IV criteria for Generalized Anxiety Disorder, which has been shown to have good reliability and validity [44]. The GAD-7 has been used extensively in both cancer patients and cancer caregivers (e.g., [50, 52]). Each item is rated from 0 to 3, then all 7 items are summed to create a total score ranging from 0 to 21, with higher scores indicating worse anxiety symptoms; established cutoffs exist [44, 51]. The GAD-7 has been shown to be sensitive to change [53].
Health Care Utilization is measured using a self-reported health care utilization questionnaire developed in previous research [54]. Caregivers will be asked to report on their own and the patient’s health care utilization including use of social work or other support services, outpatient appointments, ER visits, and hospitalization, and whether use was related to the caregiver role or stress/ the patient’s cancer.
Sample size
A power analysis for an independent-groups t-test with a medium effect size (Cohen’s d = 0.5) shows that a final sample of 160 (80 per group) would have Power > .80 with alpha = .05 and a two-tailed test. Based on an estimated 75% recruitment and 15% attrition rate at each follow up, we plan to approach 300 caregivers and enroll 225 to achieve a final sample of 160 dyads completing the 8-week assessment for analyses of hypotheses (80 participants in intervention, and 80 in control conditions).
Analysis
Our primary end point is 8-weeks; thus our population of interest is caregivers paired with a patient who survives at least 8 weeks. Recruitment will target those early in the care trajectory. Most patients are expected to survive the 8-week study period. The median survival time for patients with glioblastoma, the most common primary malignant brain tumor in adults, is 14.6 months [55]. In the unlikely event patients no longer receive care at Moffitt (transition to palliative/hospice care, change providers, death) participants will not be dropped.
Missing data
Follow-up data may be missing for a variety of reasons. In general, an intent-to-treat approach has been adopted and study resources will remain available for caregivers and patients whether or not study data is acquired. For example, participants who do not provide data at F1 or who decline navigation will continue to be contacted at F2 to complete questionnaires. Unless the patient and caregiver choose to withdraw from the study, attempts will be made to acquire follow-up data.
Preliminary analysis
Basic descriptive analyses will be conducted. Chi-square and t-tests will be conducted to determine if the intervention (eSNAP + Caregiver Navigator) or waitlist control groups significantly differ on demographics, patient medical characteristics, or outcome measures at baseline. Any measure with a group differences of p < .10 will be controlled for in primary analyses. Group differences and predictors of attrition will be examined using logistic regression. These results will be used to guide interpretation of primary analyses.
Primary analysis
The primary analysis will evaluate the efficacy of eSNAP + Navigator support intervention on caregiver well-being. This can be performed most simply by using independent-samples t-tests, if randomization is fully successful and missing data are Missing Completely at Random. However, Generalized Estimating Equations (GEE) will be used to test the effects of the intervention at F1 and F2 in models that also include the baseline measure of the outcome variable and, if necessary, potential confounds determined by preliminary analyses. This approach manages missing data under the Missing At Random (MAR) assumption and permits analyses beyond those focusing on the outcome variable at 8 weeks (e.g. determining if intervention has an early impact or if effect matures over time). The primary test will be a planned contrast of the 2 conditions at the 8-week assessment within the entire model. The GEE will also assess (1) intervention differences in the primary outcome averaged across the 4 and 8 week assessments (2), change from 4 to 8 weeks, and (3) group differences in change over time (intervention by time interaction term in the model).
Other analyses will evaluate the interdependent effects of eSNAP + Navigator on patient well-being and health care utilization, hypothesizing that patients with caregivers in the intervention group will report greater well-being and less utilization of health care. The primary outcomes are (1) burden, anxiety, and depression scores as (inverse) measures of well-being, and (2) a set of measures for healthcare utilization: the presence of and number of unplanned outpatient visits, the presence of and number of unplanned hospitalizations, and the number of bed days of care in unplanned hospitalizations. For well-being in patients at 8 weeks, linear regression will be used to assess differences between the intervention arms in the context of any potential confounds. For healthcare utilization, the distributions of the outcome variables are expected to warrant 2-stage and/or Poisson regression analyses to assess intervention differences.
Ethical considerations
Ethical approvals have been sought and granted by the Moffitt Scientific Review Committee (MCC 19731) and the Advarra Institutional Review Board (Pro00029204). Informed consent will be obtained from all study participants, in accordance with the Helsinki Declaration. Data will be stored on a secure network, protected by password, and only accessed by researchers involved in this study. This investigation will be carried out in compliance with all human subjects research ethical regulations and guidelines. This study protocol has been registered at clinicaltrials.gov (NCT04268979).
Discussion
Our protocol will test a novel social support intervention that pairs eSNAP, a web-based social network visualization tool and resource list, and caregiver navigation by telephone to address the support needs of caregivers of patients with primary malignant brain tumor and improve caregiver and patient well-being and health care utilization. This is a high-need population that has not received much attention from research. Our proposed intervention contributes to a family-centered model of care, focusing on patient and caregiver outcomes and facilitating caregiver integration with the health care system. We also reduce the burden of participation in part through the use of technology. We meet all recommendations from a recent joint report from the National Cancer Institute and National Institute of Nursing Research on research and clinical priorities for informal caregiving research [56]. This project is an important step to improving support for caregivers of patients with primary malignant brain tumor, providing appropriate levels of health care utilization, and improving the quality of life for families affected by this disease.
Availability of data and materials
The datasets generated and/or analysed during the current study will be available from the corresponding author on reasonable request.
Abbreviations
- eSNAP:
-
Electronic Social Network Assessment Program
- REDCap:
-
Research electronic data capture
- DSM-IV:
-
Diagnostic and Statistical Manual, 4th edition
References
Family Caregiver Alliance. Fact sheet: Selected caregiver statistics 2012. Available from: http://www.caregiver.org/caregiver/jsp/home.jsp.
Shaji KS, Reddy MS. Caregiving: a public health priority. Indian J Psychol Med. 2012;34(4):303–5. https://doi.org/10.4103/0253-7176.108191.
Redfoot D, Feinberg L, Houser A. The aging of the baby boom and the growing care gap: a look at future declines in the availability of family caregivers. AARP Public Policy Institute; 2013.
American Cancer Society. What is a cancer caregiver? 2016 [updated 6/6/2016. Available from: https://www.cancer.org/treatment/caregivers/what-a-caregiver-does/who-and-what-are-caregivers.html.
Taylor DH Jr, Ostermann J, Van Houtven CH, Tulsky JA, Steinhauser K. What length of hospice use maximizes reduction in medical expenditures near death in the US Medicare program? Soc Sci Med. 2007;65(7):1466–78. https://doi.org/10.1016/j.socscimed.2007.05.028.
National Alliance for Caregiving. Cancer caregiving in the US – an intense, episodic, and challenging care experience. National Alliance for Caregiving in partnership with the National Cancer Institute Cancer Support Community; 2016.
Bevans M, Sternberg EM. Caregiving burden, stress, and health effects among family caregivers of adult cancer patients. JAMA. 2012;307(4):398–403. https://doi.org/10.1001/jama.2012.29.
Burton AM, Sautter JM, Tulsky JA, Lindquist JH, Hays JC, Olsen MK, et al. Burden and well-being among a diverse sample of cancer, congestive heart failure, and chronic obstructive pulmonary disease caregivers. J Pain Symptom Manag. 2012;44(3):410–20. https://doi.org/10.1016/j.jpainsymman.2011.09.018.
Doorenbos AZ, Given B, Given CW, Wyatt G, Gift A, Rahbar M, et al. The influence of end-of-life cancer care on caregivers. Res Nurs Health. 2007;30(3):270–81. https://doi.org/10.1002/nur.20217.
Given B, Given CW. Patient and family caregiver reaction to new and recurrent breast cancer. J Am Med Women's Assoc (1972). 1992;47(5):201.
Kurtz ME, Given B, Kurtz JC, Given CW. The interaction of age, symptoms, and survival status on physical and mental health of patients with cancer and their families. Cancer. 1994;74(7 Suppl):2071–8. https://doi.org/10.1002/1097-0142(19941001)74:7+<2071::AID-CNCR2820741715>3.0.CO;2-R.
Robison J, Fortinsky R, Kleppinger A, Shugrue N, Porter M. A broader view of family caregiving: effects of caregiving and caregiver conditions on depressive symptoms, health, work, and social isolation. J Gerontol Ser B Psychol Sci Soc Sci. 2009;64(6):788–98.
Ankuda CK, Maust DT, Kabeto MU, McCammon RJ, Langa KM, Levine DA. Association between spousal caregiver well-being and care recipient healthcare expenditures. J Am Geriatr Soc. 2017;65(10):2220–6. https://doi.org/10.1111/jgs.15039.
Jacobs JM, Shaffer KM, Nipp RD, Fishbein JN, MacDonald J, El-Jawahri A, Pirl WF, Jackson VA, Park ER, Temel JS, Greer JA. Distress is interdependent in patients and caregivers with newly diagnosed incurable cancers. Ann Behav Med. 2017;51(4):519–31. https://doi.org/10.1007/s12160-017-9875-3. PMID: 28097515; PMCID: PMC5513787.
Dionne-Odom JN, Hull JG, Martin MY, Lyons KD, Prescott AT, Tosteson T, et al. Associations between advanced cancer patients' survival and family caregiver presence and burden. Cancer Med. 2016;5(5):853–62. https://doi.org/10.1002/cam4.653.
Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30(5):583–94. https://doi.org/10.1093/geront/30.5.583.
Baron RS, Cutrona CE, Hicklin D, Russell DW, Lubaroff DM. Social support and immune function among spouses of cancer patients. J Pers Soc Psychol. 1990;59(2):344–52. https://doi.org/10.1037/0022-3514.59.2.344.
Newberry A, Kuo J, Donovan H, Given B, Given CW, Schulz R, et al. Identifying family members who are likely to perceive benefits from providing care to a person with a primary malignant brain tumor. Oncol Nurs Forum. 2012;39(3):E226–32. https://doi.org/10.1188/12.ONF.E226-E232.
Nabors N, Seacat J, Rosenthal M. Predictors of caregiver burden following traumatic brain injury. Brain Injury. 2002;16(12):1039–50. https://doi.org/10.1080/02699050210155285.
Northouse L, A-L Williams, Given B, McCorkle R. Psychosocial care for family caregivers of patients with cancer. J Clin Oncol. 2012;30(11):1227–34. https://doi.org/10.1200/JCO.2011.39.5798.
Cannuscio CC, Colditz GA, Rimm EB, Berkman LF, Jones CP, Kawachi I. Employment status, social ties, and caregivers' mental health. Soc Sci Med. 2004;58(7):1247–56. https://doi.org/10.1016/S0277-9536(03)00317-4.
Kim Y, Kashy DA, Wellisch DK, Spillers RL, Kaw CK, Smith TG. Quality of life of couples dealing with cancer: dyadic and individual adjustment among breast and prostate cancer survivors and their spousal caregivers. Ann Behav Med. 2008;35(2):230–8. https://doi.org/10.1007/s12160-008-9026-y.
Kershaw T, Ellis KR, Yoon H, Schafenacker A, Katapodi M, Northouse L. The interdependence of advanced cancer patients' and their family caregivers' mental health, physical health, and self-efficacy over time. Ann Behav Med. 2015;49(6):901–11. https://doi.org/10.1007/s12160-015-9743-y.
Meyler D, Stimpson JP, Peek MK. Health concordance within couples: a systematic review. Soc Sci Med. 2007;64(11):2297–310. https://doi.org/10.1016/j.socscimed.2007.02.007.
Bidwell JT, Lyons KS, Lee CS. Caregiver well-being and patient outcomes in heart failure: A Meta-analysis. J Cardiovasc Nurs. 2017;32(4):372–82. https://doi.org/10.1097/JCN.0000000000000350. PMID: 27617564; PMCID: PMC5346066.
Bonin-Guillaume S, Durand A-C, Yahi F, Curiel-Berruyer M, Lacroix O, Cretel E, et al. Predictive factors for early unplanned rehospitalization of older adults after an ED visit: role of the caregiver burden. Aging Clin Exp Res. 2015;27(6):883–91. https://doi.org/10.1007/s40520-015-0347-y.
Longacre ML, Wong Y-N, Fang CY. An integrative review of US studies: caregiver psychological health and hospitalization characteristics of older adult care recipients. Res Gerontol Nurs. 2014;7(3):139–47. https://doi.org/10.3928/19404921-20140127-01.
Boele FW, van Uden-Kraan CF, Hilverda K, et al. Neuro-oncology family caregivers' view on keeping track of care issues using eHealth systems: it's a question of time. J Neurooncol. 2017;134(1):157–67. https://doi.org/10.1007/s11060-017-2504-y.
Berry LL, Dalwadi SM, Jacobson JO. Supporting the supporters: what family caregivers need to care for a loved one with cancer. J Oncol Pract. 2016;13(1):35–41. https://doi.org/10.1200/JOP.2016.017913.
Reblin M, Wu YP, Pok J, Kane L, Colman H, Cohen AL, et al. Development of the electronic social network assessment program using the center for eHealth and wellbeing research roadmap. JMIR Human Fact. 2017;4(3):e23. https://doi.org/10.2196/humanfactors.7845.
Montgomery RJ, Marquis J, Schaefer JP, Kosloski K. Profiles of respite use. Home Health Care Serv Q. 2002;21(3–4):33–63. https://doi.org/10.1300/J027v21n03_03.
Montgomery R, Kwak J. Tcare: tailored caregiver assessment and referral. J Soc Work Educ. 2008;44(sup3):59–64.
Sherwood PR, Given BA, Given CW, Schiffman RF, Murman DL, Lovely M, et al. Predictors of distress in caregivers of persons with a primary malignant brain tumor. Res Nurs Health. 2006;29(2):105–20. https://doi.org/10.1002/nur.20116.
Sherwood P, Given B, Given C, Schiffman R, Murman D, Lovely M. Caregivers of persons with a brain tumor: a conceptual model. Nurs Inq. 2004;11(1):43–53. https://doi.org/10.1111/j.1440-1800.2004.00200.x.
Sherwood PR, Given BA, Donovan H, Baum A, Given CW, Bender CM, et al. Guiding research in family care: a new approach to oncology caregiving. Psycho-Oncol. 2008;17(10):986–96. https://doi.org/10.1002/pon.1314.
Schmer C, Ward-Smith P, Latham S, Salacz M. When a family member has a malignant brain tumor: the caregiver perspective. J Neurosci Nurs. 2008;40(2):78–84. https://doi.org/10.1097/01376517-200804000-00006.
Schubart JR, Kinzie MB, Farace E. Caring for the brain tumor patient: family caregiver burden and unmet needs. Neuro-oncology. 2008;10(1):61–72. https://doi.org/10.1215/15228517-2007-040.
Gregg N, Arber A, Ashkan K, Brazil L, Bhangoo R, Beaney R, et al. Neurobehavioural changes in patients following brain tumour: patients and relatives perspective. Support Care Cancer. 2014;22(11):2965–72. https://doi.org/10.1007/s00520-014-2291-3.
McConigley R, Halkett G, Lobb E, Nowak A. Caring for someone with high-grade glioma: a time of rapid change for caregivers. Palliat Med. 2010;24(5):473–9. https://doi.org/10.1177/0269216309360118.
Sherwood PR, Cwiklik M, Donovan HS. Neuro-oncology family caregiving: review and directions for future research. CNS Oncol. 2016;5(1):41–8. https://doi.org/10.2217/cns.15.43.
Reblin M, Ketcher D, Forsyth P, Mendivil E, Kane L, Pok J, et al. Feasibility of implementing an electronic social support and resource visualization tool for caregivers in a neuro-oncology clinic. Support Care Cancer. 2018;26(12):4199–206. https://doi.org/10.1007/s00520-018-4293-z.
Bedard M, Molloy DW, Squire L, Dubois S, Lever JA, O'Donnell M. The Zarit burden interview: a new short version and screening version. Gerontologist. 2001;41(5):652–7. https://doi.org/10.1093/geront/41.5.652.
Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–73. https://doi.org/10.1016/j.jad.2008.06.026.
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. https://doi.org/10.1001/archinte.166.10.1092.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010. Epub 2008 Sep 30. PMID: 18929686; PMCID: PMC2700030.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010.
Freund KM, Battaglia TA, Calhoun E, Dudley DJ, Fiscella K, Paskett E, et al. National Cancer Institute Patient Navigation Research Program. Cancer. 2008;113(12):3391–9. https://doi.org/10.1002/cncr.23960.
Higginson IJ, Gao W, Jackson D, Murray J, Harding R. Short-form Zarit caregiver burden interviews were valid in advanced conditions. J Clin Epidemiol. 2010;63(5):535–42. https://doi.org/10.1016/j.jclinepi.2009.06.014.
Gaugler JE, Mittelman MS, Hepburn K, Newcomer R. Clinically significant changes in burden and depression among dementia caregivers following nursing home admission. BMC Med. 2010;8(1):85. https://doi.org/10.1186/1741-7015-8-85.
Mosher CE, Winger JG, Hanna N, Jalal SI, Einhorn LH, Birdas TJ, et al. Randomized pilot trial of a telephone symptom management intervention for symptomatic lung cancer patients and their family caregivers. J Pain Symptom Manag. 2016;52(4):469–82. https://doi.org/10.1016/j.jpainsymman.2016.04.006.
Kroenke K, Spitzer RL, Williams JB, Löwe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–59. https://doi.org/10.1016/j.genhosppsych.2010.03.006.
Ullrich A, Ascherfeld L, Marx G, Bokemeyer C, Bergelt C, Oechsle K. Quality of life, psychological burden, needs, and satisfaction during specialized inpatient palliative care in family caregivers of advanced cancer patients. BMC Palliative Care. 2017;16(1):31. https://doi.org/10.1186/s12904-017-0206-z.
Dear BF, Titov N, Sunderland M, McMillan D, Anderson T, Lorian C, et al. Psychometric comparison of the generalized anxiety disorder scale-7 and the Penn State worry questionnaire for measuring response during treatment of generalised anxiety disorder. Cogn Behav Ther. 2011;40(3):216–27. https://doi.org/10.1080/16506073.2011.582138.
Byrne MM, Koru-Sengul T, Zhao W, Weissfeld JL, Roberts MS. Healthcare use after screening for lung cancer. Cancer. 2010;116(20):4793–9. https://doi.org/10.1002/cncr.25466.
National Brain Tumor Society. Quick Brain Tumor Facts. http://braintumor.org/brain-tumor-information/brain-tumor-facts/.
Kent EE, Rowland JH, Northouse L, Litzelman K, Chou W-YS, Shelburne N, et al. Caring for caregivers and patients: research and clinical priorities for informal cancer caregiving. Cancer. 2016;122(13):1987–95. https://doi.org/10.1002/cncr.29939.
Acknowledgements
The authors would like to thank Richard Roetzheim, Amy Otto, Laura Rodriguez, and Kerie Walters.
Funding
Study activities and effort for MR, RM, VBM, SKS, BZ, KJW, SS, PF, and MMB are funded by the National Cancer Institute award R01 CA236034–01 (PI Reblin); DK’s time is funded by award 5T32CA090314–16 (PI Brandon/Vadaparampil). The study protocol has been independently peer-reviewed by the funder. This funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Author information
Authors and Affiliations
Contributions
MR conceptualized and designed the study with contributions from KJW, MMB, SKS, SS, PF, and BZ; MR was awarded funding to support the work. VBM, RM, and DK wrote the first draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approvals for human subjects research have been sought and granted by the Advarra Institutional Review Board (Pro00029204). Verbal informed consent will be obtained from all study participants and documented in study databases by consenting staff. All participants receive an information sheet and are reminded of their rights as participants prior to submission of questionnaire data. As a low-risk study and in consideration of remote research procedures due to the COVID-19 pandemic, written consent was waived. All study activities will be conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Reblin, M., Ketcher, D., McCormick, R. et al. A randomized wait-list controlled trial of a social support intervention for caregivers of patients with primary malignant brain tumor. BMC Health Serv Res 21, 360 (2021). https://doi.org/10.1186/s12913-021-06372-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-021-06372-w