Abstract
Background
Many low- and middle-income countries (LMIC) are moving towards enforcing prescription-only access to antibiotics. This systematic literature review aims to assess the interventions used to enforce existing legislation prohibiting over-the-counter (OTC) sales of antibiotics in LMICs, their impact and examine the methods chosen for impact measurement including their strengths and weaknesses.
Methods
Both PubMed and Embase were systematically searched for studies reporting on impact measurement in moving towards prescription only access to antibiotics in LMICs. The PRISMA methodological review framework was used to ensure systematic data collection and analysis of literature. Narrative data synthesis was used due to heterogeneity of study designs.
Results
In total, 15 studies were included that assessed policy impact in 10 different countries. Strategies employed to enforce regulations prohibiting OTC sales of systemic antibiotics included retention of prescriptions for antibiotics by pharmacies, government inspections, engaging pharmacists in the design of interventions, media campaigns for the general public and educational activities for health care workers. A variety of outcomes was used to assess the policy impact; changes in antimicrobial resistance rates, changes in levels of antibiotic use, changes in trends of antibiotic use, changes in OTC supply of antibiotics, and changes in reported practices and knowledge of pharmacists, medicine sellers and the general public. Differences in methodological approaches and outcome assessment made it difficult to compare the effectiveness of law enforcement activities. Most effective appeared to be multifaceted approaches that involved all stakeholders. Monitoring of the impact on total sales of antibiotics by means of an interrupted time series (ITS) analysis and analysis of pharmacies selling antibiotics OTC using mystery clients were the methodologically strongest designs used.
Conclusions
The published literature describing activities to enforce prescription-only access to antibiotics in LMICs is sparse and offers limited guidance. Most likely to be effective are comprehensive multifaceted interventions targeting all stakeholders with regular reinforcement of messages. Policy evaluation should be planned as part of implementation to assess the impact and effectiveness of intervention strategies and to identify targets for further activities. Robust study designs such as ITS analyses and mystery client surveys should be used to monitor policy impact.
Similar content being viewed by others
Background
Antimicrobial resistance (AMR) is a rapidly growing threat for global health [1]. Higher rates of AMR are observed in countries with high consumption of antibiotics. The higher resistance rates in southern and eastern Europe compared to northern Europe, for example, likely relate to the higher consumption of antibiotics in these regions [2]. Inappropriate prescribing and self-medicating are factors contributing to inappropriate use of antibiotics and may promote the emergence of resistant bacteria [3]. Self-medication with antibiotics is associated with incorrect self-diagnosis, short duration of treatment and inappropriate choice of therapeutic class and dosage [4,5,6,7]. Non-prescription use of antibiotics may also result from poor guidance regarding their use and safety by the pharmacist [8].
In many countries over-the counter (OTC) sales of antibiotics is prohibited by law [9], although that does not mean that antibiotics are not sold OTC in those countries [10]. Law enforcement requires adequate resourcing with well-functioning and effective registration systems for medicines and medicine suppliers, sufficient inspection capacity and a legal system able to impose penalties for breaches of regulations and these are not in place in many countries. Also, strict prohibition of OTC sales of antibiotics could lead to worse access to medicines in rural areas and amongst the poorest populations since pharmacies and chemical shops are often their first line of care [11]. Many low- and middle-income countries (LMIC) are currently moving towards prescription-only access to antibiotics [12]. They announce and undertake a variety of activities to support enforcement of existing laws and regulations, including governmental inspections, media campaigns, and educational activities.
Assessing the impact of a policy intervention is of utmost importance to identify whether the intended effects have been achieved and/or whether unintended effects occur [13]. In many cases this assessment is not planned nor carried out. Additionally, policy makers or researchers may be faced with methodological challenges if they wish to assess the impact of interventions. For example, there may be a lack of baseline data for before-after assessments, insufficient data points for a robust analysis or poor quality of data. Measurement of the effectiveness of interventions to enforce prescription-only access to antibiotics provides a useful example to examine the strengths and weaknesses of different methodological approaches to assessing the effectiveness of policy interventions, in this case, to ban or limit OTC sales of antibiotics. This systematic literature review aims to identify studies conducted in LMICs to enforce existing legislation and regulations prohibiting the OTC sales of antibiotics, assess the interventions used, their impact and examine the methods chosen for impact measurement including their strengths and weaknesses.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodological review framework was used to ensure systematic data collection and analysis of literature [14].
Search strategy
A systematic literature search for studies reporting interventions supporting law enforcement of prohibiting OTC sales of antibiotics in LMICs was conducted in August 2018, in both PubMed and Embase. A combination of both Medical Subject Headings (MeSH) and non-MeSH key term for OTC sales, antibiotics and legislation using Boolean operators, were used for Pubmed. The full search strategy is included as an online resource (see Additional file 1). Also, reference lists of retrieved articles were searched for relevant articles by means of the snowball technique. All titles and abstracts were exported into ProQuest RefWorks to identify and remove duplicates.
Inclusion and exclusion criteria
Eligible studies had to report on the impact of single or multiple interventions supporting enforcement of laws prohibiting OTC sales of antibiotics. In this paper, these interventions are referred to as ‘law enforcement activities’. Studies were excluded if they did not contain information about the impact of law enforcement activities, did not focus on LMICs, were published before 2000 or both the title and abstract were not available in English. LMIC status was defined as countries having a LMIC status during the policy intervention (source: Worldbank). Both ‘OTC status’ and ‘non-prescription sales’ refer to medicines sold directly to a consumer without a prescription. These definitions do not include selling of antibiotics scheduled for pharmacist-initiated use. The articles were not selected based on quality of the study or risk of bias. A formal risk of bias assessment (e.g. using the GRADE approach) is not provided. Strengths and weaknesses of the studies were discussed and taken into consideration in the interpretation of the results as part of the main study aim.
Data analysis
Heterogeneity of study designs, settings and outcome measures precluded any formal pooling of quantitative results. Therefore, a narrative synthesis was conducted. Information about study location, year of intervention, law enforcement activities undertaken, data sources, outcome measures, size of study, and study results were extracted from the studies. In addition, we report the strengths and limitations of the different methodological approaches used.
Results
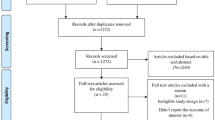
The literature search resulted in 966 hits (335 hits in PubMed, 631 in Embase). Additionally, the snowballing method resulted in three additional hits. After excluding duplicates, 836 unique hits were screened for relevance based on titles. This resulted in 152 potentially relevant articles. After screening abstracts and/or full texts, 15 studies met the inclusion criteria (Fig. 1). In one case, only the abstract of the article was used because the full text was written in Spanish [15]. Several studies examined data from more than one country. The included studies reported on law enforcement in Brazil (n = 5) [16,17,18,19,20], Mexico (n = 2) [18, 19], Chile (n = 2) [15, 21], Colombia (n = 1) [21], Venezuela (n = 1) [21], Bosnia and Herzegovina (n = 2) [22, 23], Azerbaijan (n = 1) [24], North Macedonia (n = 1) [25], Vietnam (n = 3) [5, 26, 27] and Thailand (n = 2) [27, 28]. Countries were grouped by WHO region to account for potential regional and cultural differences in antibiotic use and implementation of regulations. The main characteristics of the studies including law enforcement activities, the method(s) of impact measurement and the key results are displayed in Table 1.
All studies focused on the prohibition of OTC antibiotics; in the Venezuelan case [21], the prohibition only applied to selected classes of antibiotics. The most common interventions were media campaigns (seven countries), government inspections (eight countries) and retention of prescriptions (eight countries). Education of pharmacists formed part of the suite of interventions in three countries. Only in Chile and Thailand, pharmacists or grocery store owners were involved in designing the law enforcement activities (Table 2).
Five main outcomes were reported in the 15 eligible studies: changes in AMR trends, changes in volumes of antibiotic use, change in patterns of antibiotic use, changes in level of OTC supply of antibiotics and changes in reported practice and knowledge about prescription-only access to antibiotics. One study used AMR data. Eight used antibiotic sales data by means of an interrupted-time-series (ITS) analysis or an observational study design. An ITS analysis is a quasi-experimental design, which applies statistical techniques such as segmented regression analysis to estimate both the direct policy impact on sales data (change in levels of consumption) as well as the impact on trends of consumption of antibiotics over time (e.g. does the slope differ from what was expected in the absence of the intervention). ITS studies can be conducted with or without a control group, the latter capturing changes in the prescribing environment apart from the intervention. Studies using an observational design, analysed annual antibiotic sales data by means of a trend analysis to see whether total antibiotic sales increased, decreased, or did not change during the period of analysis. It differs from the trend analysis in ITS studies in that ITS studies compare trends before and after the intervention, whilst these observational studies assess only one trend starting at the point of intervention. Two studies used surveys to assess the impact of an intervention on knowledge about antibiotic use and self-reported practice regarding antibiotics. The mystery client method was used in three studies; either by using before-and-after measurement or in a quasi-experimental controlled trial setting in which intervention groups were compared with non-intervention groups. One study used both the mystery client method and questionnaires.
Main study results
All obtained information is grouped by country and WHO region. First, a short description of all reported policy measures is provided, followed by a description of the impact of the law enforcement as reported in the literature. A summary of all included studies, the methods used, and interpretation of the results is given in Table 1. Table 2 provides an overview of all law enforcement activities undertaken, and the reported impact is provided for each country.
The Americas
The Brazilian Health Regulatory Agency (ANVISA) implemented a policy enforcing the prohibition of OTC sales of all systemic antibiotics in 2010. They announced regular inspections of pharmacies to check the pharmacy’s compliance with the new policy. ANVISA did not carry out any information campaigns to inform patients about the policy change or to reduce inappropriate use of antibiotics. The effect of the enforcement in 2010 was measured in five different studies, which all reported a decrease in total antibiotic sales, based on both private commercial sales data from IMS Health and sales data reported by 3000 pharmacies in the private sector [16,17,18,19,20]. A decrease in sales of penicillins, sulphonamides, and macrolides [17, 18, 20] was reported, while the sales of fluoroquinolones seemed to increase [20]. The effect on antibiotic sales trend was assessed by means of an ITS analysis, however, the results differed between the two studies. Santa-Ana-Tellez et al. found no difference in antibiotic sales trend before and after the policy change [18], whereas Moura et al. reported a significant decrease in trend [17]. Moura et al. used quarterly antibiotic sales between January 2007 and June 2012, while Santa-Ana-Tellez et al. used monthly sales data between January 2008 and December 2012. The exact cause of this discrepancy in results is not clear. In addition, Santa-Ana-Tellez et al. (2015) investigated the impact of the policy intervention on appropriate antibiotic use by using seasonal variation of penicillin sales between summer and winter, and no difference was found [19]. Mattos et al. assessed the effect of the restriction of OTC sales of antibiotics on bacterial resistance trends in Brazil [16]. Annual resistance data of urinary Escherichia coli cultures from outpatients with a suspected urinary tract infection between 2009 and 2015 were compared with annual sales data of antibiotics (2008–2012) to study the impact of the policy intervention on bacterial resistance. Although an immediate decrease in antibiotic sales was seen, it did not appear to have any influence on the increasing bacterial resistance trend [16].
Law enforcement activities to reduce non-prescription sales of antibiotics started in 2010 and focused on both pharmacists and the general public in Mexico. Pharmacies had to retain and register all prescription data for systemic antibiotics. The ministry of health (MoH) announced significant penalties and revocation of licenses in case of non-compliance [32]. In addition, patients were informed about the policy change by means of a media campaign [33]. Santa-Ana-Tellez et al. reported a direct reduction in antibiotic sales in the private sector (assessed using IMS Health data) after the intervention [18]. Sales of penicillins and sulphonamides decreased significantly [18]. Moreover, the seasonal variation decreased significantly from 1.1 defined daily dose per thousand inhabitants per day (DID) to 0.7 DID [19]. While there was a change in the level of consumption, there was no change was seen in trends of antibiotic sales over time before and after the intervention [18].
Chile moved towards prescription-only access to antibiotics in 1999. The MoH targeted both pharmacies and the general public in enforcing the regulation. In addition, pharmacists were involved in the design of the enforcement. Antibiotics were removed from a list of medicines having sales incentives provided for pharmacy staff. Wirtz et al. assessed the impact on changes in antibiotic use and changes in patterns of antibiotic use in three different countries by means of an ITS analysis. They reported a considerable decrease in overall private antibiotic sales (− 5.56 DID; baseline: 14.5 DID) after the intervention [21]. Moreover, the trend in antibiotic sales over time seemed to reduce in the 3 years after the implementation compared to before the intervention [21]. However, there was no evidence of persisting long-term effects of the intervention. After 2002 the sales increased again, resulting in similar numbers of antibiotic sales in 2008 compared to before the intervention [15].
In 2005, Colombia only focussed on the district of Bogota for reducing OTC sales of antibiotics. An information campaign targeted the general public both before and during the intervention. However, no information about retaining prescriptions and inspections in pharmacies was reported [21]. A small decrease in private antibiotic sales (− 1.00 DID; baseline 8 DID) was observed after the intervention by means of an ITS analysis. The total sales of antibiotics in the private sector decreased over time, although no change in trend of antibiotic sales was seen that could be attributed to the intervention [21].
OTC sales of quinolones, macrolides-lincosamides, third-generation cephalosporins and rifampicin were prohibited from 2006 onwards in Venezuela. Law enforcement only targeted pharmacist by obliging them to retain prescriptions. However, no records of actual inspections were found. The new regulation seemed to have no effect on reducing antibiotic sales, while penicillin use sharply increased in Venezuela [21].
In summary, all studies assessing policy impact in The Americas used antibiotic sales data. In Brazil, a direct reduction in antibiotic sales in the private sector was reported after the policy intervention. There was inconsistency in evidence about differences in trends of consumption of antibiotics before and after the intervention in Brazil. No impact was found on seasonal variation Brazil, whereas the seasonal variation decreased in Mexico. In both Mexico and Colombia, the antibiotic sales decreased after the intervention, but no impact on sales trends was found. The largest direct impact on antibiotic sales was seen in Chile, whereas no impact was reported in Venezuela.
Europe
A multifaceted program targeting patients, pharmacists and other healthcare workers was implemented by the regulatory authorities in the Republika Srpska (Bosnia and Herzegovina) from 2010 onwards. Media campaigns were initiated, and a guideline was developed for pharmacy workers to assist them in decisions regarding providing legal OTC medicines or referral of the patient to a doctor. The impact on OTC supply of antibiotics was assessed by means of the mystery client method. The number of pharmacies selling antibiotics significantly decreased between 2010 and 2015 (from 58 to 18.5%) [22]. However, no effect was seen on total sales of antibiotics reported by the Bosnia and Herzegovina Public Health Institute between these years; the total antibiotic sales remained stable, which might suggest that more patients visited doctors to obtain a prescription [23].
Azerbaijan also implemented a multifaceted program targeting pharmacists and the general public from 2011 onwards to reduce OTC sales of antibiotics. Throughout the period of analysis, media campaigns were set-up every year in April and November intended to educate pharmacists and the general public about appropriate antibiotic use. In addition, pharmacists were educated about the changed regulation during inspections by governmental workers. A substantial decrease in total sales of antibiotics (based on import records) was reported between 2011 and 2015 (from 17.1 DID to 8.02 DID).
The policy intervention in North Macedonia took place in 2014 and 2015 and targeted both health care workers and the general public. Ivanovska et al. assessed change in patient knowledge and self-reported practices regarding non-prescription use of antibiotics by the parents and their children [25]. A survey of the general public was conducted three times; before the interventions (2014, n = 403), after a national media campaign about antibiotic use (2015, n = 400) and after implementing fines for pharmacies selling antibiotics without a prescription (2016, n = 400). This study showed an initial impact of the media campaign by an increasing percentage of children using antibiotics with a prescription (89 to 95%). However, this impact subsided during the third round of questionnaires after 1 year. No effect was seen in self-medication practices in the parents [25].
South-East Asia
In Vietnam, 29 pharmacies were exposed to a regulatory enforcement, educational, and peer influence intervention between 1998 and 1999 aimed to reduce the number of pharmacists selling antibiotics without prescription. The interventions led to a significant drop in pharmacies selling cefalexin without a prescription to a mystery client (56%) compared to reference pharmacies that were not exposed to the intervention (89%) [26]. In addition, Chalker et al. assessed the impact of the interventions on both knowledge and reported change in practice by means of a survey amongst pharmacies. The number of pharmacists indicating that they would sell cefalexin without a prescription in a questionnaire also decreased in the intervention group (20% instead of 57%) [31].
The same interventions were conducted in 39 pharmacies in Thailand between 1997 and 1999. However, no significant differences in number of pharmacies selling cefalexin without a prescription before and after the interventions were reported [27]. A second intervention that took place in Thailand around 2014 targeted grocery store owners in 20 different villages. Community leaders and government officers visited grocery owners to teach them about responsible antibiotic use and regulations regarding OTC sales of antibiotics [28]. A study by Arparsrithongsagul et al. assessed the impact of the intervention by means of a quasi-experimental design for the mystery client- and survey method. The rate of grocery stores having antibiotics in stock (penicillin in particular) decreased significantly in the villages that were exposed to the intervention (79% vs 23%) compared to the unexposed pharmacies (88% vs. 85%). These results were strengthened by an increase in knowledge of grocery store owners about antibiotic use, especially on regulation of antibiotic sales [28].
Methodological considerations
The studies illustrate a number of methodologies and outcome measures of varying robustness and utility for evaluating the impact of interventions and informing policy actions. The mystery client method closest approaches the measurement of actual practices of pharmacists. In a study conducted in Vietnam [31], only 20% of pharmacists stated that they would sell cefalexin without a prescription post-intervention, yet 56% of the same sample of pharmacies still sold cefalexin to mystery clients without a prescription [26]. This emphasises the importance of measuring actual behaviour instead of self-reported behaviour. However, mystery client studies are resource intensive undertakings. There were four examples in this review of repeat studies using mystery clients to assess changes in practices over time [22, 26,27,28]. Researchers need to be careful in both selection of time points to account for seasonal variations and clinical status of the patient to prevent erroneous conclusions.
Most studies relied on the use of sales data to assess the impact of interventions on volumes or patterns of consumption of antibiotics. To account for changes in medicine use independently of the OTC restrictions, some researchers used sales data of antihypertensives or antibiotic sales data in the public sector as a reference group assuming that the interventions did not affect use in these cases because antibiotics are only accessible by prescription in the public sector [17, 18]. Sales data have the advantage of being readily available at reasonable cost, facilitating tracking of changes and patterns over time. However, changes in antibiotic sales data will reflect the impact of all types of interventions rather than specific elements. Any changes in patients’ knowledge and practice regarding antibiotic usage would likely be attributable to the impact of media campaigns but would not be identified separately in changes in overall volumes of use. This argues for a suite of assessment tools to enable assessment of the impact of the different strategies applied.
Changes in sales of specific antibiotic classes or seasonal variation in penicillin sales will facilitate the evaluation of more targeted interventions to change practice. An important corollary is the need to consider unintended consequences. Shifts away from supply of some classes of antibiotics may be offset by changes to other classes, such as quinolones or third- and fourth-generation cephalosporins that may also be undesirable. System impacts should also be monitored.
Where a policy intervention is implemented at a point in time, an ITS analysis can be conducted [34]. This analysis makes it possible to attribute the observed effect to the intervention and makes this method more robust compared to an observational study design using annual antibiotic sales data. In the studies included in this literature review, only the law enforcement activities in the South American countries could be defined as such. The multifaceted interventions in Azerbaijan and Bosnia and Herzegovina were conducted throughout the whole period of analysis, which made it impossible to define one moment in time. Therefore, time trends in antibiotic use were conducted in the studies assessing the impact of the interventions in these countries. However, many other factors can contribute to changes in antibiotic usage and therefore conclusions on the effectiveness of the interventions need to be more confined.
Multiple studies used a quasi-experimental controlled design in assessing impact on changes in OTC supply of antibiotics or self-reported practice and knowledge. These studies compared pharmacies that were exposed to interventions with unexposed pharmacies to correct for other variables that can affect the outcome. However, these studies [26,27,28, 31] usually include small sample sizes which is unfavourable for the generalisability of the study. Studies using before-and-after measurements to assess the impact on OTC supply of antibiotics included larger samples compared to the ones using a quasi-experimental study design, making the sample more representative for the whole country.
Discussion
This systematic literature review shows that research on the impact of law enforcement activities to reduce OTC access to antibiotics in low and middle-income countries is sparse. We found 15 eligible studies that reported the impact of interventions undertaken in 10 LMICs. Most law enforcement activities included regulatory (governmental inspections), managerial (involvement of pharmacists in designing interventions and retention of prescriptions) and educational (media campaigns and education of pharmacists) interventions. The impact of law enforcement activities varied greatly between countries. This can be explained in part by differences in policy measures and strictness of enforcement, differences in socio-economic factors, or differences in overall medication use. Table 2 shows that countries where no impact of interventions was shown were the countries which implemented least interventions (e.g. Venezuela and Thailand (intervention 1997–1999)). This points towards the need of implementing comprehensive multifaceted interventions. In addition, the study from North-Macedonia clearly showed that the effect ceased after the campaign had stopped. A wide range of methods and outcomes measures to quantify the effect of the enforcement was found. This makes it difficult to quantify the differences in actual impact of law enforcement between studies, countries and therefore between measures that were undertaken to enforce prohibition of OTC sales of antibiotics. It is for example very difficult to compare data on antibiotic use with a decrease in the number of pharmacies selling antibiotics without a prescription. The Bosnia and Herzegovina case indicated that a decrease in pharmacies selling antibiotics not necessarily leads to a decrease in total sales of antibiotics or specific antibiotic classes in a country [22, 23]. Whether the policy interventions can still be considered effective in this country depends on which outcome measure is regarded most important. Using multiple outcomes is essential in assessing policy impact. When looking only at OTC sales of medicine dispensers, for example, one might miss other system impacts such as doctors writing prescriptions or patients using antibiotics more appropriately. Therefore, the policy conclusions and recommendations from the studies (see Table 3) are based on consistency of effect in a positive direction.
In most countries, law enforcement related to the non-prescription sale of all systemic antibiotics. Restriction of OTC sales of only a selected group of systemic antibiotics (reserve antibiotics and tuberculosis medicines) led to an increase in sales of penicillins in Venezuela [21]. A similar effect might be expected in India, since the recent prohibition of OTC sales of a selected group of antibiotics [35]. Since broad-spectrum penicillins and other broad-spectrum antibiotics are the most frequently sold antibiotic classes without a prescription [3, 36], it is important to include these classes to accomplish a significant reduction of antibiotic sales. In LMICs, however, children are still more likely to die due to a lack of access to effective and affordable antibiotics compared to antibiotic resistance [37]. Therefore, a balance has to be struck between reducing excess use of antibiotics and improving access to needed antibiotics in moving towards prescription-only access to antibiotics. This balance cannot be accomplished by only prohibiting OTC sales. There is a need for public information campaigns on more appropriate use of antibiotics [38] and improvement of prescription practices by doctors to reduce unnecessary antibiotic usage [39].
Both media campaigns and legislation requiring retention of antibiotic prescriptions seemed to have impact only when undertaken as longer-term interventions. In Azerbaijan, the public campaign was conducted twice yearly for multiple years [24]. This might have contributed to the significant, persisting decrease in antibiotic sales between 2011 and 2015. A repeating commercial or advertisement makes the population familiar with the content and is more likely to have impact [38, 40]. In contrast, the media campaign in Chile was comprehensive but only operated before and during the implementation of the policy change. This might have influenced the increase in antibiotic sales that was seen 3 years after the law enforcement started [15]. To make a difference, public campaigns should focus on changing the public attitude and behaviour regarding antibiotics [38]. Moreover, public health campaigns in other disciplines have shown that repeated exposure to the campaign often results in a sustained impact.
Only studies conducted in Azerbaijan, Vietnam and Thailand included education of pharmacists, and most of them were considered to be successful. In Azerbaijan, pharmacists were educated about the prescription-only status of antibiotics when they were visited by the governmental inspectors [24]. It is time-consuming to educate all pharmacists face-to-face. However, pharmacies needed to be visited by a governmental inspector anyway to be checked for compliance. Taking the opportunity to educate pharmacists at the same time is an interesting intervention that seemed to contribute to a reduction in total antibiotic sales. In Thailand, influential village people were trained to educate medical sellers in their village about responsible antibiotic use and regulatory measures [28]. This could be a very effective way to reach all untrained medical sellers in rural areas. Retention of prescriptions was mandatory in many included cases. The thoroughness of the inspections of the retained antibiotic prescriptions was unfortunately poorly described in literature. Unsuccessful law enforcement initiatives, like the Venezuela case, failed to impose sanctions for non-compliance [21]. However, in Vietnam and Azerbaijan, the government conducted visits by regulatory inspectors, but no fines were to be given. Despite the lack of sanctions, the law enforcement was effective in both countries [24, 26]. There is insufficient evidence to draw a conclusion about the impact of penalties on behavior of pharmacies in this case.
Ultimately, the policy changes and enforcement of prescription-only access would be expected to encourage more rational use of antibiotics and positively affect antimicrobial resistance patterns in a country. A potential explanation for the lack of impact on AMR patterns in Brazil is that the policy measures did have a direct effect on the level of use as confirmed in some of the other studies in this review [17, 18], but could not change the underlying trend of an overall increase in antibiotic sales. A recent study about the influence of anthropological and socioeconomic factors on global AMR concluded that reduction of antibiotic consumption will not be sufficient to control AMR [29]. In addition to improving antibiotic dispensing practices, countries should focus on improving sanitation, increasing access to clean water, and increasing public health-care expenditure [29]. AMR patterns therefore do not seem the most appropriate outcome measure to assess the impact of interventions aiming at prohibiting OTC sales of antibiotics.
Most studies included in this review assessed policy impact based on antibiotic sales data from the private sector. This sector is suggested to be most affected by the law enforcement activities, since patients already need a prescription when getting medicines dispensed in the public sector. Moura et al. confirmed the absence of an impact of law enforcement in the public sector [17]. The ITS analysis is the preferred statistical method to assess policy impact by means of total antibiotic sales, since it is a strong quasi-experimental approach for evaluating direct changes in levels of consumption as well as the long-term effect and relates the impact to the policy intervention [34]. Moreover, it covers impact on both change in levels of consumption as well as the impact on trends of consumption of antibiotics over time due. However, many factors can influence the total amount of antibiotic sales. For example, accessibility, availability, affordability, reimbursement and other policy changes involving antibiotics can affect the total use of certain medicines [41].
Antibiotic sales data can also be used as a proxy measure for the quality of antibiotic use in a certain country by means of shifts in antibiotic sales and seasonal variation [30]. Moreover, sales data can be used to measure unintended effects of restricting OTC sales of antibiotics. Santa-Ana-Tellez et al. also identified increasing sales of non-steroidal anti-inflammatory drugs (NSAIDs) and cough and cold medications which coincided with decreasing antibiotics sales [42]. Since NSAID may have severe side effects such as stomach bleedings [43], this increase is potentially unintended. Few studies included in this review reported on unintended consequences. However, it is an important feature in designing the evaluation of policy interventions. In this case important consequences are shifts to other classes of antibiotics or other medicines [19, 23, 24], compensatory changes in the health system with greater burden on PHC for doctors to write prescriptions [44], employment of doctors by pharmacies [32, 45], the emergence of black markets [33], or limited access to antibiotic prescriptions in poor rural communities [46].
The mystery client method makes it possible to measure the impact on actual practice of pharmacists in selling antibiotics OTC [47]. However, the number of pharmacies selling antibiotics OTC highly depends on the patient that is asking for the antibiotics and the clinical condition. A study conducted in Spain revealed that patients visiting a pharmacy with symptoms corresponding to urinary tract infection were far more likely to receive antibiotics OTC compared to patients having complaints indicating acute bronchitis (81.1 vs 32.9%) [48]. Moreover, it can be a costly and time-consuming method, does not provide continuous information over time, and often acquires data from a small part of the pharmaceutical system of a country. However, interventions can be targeted at the pharmacy sector if OTC sales of antibiotics are common. Surveys to assess pharmacists’ self-reported practices regarding OTC sales of antibiotics are an inaccurate method to test the impact of law enforcement. It can be assumed that pharmacists tend to give socially desirable answers to the question if they would sell antibiotics OTC, because they know it is prohibited by law. The studies conducted in Vietnam confirmed this, since a large difference was found between self-reported practice and actual practice in the same study population [26, 31]. In addition, the study by Ivanovska et al. showed the importance of the period of time in which the impact is measured, since it might fade over time [25].
Most countries were classified as middle-income countries during the period of law enforcement, except Vietnam and Thailand in 1997 and 1999. It is clear that there is a paucity in data from lower income countries (LICs) and countries from the African and Middle Eastern WHO regions. Multiple studies have reported about the issue of OTC sales of antibiotics in these countries [10], but no initiatives to enforce laws regarding non-prescription sales of antibiotics in Africa have been described according to our review. A possible explanation for this is that LICs often lack regulatory resources, systems to track antibiotic sales, healthcare capacity and access to healthcare to strictly enforce laws and monitor the impact of their measures [49].
The period during which law enforcement took place varied greatly between countries over a period of around 20 years (1997–2016). In this period, methods for communication of policy changes, general knowledge of antibiotics and antibiotic treatment strategies have changed. This makes it difficult to generalize findings regarding the impact of communication strategies. This review only included articles written in English. Therefore, we were not able to include the full text of the paper by Bavestrello et al., but only the English abstract [15]. Another potential limitation of this review is that only two databases were searched, i.e. PubMed and Embase. Although the snowballing technique was used, we might have missed articles on this topic as well as grey literature. Many different study designs have been used to measure policy impact. Due to heterogeneity of study designs it was impossible to pool data from the different studies, which is a limitation of this study. However, the wide range of different included studies also reflects the broad literature search that has been conducted. The strength of this review is that it is the first to provide a full overview of all different methods that have been used to assess impact of law enforcement activities in moving towards prescription-only access to antibiotics.
Conclusions
There are only a few published studies assessing the impact of law enforcement to reduce OTC sales of antibiotics in LMICs, and these offer limited guidance to other authorities who may wish to enforce existing legislation in their own settings. Multiple methods with different outcome measures have been used to assess impact of these interventions. These differences make it hard to compare outcomes and determine the effectiveness of specific law enforcement activities. However, involvement of pharmacists in the design of interventions, education of medicine dispensers, media campaigns targeted at the general public conducted on a regular basis over a period of time and inspections by governmental workers seemed most effective when implemented as part of a package of measures. ITS analysis and the mystery client method are preferred methods for evaluation of the impact of interventions because they allow for measuring long-term policy impact and actual practice of medicine dispensers, respectively. The appropriate methods should be chosen based on available resources and duration of law enforcement activities. Evaluation of policy impact should be planned and undertaken prior to implementing policy interventions to inform on policy development and guide targets for additional interventions.
Availability of data and materials
Most of the studies included in the literature review are publicly available and can be obtained online. The other papers can be requested from the authors of the document. Aukje Mantel-Teeuwisse can be contacted for questions regarding the data we used.
Abbreviations
- AMR:
-
Antimicrobial resistance
- DID:
-
Defined daily dose per thousand inhabitants per day
- ITS:
-
Interrupted-time-series
- LMIC:
-
Low- and middle-income countries
- MoH:
-
Ministry of health
- NSAID:
-
Non-steroidal anti-inflammatory drugs
- OTC:
-
Over-the-counter
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- WHO:
-
World Health Organization
References
Ventola CL. The antibiotic resistance crisis: part 1 causes and threats. Pharm Ther. 2015;40(4):277–83.
Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–87.
Paget J, Lescure D, Versporten A, Goossens H, Schellevis F, van Dijk L. Antimicrobial resistance and causes of non-prudent use of antibiotics in human medicine in the EU. Euro Commission. 2017; Available from: https://ec.europa.eu/health/amr/sites/amr/files/amr_arna_report_20170717_en.pdf.
Awad A, Eltayeb I, Matowe L, Thalib L. Self-medication with antibiotics and antimalarials in the community of Khartoum State. Sudan J Pharm Pharm Sci. 2005;8(2):326–31.
Chalker J, Chuc NTK, Falkenberg T, Do NT, Tomson G. STD management by private pharmacies in Hanoi: practice and knowledge of drug sellers. Sex Transm Infect. 2000;76(4):299–302.
Hadi U, Duerink DO, Lestari ES, Nagelkerke NJ, Werter S, Keuter M, et al. Survey of antibiotic use of individuals visiting public healthcare facilities in Indonesia. Int J Infect Dis. 2008;12(6):622–9.
Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5(8):481–93.
Guinovart MC, Figueras A, Llor C. Selling antimicrobials without prescription – far beyond an administrative problem. Enferm Infecc Microbiol Clin. 2018;36(5):290–2.
Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis. 2011;11(9):692–701.
Auta A, Hadi MA, Oga E, Adewuyi EO, Abdu-Aguye SN, Adeloye D, et al. Global access to antibiotics without prescription in community pharmacies: a systematic review and meta-analysis. J Inf Secur. 2018;12(59):1–11.
Alhomoud F, Aljamea Z, Almahasnah R, Alkhalifah K, Basalelah L, Kais F. Self-medication and self-prescription with antibiotics in the Middle East—do they really happen ? A systematic review of the prevalence, possible reasons, and outcomes. Int J Infect Dis. 2017;57:3–12.
Iwamoto K, Pedersen HB, Tello JE, Lo Fo Wong D, Robertson J. Cross-programmatic consultation on the role of primary care in the responsible use of medicines and the reduction of antimicrobial resistance. Expert Rev Anti-Infect Ther. 2019;00(00):1–4.
Theobald S, Brandes N, Gyapong M, El-Saharty S, Proctor E, Diaz T, et al. Implementation research: new imperatives and opportunities in global health. Lancet. 2018;0(0):2214–28.
Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):1–6.
Bavestrello FL, Cabello MÁ. Consumo comunitario de antimicrobianos en Chile, 2000-2008. Rev Chil Infectol. 2011;28(2):107–12.
Mattos KPH, Visacri MB, Quintanilha JCF, Lloret GR, Cursino MA, Levin AS, et al. Brazil’s resolutions to regulate the sale of antibiotics: impact on consumption and Escherichia coli resistance rates. J Glob Antimicrob Resist. 2017;10:195–9.
Moura ML, Boszczowski I, Mortari N, Barrozo LV, Neto FC, Lobo RD, et al. The impact of restricting over-the-counter sales of antimicrobial drugs: preliminary analysis of national data. Medicine (Baltimore) 2015;94(38):e1605.
Santa-Ana-Tellez Y, Mantel-Teeuwisse AK, Dreser A, Leufkens HGM, Wirtz VJ. Impact of over-the-counter restrictions on antibiotic consumption in Brazil and Mexico. PLoS One. 2013;8(10):6–11.
Santa-Ana-Tellez Y, Mantel-Teeuwisse AK, Leufkens HGM, Wirtz VJ. Seasonal variation in penicillin use in Mexico and Brazil: analysis of the impact of over-the-counter restrictions. Antimicrob Agents Chemother. 2015;59(1):105–10.
Lopes-Júnior R, de Sá Del Fiol F, Araujo JLO, de Toledo MI, Barberato-Filho S. Decrease in penicillin sales in Brazil after over-the-counter restrictions. Antimicrob Agents Chemother. 2015;59(9):5862–3.
Wirtz VJ, Herrera-Patino JJ, Santa-Ana-Tellez Y, Dreser A, Elseviers M, Vander Stichele RH. Analysing policy interventions to prohibit over-the-counter antibiotic sales in four Latin American countries. Tropical Med Int Health. 2013;18(6):665–73.
Marković-Peković V, Grubiša N, Burger J, Bojanić L, Godman B. Initiatives to Reduce Nonprescription Sales and Dispensing of Antibiotics: Findings and Implications. J Res Pharm Pract | Publ by Wolters Kluwer-Medknow. 2017;6(2):120–5.
Bojanić L, Marković-Peković V, Škrbić R, Stojaković N, Dermanović M, Bojanić J, et al. Recent initiatives in the Republic of Srpska to enhance appropriate use of antibiotics in ambulatory care; Their influence and implications. Front Pharmacol. 2018;9(MAY):1–13.
Abilova V, Kurdi A, Godman B. Ongoing initiatives in Azerbaijan to improve the use of antibiotics; findings and implications. Expert Rev Anti-Infect Ther. 2017;16(1):77–84.
Ivanovska V, Angelovska B, van Dijk L, Zdravkovska M, Leufkens HG, Mantel-Teeuwisse AK. Change in parental knowledge, attitudes and practice of antibiotic use after a national intervention programme. Eur J Public Health. 2018;28(4):724–29.
Chuc NT, Larsson M, Do NT, Diwan VK, Tomson GB, Falkenberg T. Improving private pharmacy practice: a multi-intervention experiment in Hanoi, Vietnam. J Clin Epidemiol. 2002;55(11):1148–55.
Chalker J, Ratanawijitrasin S, Chuc NTK, Petzold M, Tomson G. Effectiveness of a multi-component intervention on dispensing practices at private pharmacies in Vietnam and Thailand - a randomized controlled trial. Soc Sci Med. 2005;60(1):131–41.
Arparsrithongsagul S, Kulsomboon V, Zuckerman IH. Multidisciplinary perspective intervention with community involvement to decrease antibiotic sales in village groceries in Thailand. Asia-Pacific J Public Heal. 2015;27(2):NP2480–8.
Collignon P, Beggs JJ, Walsh TR, Gandra S, Laxminarayan R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Heal. 2018;2(9):e398–405.
Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Taylor TH. Trends and seasonal variation in outpatient antibiotic prescription rates in the United States, 2006 to 2010. Antimicrob Agents Chemother. 2014;58(5):2763–6.
Chalker J, Chuc NTK, Falkenberg T, Tomson G. Private pharmacies in Hanoi Vietnam: a randomised trial of a 2 year multi-component intervention on knowledge and stated practice regarding ARI, STD and antibiotic/steroid requests. Tropical Med Int Health. 2001;7(9):803–10.
Zaidi MB, Dreser A, Figueroa IM. A collaborative initiative for the containment of antimicrobial resistance in Mexico. Zoonoses Public Health. 2015;62(s1):52–7.
Dreser A, Vázquez-Vélez E, Treviño S, Wirtz VJ. Regulation of antibiotic sales in Mexico: an analysis of printed media coverage and stakeholder participation. BMC Public Health. 2012;12:1051.
Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309.
Hazra A, Bengal W, Hazra A, Bengal W. Schedule H1: Hope or hype? Indian J Pharm. 2014;46(4):361–2.
Dameh M, Green J, Norris P. Over-the-counter sales of antibiotics from community pharmacies in Abu Dhabi. Pharm World Sci. 2010;32(5):643–50.
Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen J, Klugman K, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387:168–75.
Huttner B, Goossens H, Verheij T, Harbarth S. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis. 2010;10(1):17–31.
Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–98.
Saam M, Huttner B, Harbarth S. Evaluation of antibiotic awareness campaigns. Geneva; 2016. Available from: http://www.who.int/selection_medicines/committees/expert/21/applications/s6_antibiotic_awareness_campaigns.pdf
Khatib R, McKee M, Shannon H, Chow C, Rangarajan S, Teo K, et al. Availability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet. 2016;387(10013):61–9.
Santa-Ana-Tellez Y, Mantel-Teeuwisse AK, Leufkens HGM, Wirtz VJ. Effects of over-the-counter sales restriction of antibiotics on substitution with medicines for symptoms relief of cold in Mexico and Brazil: time series analysis. Health Policy Plan. 2016;31(9):1291–6.
García Rodríguez L, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343(8900):769–72.
Paninchukunnath A. OTC drug marketing – global trends and Indian experiences. Health care & marketing; 2007. Available from: https://www.google.com/url?sa=t&source=web&rct=j&url=http://citeseerx.ist.psu.edu/viewdoc/download%3Fdoi%3D10.1.1.460.9723%26rep%3Drep1%26type%3Dpdf&ved=2ahUKEwiek4COh8zjAhWqmIsKHWR3AwoQFjAAegQIAhAB&usg=AOvVaw1ozRSrK7fUnjUhpIN3Os.
Pérez-Cuevas R, Doubova SV, Wirtz VJ, Servan-Mori E, Dreser A, Hernández-Ávila M. Effects of the expansion of doctors’ offices adjacent to private pharmacies in Mexico: Secondary data analysis of a national survey. BMJ Open. 2014;4(5):e004669.
Heyman G, Cars O, Bejarano MT, Peterson S. Access, excess, and ethics-towards a sustainable distribution model for antibiotics. Ups J Med Sci. 2014;119(2):134–41.
Jacob S, Schiffino N, Biard B. The mystery shopper: a tool to measure public service delivery? Int Rev Adm Sci. 2018;84(1):164.
Guinovart MC, Figueras A, Llop JC, Llor C. Obtaining antibiotics without prescription in Spain in 2014: even easier now than 6 years ago. J Antimicrob Chemother. 2014;70(4):1270–1.
Orach CG. Health equity: challenges in low income countries. Afr Health Sci. 2009;9(2):49–51.
Acknowledgements
Not applicable
Funding
The research was commissioned by the World Health Organization (WHO) Regional Office for Europe, Copenhagen, Denmark. The views expressed in this paper are those of the authors and should not be interpreted as the views of the WHO Regional Office for Europe. The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
TJ, JR and AM designed the study. TJ performed the systematic literature review and wrote the manuscript. HH, KI and HP were actively involved in the study progress and reviewed drafts of the manuscript. TJ, AM, JR, HH, KI and HP read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Three co-authors are affiliated with the WHO Regional Office for Europe. All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1
Search strategy. Full search strategy for PubMed. (DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jacobs, T.G., Robertson, J., van den Ham, H.A. et al. Assessing the impact of law enforcement to reduce over-the-counter (OTC) sales of antibiotics in low- and middle-income countries; a systematic literature review. BMC Health Serv Res 19, 536 (2019). https://doi.org/10.1186/s12913-019-4359-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-019-4359-8