Abstract
Background
Comparison of services and identification of factors important for favourable patient outcomes in emergency medical services (EMS) is challenging due to different organization and quality of data. The purpose of the present study was to evaluate the feasibility of physician-staffed EMS (p-EMS) to collect patient and system level data by using a consensus-based template.
Methods
The study was an international multicentre observational study. Data were collected according to a template for uniform reporting of data from p-EMS using two different data collection methods; a standard and a focused data collection method. For the standard data collection, data were extracted retrospectively for one year from all FinnHEMS bases and for the focused data collection, data were collected prospectively for six weeks from four selected Norwegian p-EMS bases. Completeness rates for the two data collection methods were then compared and factors affecting completeness rates and template feasibility were evaluated. Standard Chi-Square, Fisher’s Exact Test and Mann-Whitney U Test were used for group comparison of categorical and continuous data, respectively, and Kolomogorov-Smirnov test for comparison of distributional properties.
Results
All missions with patient encounters were included, leaving 4437 Finnish and 128 Norwegian missions eligible for analysis. Variable completeness rates indicated that physiological variables were least documented. Information on pain and respiratory rate were the most frequently missing variables with a standard data collection method and systolic blood pressure was the most missing variable with a focused data collection method. Completeness rates were similar or higher when patients were considered severely ill or injured but were lower for missions with short patient encounter. When a focused data collection method was used, completeness rates were higher compared to a standard data collection method.
Conclusions
We found that a focused data collection method increased data capture compared to a standard data collection method. The concept of using a template for documentation of p-EMS data is feasible in physician-staffed services in Finland and Norway. The greatest deficiencies in completeness rates were evident for physiological parameters. Short missions were associated with lower completeness rates whereas severe illness or injury did not result in reduced data capture.
Similar content being viewed by others
Background
Systems for pre-hospital critical care exist worldwide, but emergency medical service (EMS) systems differ in resources, organizational and operational models; from simple systems providing basic life support to sophisticated systems providing critical care [1,2,3,4,5,6,7,8,9,10].
Treatment and diagnostic options in pre-hospital care are increasing and several in-hospital techniques are currently being applied in the pre-hospital setting [10,11,12,13,14]. To enable more point-of-care diagnostics and increase in advanced interventions, some EMS systems, especially in Europe and Australasia, have introduced helicopters and rapid-response cars staffed with specially trained physicians [10, 15]. The effect of physician-staffed EMS (p-EMS) is debated and studies report contradicting results [16,17,18,19,20,21,22,23,24,25,26,27,28]. A substantial challenge to assess quality of health care is lack of uniform documentation, this is also pertinent to p-EMS [29, 30].
The concept of consensus-developed condition-specific datasets has proven useful for research and quality assessment in several areas of critical care [30,31,32,33]. To evaluate the effect and efficiency of p-EMS, a template for uniform reporting of data from p-EMS was published [34]. However, to implement a template for documentation, feasibility of the template to collect the requested data in the context intended should be demonstrated [29, 35,36,37,38,39].
In Scandinavia, p-EMS is well established, and services are relatively similar, thus joint research efforts may be valuable [5, 34]. Finland is currently the only country where the template for documenting and reporting from p-EMS is implemented, thus the only country able to provide routinely collected template data. To evaluate template feasibility, we wanted to compare two different data collection methods in two similar systems. P-EMS in Finland and Norway employ the same operational and medical concept and differences between services are mainly seen in time variables, patient volume and service area [5]. We considered comparison of Finland and Norway to be feasible; thus, we decided to include these two countries for the present study.
The aim of the present study was to evaluate the feasibility of pre-hospital physicians to collect patient and system level data by using the template for uniform reporting of data from p-EMS [34], comparing data collection from a standard to a focused data collection method.
Methods
Study design
The study was an international multicentre feasibility study including two physician-staffed pre-hospital services. As the aim of the study was to examine the feasibility of collecting template data in a standard operational pre-hospital context, we designed a two-method collection protocol. We hypothesized that by using a dedicated and motivated group of physicians (focused data collection method), we would achieve a robust indication of whether the template data were possible to collect in general. By comparing data collected with the focused collection method to routinely collected data (standard data collection method) we could assess whether both methods were feasible, or if data collection was feasible for specially dedicated physicians only.
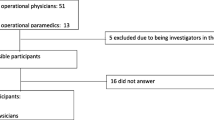
For the standard data collection method, data from the five p-EMS bases administered by FinnHEMS (the national operator of p-EMS in Finland), covering a total population of 3.7 million inhabitants, were extracted from their database for a period of 12 months (March 2013 through February 2014). The physicians were not informed that data were extracted, thus completeness rates represents routinely collected data for FinnHEMS.
For the focused data collection method, template data were collected prospectively for six weeks in Norway (January through March 2014) by 16 physicians from four p-EMS bases, covering a total population of 1.75 million inhabitants. Each participating physician was asked to collect template data as complete as possible on a predefined form and all physicians were informed that this was a study of completeness rates. Emphasis was on keeping the data collection period short to avoid study-fatigue. Data were placed in standardized categories and data sets from Finland and Norway were then merged.
Feasibility of the two data collection methods were assessed by comparing completeness rates on several variables. Variables that proved difficult to collect were identified and reasons for different completeness rates were sought by comparing completeness rates for different patient groups and operational settings. Data were stratified according to medical problem, p-EMS escort to hospital, severity of the patient’s condition, patient age, time from p-EMS arrival on scene to delivery at hospital and mode of transportation.
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [40] and Standards for Quality Improvement Reporting Excellence (SQUIRE) [41] guidelines were consulted when drafting the manuscript.
Data variables
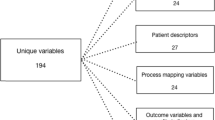
The template for data collection consists of five main sections [34]. The first section, “Fixed system variables” contain data about service area, organization and activation criteria and is identical to all missions for each base, hence this section was not included in the study. The second section, “Event operational descriptors”, contain time data, data on dispatch and type of transportation. The third section, “Patient descriptors”, contain patient data, data on patient physiology and medical problem. The fourth section, “Process mapping data”, contain data on medication and procedures performed during the mission and the fifth section, “Outcome measures”, contain data on mission outcome. A full description of all variables is provided in Additional file 1. Physicians in Norway were instructed to register event operational and patient descriptors, process mapping and outcome measures. In total 33 variables were registered. Information on gender was omitted to de-identify patients. Further, the outcome measure “Physiological improvement” was also omitted, as this is a proposed quality indicator yet to be validated. The corresponding variables were extracted from the FinnHEMS database. For the standard data collection method, all process mapping data and data on unit arrival at scene, type and result of dispatch, comorbidity and medical problem were mandatory to register to complete patient records. For the focused data collection method, no variable was mandatory.
Statistical analysis
The two data collection methods were compared by comparing completeness rates on several variables. Variables that proved difficult to collect were identified and reasons for different completeness rates were sought by comparing completeness rates for different patient groups and operational settings. Data were stratified according to medical problem, p-EMS escort to hospital, severity of the patient’s condition, patient age, time from p-EMS arrival on scene to delivery at hospital and mode of transportation. Categorical data are presented as counts (n) and proportions (%) while continuous data are presented as median and interquartile range (IQR). Standard Chi-Square, Fisher’s Exact Test and Mann-Whitney U Test were used for group comparison of categorical and continuous data. Kolomogorov-Smirnov test [42] was used for comparison of distributional properties. Data were analyzed using IBM SPSS statistics version 22 and R 3.1.0.
Results
Study material
FinnHEMS submitted data from 12,486 missions. Of these, 8049 (64%) missions were excluded due to no patient encounter (supervision or advice only or due to a concurrent mission, weather or technical conditions), leaving 4437 (36%) missions eligible for further analyses. Norwegian p-EMS submitted data from 177 missions. Of these, 49 (28%) missions were excluded because of no patient encounter (due to weather or technical conditions), leaving 128 (72%) missions eligible for further analyses. The physicians in Norway registered on average 8 forms each, which is 1–2 forms per shift.
Patient and mission characteristics
Patient and mission characteristics are summarized in Table 1. In both countries the majority of dispatches were for medical missions. Finland had more trauma dispatches than Norway but fewer inter-hospital transfers. In both countries, trauma was the single most common medical problem, followed by cardiac arrest in Finland and chest pain in Norway. In Finland, p-EMS physicians were transported to the scene by helicopter, but most patients were transported to the hospital by ground ambulances accompanied by the p-EMS physician. Most Norwegian patients were transported to the hospital by helicopter. Finland had significantly longer median on-scene time and median time from origin call to patient arriving hospital compared with Norway, but there was no difference in transport time or time from when call was received at the emergency medical communication centres to p-EMS arrival at scene. Significantly more advanced procedures were performed in Finland compared to Norway, but there was no difference in the number of patients receiving medication.
Completeness of patient-level core data
With the standard data collection method, all 13 mandatory variables were 100% complete (Table 2) while further four of the variables were > 80% complete. Ten variables had < 50% completeness. Six out of ten physiological variables (first and last value of heart rate, systolic blood pressure, heart rhythm, oxygen saturation and respiratory rate) had < 50% completeness. With the focused data collection method, seven variables were 100% complete, and overall 29 variables were > 80% complete. Two variables were < 50% complete. Except from the two variables reporting first and last systolic blood pressure, all physiological variables were > 80% complete.
Completeness rate and patient characteristics
Completeness rates were affected by clinical problems encountered and mission characteristics. Significantly more variables were collected with a focused data collection method than with a standard data collection method, both for different medical conditions, when patients were severely ill or injured and when patient care was less than 20 min. An additional file (Additional file 2) depicts our definition of a severely ill or injured patient. More variables were collected with focused data collection, regardless of transport mode. Completeness rate variations among different clinical problems are depicted in Figs. 1 and 2 and Table 3.
Completeness rates for standard and focused data collection method. Figure depicting completeness rates for all variables with a standard and a focused data collection method. Each dot represents one variable, and the corresponding percent of core data collected for that variable. For perfect collection, the figure would be a vertical line of dots a 100%
Completeness rate variations for different subgroups. Figure depicting completeness rates for different patient groups, operational characteristics and medical conditions for standard and focused data collection methods. Each dot represents one variable, and the corresponding percent of core data collected for that variable. For perfect collection, the figure would be a vertical line of dots a 100%
When comparing different patient groups for each data collection method, we found that for both methods, significantly less variables were collected when patient care was less than 20 min than when patient care was more than 20 min. Further, to be escorted by a physician to hospital resulted in more reported variables than when patients were treated by physicians on-scene and transported without physician. For children under 10 years of age, less variables were collected than when patients were older. Transport by helicopter resulted in significantly higher completeness rates with a standard data collection method, but there was no significant difference regarding transport mode with a focused data collection method. With standard data collection, significantly more variables were collected when patients were severely ill or injured compared to not severely ill or injured patients. There was no significant difference among these patient groups with a focused data collection. Differences in completeness rates among different patient groups with the two data collection methods are summarized in Table 4.
Discussion
When efforts are optimized, p-EMS can achieve high completeness rates in collecting prospective data using a template. Motivation and focus on documentation, rather than operational context, seems to affect data completeness rates most.
Lack of documentation is often highlighted as a limitation for research in emergency medicine, especially for retrospective registry studies [5, 35, 43,44,45]. Putting attention to increase the quality of routinely collected data may enable such data to be an important and effective source to monitor and compare services. As such, strategies to increase data capture should be sought [30, 43, 45,46,47,48]. Training programs may increase data capture, most likely by increasing attention to documentation [43, 48]. In our study the effect of motivation was evident, where significantly more data were registered with a focused data collection method than with a standard data collection method. Feedback on how high-quality research or quality assurance will benefit from complete data registration can make physicians more aware of the importance of data registration, thereby increasing data capture.
Echoing our results, several studies have found physiological variables to be the least documented variables [44, 45, 49]. Laudermilch et al. [44] found that 28% of patient records had missing physiological data and Bergrath et al. [45] reports vital parameters necessary to document Mainz Emergency Evaluation Score (MEES) to be present at two time points in only 31.08% of patients. Gravel et al. reports from the paediatric population that high rates of vital signs data are missing [50]. With a standard and a focused data collection method, 48 and 85% of physiological variables were registered, respectively, indicating that high completeness rates are achievable. However, physiological data were not complete, even with a focused data collection method. Good clinical assessment depends on correct evaluation of vital signs; thus, documentation of physiological variables is important [48, 50] and strategies for improvement of reporting should be sought.
Physiological data change according to patient state and repeated registrations of the same variable capture trends and reveal changes in patient condition and the effect of treatment [30,31,32, 46, 47, 51]. The p-EMS template requests documentation of physiological variables at two time points. For all repeated parameters we found the first value to be more complete than the last value, thereby complicating intervention comparison and comparison of changes in patient state. This is comparable with the findings of Bergrath et al. [45]. Medical directors should emphasize the statutory requirement for temporal documentation of physiological parameters and that this also pertains to p-EMS [52].
Ideally data capture in pre-hospital critical care should be simple, accurate and fast. For both cohorts, clinical data are registered on paper during the mission and are later digitally registered. This process is time-consuming, inexpedient and carries a risk for recall-bias and documentation fatigue. Automated data capture from monitors may increase completeness rates and is widely used in anaesthetic services documenting every change in the patient state [53]. Implementation of these readily available concepts to the pre-hospital environment is increasing [54]. Although there are still challenges, automated data capture may reduce administrative workload, improve patient focus and transferal of patient documentation to the next level of care [54,55,56,57,58].
Laudermilch et al. [44] suggests that datasets are less complete for more severely injured patients and that increased workload reduce data capture. This is in contrast to our findings, where data capture was increased or remained equal for patients with a critical condition (Table 3). Corresponding with our findings, Bergrath et al. report calculability of MEES to improve with increasing medical severity [45]. Patients with minor complaints might be considered to require less attention and thereby an increased amount of missing data occurs [49, 59, 60]. However, with a focused data collection method, we found no differences in data completeness for less critical patients.
Time available for data capture may affect completeness rates. We found missions with less than 20 min of patient encounter were associated with lower completeness rates than missions lasting more than 20 min. This may reflect increased workload. For children below 10 years of age, we found lower completeness rates of vital parameters than for patients above 10 years of age. For less severely ill or injured children, measuring blood pressure can be uncomfortable and doctors may be reluctant to perform the measurements, resulting in lower completeness rates.
Categorization of data may increase data capture compared to registering exact values [59]. In Finland, where data were collected by a standard method, the template has been modified and pain was reported using a scale from 1 to 10 instead of using the original three-parted scale described in the template; no pain, moderate pain and severe pain. Jennings et al. [61] recommends the verbal numerical rating scale to measure pain in the pre-hospital setting, corresponding with the FinnHEMS template modification. In Norway, where a focused data collection method was used, pain was reported according to the original template. With the standard data collection method, we found that completeness rates for data on pain were low while with the focused data collection method, data on pain were almost complete, supporting a reduced number of categories to increase data capture. However, fewer categories reduce precision, leading to imprecise estimates, and must be weighed against the need for accuracy.
Outcome comparison often adjust for on-scene time, making low documentation completeness or imprecise registrations of this variable a limitation for research [62,63,64]. Eckstein et al. [65] found on-scene time being documented in 70% in a cohort of major trauma patients. In our study, on-scene time was documented in 56% an 91% of the cases with a standard and focused data collection method respectively, indicating that high completeness rates are achievable when attention is directed towards documentation. In Norway, the emergency medical communication centres automatically documented the origin time data whereas the response units registered other time variables on paper or non-portable devices [59]. Due to weather and operational conditions, paper registration was often not feasible, and variables were often registered in retrospect, increasing the risk for imprecise registrations. Portable devices available for registrations on site could further increase completeness rates and accuracy of data.
In our data Finland report significantly longer median on-scene times than in Norway (22 versus 12 min), still on-scene times are considerable shorter than reported from German (32 min) and Dutch (27.2 min) services [66]. For trauma patients, the concept of aiming for a pre-hospital time period less than one hour (“The golden hour”) and of keeping on-scene times to not more than 10 min, have been directional for organization of pre-hospital care [67]. In recent years these concepts have been challenged [67,68,69]. Harmsen et al. conclude that emphasis should be on making sure the patient receives proper pre-hospital care rather than on getting the patient to hospital as fast as possible [70]. In our data, physicians in Finland are providing significantly more advanced procedures than in Norway. This may explain the longer on-scene times in Finland. We do not know, for our system, which advanced procedures should be performed by pre-hospital physicians to improve patient care. However, we believe that uniform documentation may enable us, in the future, to identify procedures beneficial in p-EMS.
All process mapping data (procedures performed, and medication administered) are mandatory in Finland, possibly explaining the 100% completeness rates. In Norway, where no data points are mandatory, completeness vary between 26 and 99% for process data.
Two variables showed particularly low completeness rates in Norway: “Diagnostic procedures” and “Breathing – procedures used”. For these two variables there is no option for choosing “none” or “not relevant”, and when no procedure is performed these data fields will appear as missing. We suggest this to be revised in the template.
Comparing data from two countries had some practical challenges. Although both data collection methods collected data according to the same template, the data were registered in different data formats and in different language. To be able to compare the datasets a work-intensive data management job was needed to standardize categories. Thus, to allow rapid and accurate comparison we recommend data to be registered in the same data format. This is achievable and one might suggest a digital template with predefined names and categories to be implemented. This means that data, when transformed into statistical analysis software, must have the same properties, names and limitations to be able to be easily merged into the same database and analyzed. Adaptions where additional variables are included for local purposes can easily be managed within such a digital template without hampering template comparisons. We believe that simplifying the comparison processes by standardizing data entry will generate more multi-centre research.
Limitations
The present study has several limitations. We did not include a formal questionnaire to investigate reasons for missing data, although most physicians in Norway provided informal information regarding this. A questionnaire could have been useful to discover reasons for registration failure of importance to aid revision of the template. In Finland, the physicians were not informed about the study in advance, so the database reflects normal documentation rates in FinnHEMS bases. In Norway, the Hawthorne effect is an obvious and wanted effect, whereas the risk for this in Finland is lower.
The data are from 2013/2014 and this may be considered old. However, documentation method or organisation of p-EMS have not changed in either Finland or Norway since 2014, thus we believe the results still are valid and that newer data would have yielded similar results.
In Norway, 16 physicians participated, and each physician had on average 5 shifts during the data collection period. Each physician registered on average 8 cases, this is on average 1,6 cases per shift. This is a low number if each physician were to be evaluated individually. Because the aim of the study was to evaluate the documentation system, not the individual physician, we find the total number of cases registered in Norway to be acceptable.
The study was conducted in two similar p-EMS settings in two high-income countries and results may not be applicable to all other EMS settings. However, documentation for the study was paper-based, not including expensive equipment. The principles for pre-hospital emergency medical treatment are generally recognized, and international expert consensus on important data to be collected in the field should apply to both low- and high-income EMS systems. The concept of using a template by motivated personnel for data collection may therefore be applicable to other less resource-intensive settings.
Thirteen of the variables are mandatory to register in Finland and electronic patient files cannot be saved unless these variables are registered; completeness rates are therefore 100%. To compare these with Norwegian data will not give an idea of what is possible to collect in an everyday setting or if implementation challenges also apply for this type of data. Finally, the challenge with possible fabricated data to finalize registrations must be addressed.
Conclusions
We found that a focused data collection method increased data capture compared to a standard data collection method. With a focused data collection method, 88% of variables were more than 80% complete. The greatest deficiencies in completeness rates were evident for physiological parameters. Short missions were associated with lower completeness rates whereas severe illness or injury did not result in reduced data capture. We find the template for p-EMS feasible but highlight motivation and training to maintain high rates of data capture after implementation.
Based on the findings in this study an international consensus-based revision of the template studied will be initiated.
Abbreviations
- ASA-PS:
-
American Society of Anaesthesiologists Physical Status Classification
- EMS:
-
Emergency Medical Service
- FinnHEMS:
-
Finnish Helicopter Emergency Medical Service
- IQR:
-
Interquartile Range
- MEES:
-
Mainz Emergency Evaluation Score
- NAAF:
-
The Norwegian Air Ambulance Foundation
- p-EMS:
-
Physician-staffed pre-hospital Emergency Medical Services
- SpO2:
-
Saturation of peripheral Oxygen
References
Ong ME, Cho J, Ma MH, Tanaka H, Nishiuchi T, Al Sakaf O, Abdul Karim S, Khunkhlai N, Atilla R, Lin CH, et al. Comparison of emergency medical services systems in the pan-Asian resuscitation outcomes study countries: report from a literature review and survey. Emerg Med Australas. 2013;25:55–63.
Di Bartolomeo S, Gava P, Truhlar A, Sandberg M. Cross-sectional investigation of HEMS activities in Europe: a feasibility study. ScientificWorldJournal. 2014;2014:201570.
Haider AH, David JS, Zafar SN, Gueugniaud PY, Efron DT, Floccard B, MacKenzie EJ, Voiglio E. Comparative effectiveness of inhospital trauma resuscitation at a French trauma center and matched patients treated in the United States. Ann Surg. 2013;258:178–83.
Kruger AJ, Lossius HM, Mikkelsen S, Kurola J, Castren M, Skogvoll E. Pre-hospital critical care by anaesthesiologist-staffed pre-hospital services in Scandinavia: a prospective population-based study. Acta Anaesthesiol Scand. 2013.
Kruger AJ, Skogvoll E, Castren M, Kurola J, Lossius HM. Scandinavian pre-hospital physician-manned emergency medical services--same concept across borders? Resuscitation. 2010;81:427–33.
Li T, Jones CMC, Shah MN, Cushman JT, Jusko TA. Methodological challenges in studies comparing prehospital advanced life support with basic life support. Prehosp Disaster Med. 2017;32:444–50.
Mould-Millman NK, Dixon JM, Sefa N, Yancey A, Hollong BG, Hagahmed M, Ginde AA, Wallis LA. The state of emergency medical services (EMS) Systems in Africa. Prehosp Disaster Med. 2017;32:273–83.
Nielsen K, Mock C, Joshipura M, Rubiano AM, Zakariah A, Rivara F. Assessment of the status of prehospital care in 13 low- and middle-income countries. Prehosp Emerg Care. 2012;16:381–9.
Sasser S, Varghese M, Kellermann A, Lormand J-D, Organization WH. Prehospital trauma care systems. Geneva: World Health Organization; 2005.
Sollid SJM, Rehn M. The role of the anaesthesiologist in air ambulance medicine. Curr Opin Anaesthesiol. 2017;30:513–7.
Hov MR, Ryen A, Finsnes K, Storflor J, Lindner T, Gleditsch J, Lund CG. Pre-hospital ct diagnosis of subarachnoid hemorrhage. Scand J Trauma Resusc Emerg Med. 2017;25:21.
O'Dochartaigh D, Douma M. Prehospital ultrasound of the abdomen and thorax changes trauma patient management: a systematic review. Injury. 2015;46:2093–102.
Ouweneel DM, Schotborgh JV, Limpens J, Sjauw KD, Engstrom AE, Lagrand WK, Cherpanath TGV, Driessen AHG, de Mol B, Henriques JPS. Extracorporeal life support during cardiac arrest and cardiogenic shock: a systematic review and meta-analysis. Intensive Care Med. 2016;42:1922–34.
Sadek S, Lockey DJ, Lendrum RA, Perkins Z, Price J, Davies GE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in the pre-hospital setting: an additional resuscitation option for uncontrolled catastrophic haemorrhage. Resuscitation. 2016;107:135–8.
Reid BO, Rehn M, Uleberg O, Kruger AJ. Physician-provided prehospital critical care, effect on patient physiology dynamics and on-scene time. Eur J Emerg Med. 2018;25:114–9.
Bakalos G, Mamali M, Komninos C, Koukou E, Tsantilas A, Tzima S, Rosenberg T. Advanced life support versus basic life support in the pre-hospital setting: a meta-analysis. Resuscitation. 2011;82:1130–7.
Fevang E, Lockey D, Thompson J, Lossius HM. The top five research priorities in physician-provided pre-hospital critical care: a consensus report from a European research collaboration. Scand J Trauma Resusc Emerg Med. 2011;19:57.
Kondo Y, Fukuda T, Uchimido R, Hifumi T, Hayashida K. Effects of advanced life support versus basic life support on the mortality rates of patients with trauma in prehospital settings: a study protocol for a systematic review and meta-analysis. BMJ Open. 2017;7:e016912.
Ryynanen OP, Iirola T, Reitala J, Palve H, Malmivaara A. Is advanced life support better than basic life support in prehospital care? A systematic review Scand J Trauma Resusc Emerg Med. 2010;18:62.
Sanghavi P, Jena AB, Newhouse JP, Zaslavsky AM. Outcomes of basic versus advanced life support for out-of-hospital medical emergencies. Ann Intern Med. 2015;163:681–90.
Timmermann A, Russo SG, Hollmann MW. Paramedic versus emergency physician emergency medical service: role of the anaesthesiologist and the European versus the Anglo-American concept. Curr Opin Anaesthesiol. 2008;21:222–7.
Andruszkow H, Lefering R, Frink M, Mommsen P, Zeckey C, Rahe K, Krettek C, Hildebrand F. Survival benefit of helicopter emergency medical services compared to ground emergency medical services in traumatized patients. Crit Care. 2013;17:R124.
Den Hartog D, Romeo J, Ringburg AN, Verhofstad MH, Van Lieshout EM. Survival benefit of physician-staffed helicopter emergency medical services (HEMS) assistance for severely injured patients. Injury. 2015;46:1281–6.
Giannakopoulos GF, Kolodzinskyi MN, Christiaans HM, Boer C, de Lange-de Klerk ES, Zuidema WP, Bloemers FW, Bakker FC. Helicopter emergency medical services save lives: outcome in a cohort of 1073 polytraumatized patients. Eur J Emerg Med. 2013;20:79–85.
Peters J, van Wageningen B, Hendriks I, Eijk R, Edwards M, Hoogerwerf N, Biert J. First-pass intubation success rate during rapid sequence induction of prehospital anaesthesia by physicians versus paramedics. Eur J Emerg Med. 2015;22:391–4.
Ringburg AN, Thomas SH, Steyerberg EW, van Lieshout EM, Patka P, Schipper IB. Lives saved by helicopter emergency medical services: an overview of literature. Air Med J. 2009;28:298–302.
Seamon MJ, Doane SM, Gaughan JP, Kulp H, D'Andrea AP, Pathak AS, Santora TA, Goldberg AJ, Wydro GC. Prehospital interventions for penetrating trauma victims: a prospective comparison between advanced life support and basic life support. Injury. 2013;44:634–8.
Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, Adams PW, Daley BJ, Miller RS, Harbrecht BG, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315–26.
Ringdal KG, Lossius HM. Feasibility of comparing core data from existing trauma registries in scandinavia. Reaching for a Scandinavian major trauma outcome study (MTOS). Scand J Surg. 2007;96:325–31.
Ringdal KG, Lossius HM, Jones JM, Lauritsen JM, Coats TJ, Palmer CS, Lefering R, Di Bartolomeo S, Dries DJ, Soreide K. Collecting core data in severely injured patients using a consensus trauma template: an international multicentre study. Crit Care. 2011;15:R237.
Dick WF, Baskett PJ. Recommendations for uniform reporting of data following major trauma--the Utstein style. A report of a working party of the international trauma Anaesthesia and critical care society (ITACCS). Resuscitation. 1999;42:81–100.
Sollid SJ, Lockey D, Lossius HM. A consensus-based template for uniform reporting of data from pre-hospital advanced airway management. Scand J Trauma Resusc Emerg Med. 2009;17:58.
Fattah S, Rehn M, Lockey D, Thompson J, Lossius HM, Wisborg T. A consensus based template for reporting of pre-hospital major incident medical management. Scand J Trauma Resusc Emerg Med. 2014;22:5.
Kruger AJ, Lockey D, Kurola J, Di Bartolomeo S, Castren M, Mikkelsen S, Lossius HM. A consensus-based template for documenting and reporting in physician-staffed pre-hospital services. Scand J Trauma Resusc Emerg Med. 2011;19:71.
Francis RC, Schmidbauer W, Spies CD, Sorensen M, Bubser F, Kerner T. Standard operating procedures as a tool to improve medical documentation in preclinical emergency medicine. Emerg Med J. 2010;27:350–4.
Davidson SJ, Zwemer FL Jr, Nathanson LA, Sable KN, Khan AN. Where's the beef? The promise and the reality of clinical documentation. Acad Emerg Med. 2004;11:1127–34.
Ringdal KG, Skaga NO, Hestnes M, Steen PA, Roislien J, Rehn M, Roise O, Kruger AJ, Lossius HM. Abbreviated injury scale: not a reliable basis for summation of injury severity in trauma facilities? Injury. 2013;44:691–9.
Ringdal KG, Skaga NO, Steen PA, Hestnes M, Laake P, Jones JM, Lossius HM. Classification of comorbidity in trauma: the reliability of pre-injury ASA physical status classification. Injury. 2013;44:29–35.
Lossius HM, Kruger AJ, Ringdal KG, Sollid SJ, Lockey DJ. Developing templates for uniform data documentation and reporting in critical care using a modified nominal group technique. Scand J Trauma Resusc Emerg Med. 2013;21:80.
Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–24.
Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2015.
Conover WJ. Practical nonparametric statistics. New York: John Wiley & Sons; 1971.
di Martino P, Leoli F, Cinotti F, Virga A, Gatta L, Kleefield S, Melandri R. Improving vital sign documentation at triage: an emergency department quality improvement project. J Patient Saf. 2011;7:26–9.
Laudermilch DJ, Schiff MA, Nathens AB, Rosengart MR. Lack of emergency medical services documentation is associated with poor patient outcomes: a validation of audit filters for prehospital trauma care. J Am Coll Surg. 2010;210:220–7.
Bergrath S, Skorning M, Rortgen D, Beckers SK, Brokmann JC, Mutscher C, Rossaint R. Is paper-based documentation in an emergency medical service adequate for retrospective scientific analysis? An evaluation of a physician-run service. Emerg Med J. 2011;28:320–4.
Gaieski DF, Abella BS, Goyal M. CPR and postarrest care: overview, documentation, and databases. Chest. 2012;141:1082–9.
Lossius HM, Sollid SJ, Rehn M, Lockey DJ. Revisiting the value of pre-hospital tracheal intubation: an all time systematic literature review extracting the Utstein airway core variables. Crit Care. 2011;15:R26.
Bird C, Shea A, Michie CA, George G. A simple intervention improves the recording of vital signs in children presenting to the emergency department. Emerg Med J. 2009;26:698–700.
O'Reilly GM, Cameron PA, Jolley DJ. Which patients have missing data? An analysis of missingness in a trauma registry. Injury. 2012;43:1917–23.
Gravel J, Opatrny L, Gouin S. High rate of missing vital signs data at triage in a paediatric emergency department. Paediatr Child Health. 2006;11:211–5.
Arrich J, Sterz F, Herkner H, Testori C, Behringer W. Total epinephrine dose during asystole and pulseless electrical activity cardiac arrests is associated with unfavourable functional outcome and increased in-hospital mortality. Resuscitation. 2012;83:333–7.
Lov om helsepersonell, kap.8, § 40 [https://lovdata.no/dokument/NL/lov/1999-07-02-64/KAPITTEL_8#KAPITTEL_8].
Rothman B, Leonard JC, Vigoda MM. Future of electronic health records: implications for decision support. Mt Sinai J Med. 2012;79:757–68.
Newgard CD, Fu R, Malveau S, Rea T, Griffiths DE, Bulger E, Klotz P, Tirrell A, Zive D. Out-of-hospital research in the era of electronic health records. Prehosp Emerg Care. 2018:1–12.
Anantharaman V, Swee HL. Hospital and emergency ambulance link: using IT to enhance emergency pre-hospital care. Int J Med Inform. 2001;61:147–61.
Landman AB, Rokos IC, Burns K, Van Gelder CM, Fisher RM, Dunford JV, Cone DC, Bogucki S. An open, interoperable, and scalable prehospital information technology network architecture. Prehosp Emerg Care. 2011;15:149–57.
Mackenzie CF, Hu P, Sen A, Dutton R, Seebode S, Floccare D, Scalea T. Automatic pre-hospital vital signs waveform and trend data capture fills quality management, triage and outcome prediction gaps. AMIA Annu Symp Proc. 2008:318–22.
Wu RC, Straus SE. Evidence for handheld electronic medical records in improving care: a systematic review. BMC Med Inform Decis Mak. 2006;6:26.
Staff T, Sovik S. A retrospective quality assessment of pre-hospital emergency medical documentation in motor vehicle accidents in South-Eastern Norway. Scand J Trauma Resusc Emerg Med. 2011;19:20.
Arbabi S, Jurkovich GJ, Wahl WL, Franklin GA, Hemmila MR, Taheri PA, Maier RV. A comparison of prehospital and hospital data in trauma patients. J Trauma. 2004;56:1029–32.
Jennings PA, Cameron P, Bernard S. Measuring acute pain in the prehospital setting. Emerg Med J. 2009;26:552–5.
Lerner EB, Moscati RM. The golden hour: scientific fact or medical "urban legend"? Acad Emerg Med. 2001;8:758–60.
Feero S, Hedges JR, Simmons E, Irwin L. Does out-of-hospital EMS time affect trauma survival? Am J Emerg Med. 1995;13:133–5.
Petri RW, Dyer A, Lumpkin J. The effect of prehospital transport time on the mortality from traumatic injury. Prehosp Disaster Med. 1995;10:24–9.
Eckstein M, Chan L, Schneir A, Palmer R. Effect of prehospital advanced life support on outcomes of major trauma patients. J Trauma. 2000;48:643–8.
Timm A, Maegele M, Lefering R, Wendt K, Wyen H. Pre-hospital rescue times and actions in severe trauma. A comparison between two trauma systems: Germany and the Netherlands. Injury. 2014;45 Suppl 3:S43–52.
Rogers FB, Rittenhouse KJ, Gross BW. The golden hour in trauma: dogma or medical folklore? Injury. 2015;46:525–7.
Kleber C, Lefering R, Kleber AJ, Buschmann CT, Bail HJ, Schaser KD, Haas NP. Rescue time and survival of severely injured patients in Germany. Unfallchirurg. 2013;116:345–50.
Newgard CD, Schmicker RH, Hedges JR, Trickett JP, Davis DP, Bulger EM, Aufderheide TP, Minei JP, Hata JS, Gubler KD, et al. Emergency medical services intervals and survival in trauma: assessment of the "golden hour" in a North American prospective cohort. Ann Emerg Med. 2010;55:235–246.e234.
Harmsen AM, Giannakopoulos GF, Moerbeek PR, Jansma EP, Bonjer HJ, Bloemers FW. The influence of prehospital time on trauma patients outcome: a systematic review. Injury. 2015;46:602–9.
Lov om helsepersonell, kap.5, § 26 [https://lovdata.no/dokument/NL/lov/1999-07-02-64#KAPITTEL_5].
The Personal Data Act, vol 529/1999 [http://www.finlex.fi/fi/laki/alkup/1999/19990523].
Acknowledgements
The authors will express their sincere gratefulness to the donors of the Norwegian Air Ambulance Foundation, who by their contributions funded this study.
The authors also thank Sigurd Heian who coordinated data collection in Ålesund and Anna Olkinuora who provided great support with all practical issues in Finland.
Funding
The Norwegian Air Ambulance Foundation (NAAF) funded this project. However, the NAAF played no part in study design, data collection, analysis, writing or submitting to publication.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
KT, AJK and HML conceived the idea. KT, AJK, ØØ and IV were involved in acquisition of data. KT analyzed the data, AJK and JR supervised the analysis. KT, MR, KGR, HML, IV, ØØ, JR and AJK were involved in discussions of interpretation of the data. KT drafted the manuscript and all authors (KT, MR, KGR, HML, IV, ØØ, JR and AJK) revised it critically during several revision rounds. All authors have approved the final version of the manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Norwegian Regional Committee for Medical and Health Research Ethics deemed the system revision to be a quality improvement initiative not in need of formal approval (REK 2013/397b). The Privacy Ombudsman at the individual health authorities in Norway gave permission for data collection (ID 2013/17 and 2013/9865). Due to the nature of the study and national regulations there were no need for written consent [71]. In Finland the study was observational in nature and the data analyzed were fully anonymized; therefore the Ethics Committee approval was not needed due to national regulations [72]. Each hospital district gave individual permission to data collection at each base (ID 85/2015, R16502, J4/16).
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Template for documenting and reporting in physician-staffed pre-hospital services. A full description of all variables listed in the template for documenting and reporting in physician-staffed pre-hospital services (DOCX 30 kb)
Additional file 2:
Definition of severely ill or injured patient. A patient is considered severely ill or injured if one of the listed items are present. (DOCX 17 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tønsager, K., Rehn, M., Ringdal, K.G. et al. Collecting core data in physician-staffed pre-hospital helicopter emergency medical services using a consensus-based template: international multicentre feasibility study in Finland and Norway. BMC Health Serv Res 19, 151 (2019). https://doi.org/10.1186/s12913-019-3976-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-019-3976-6