Abstract
Background
Irrational drug use is a global health challenge in all healthcare settings, such as hospitals. This study evaluated the impact of an intervention by the pharmaceutical care unit on the use pattern of high-value medications and their direct costs in a referral hospital.
Methods
This interventional, prospective study was carried out in clinical wards of Namazi Hospital (Shiraz University of Medical Sciences) during six months from May 2015 to October 2015. Clinical pharmacists completed the checklists for albumin, intravenous (IV) pantoprazole, and IV immune globulin (IVIG), as three high-cost medications. When ordering these medications, the physicians were asked to complete the checklists. Then, trained pharmacists examined the checklists, based on the clinical and paraclinical conditions.
Results
The total number of administered medications and their relative cost decreased by 50.76% through guideline implementation; the difference was significant (P < 0.001). In addition, the direct cost of albumin and IV pantoprazole significantly decreased (55.8% and 83.92%, respectively). In contrast, the direct cost of IVIG increased by 40.9%. After guideline implementation, the monthly direct cost of all three medications decreased by $77,720 (55.88%). The all-cause in-hospital mortality rate did not change significantly due to the intervention. The median length of hospital stay was six and seven days, respectively in the pre- and post-intervention periods.
Conclusion
Based on the findings, implementation of guidelines by the pharmaceutical care unit caused a significant reduction in albumin and IV pantoprazole consumption and reduced their direct costs in a referral center in Iran.
Similar content being viewed by others
Key points
• Guideline implementation by pharmacists significantly reduced the direct cost of albumin and IV pantoprazole.
• Guideline implementation was not an effective method for decreasing the direct cost of IVIG.
• Direct supervision of pharmaceutical care units can improve the pattern of medication use and cost-saving strategies.
Background
Irrational drug use is a global challenge in all healthcare settings, such as hospitals. Furthermore, prescribers in the community continue inappropriate hospital prescriptions. This is a concerning issue since medical as well as financial resources are limited, particularly in developing countries [1]. In developing countries, although drugs constitute up to 40% of the healthcare budget, a large number of people may not have adequate access to the most basic or essential medicines [2].
The World Health Organization has suggested different administrative, educational, and regulatory strategies to improve the drug use pattern, including continuous healthcare team training, standard clinical practice guidelines, and drug utilization evaluation [1]. Implementation of clinical practice guidelines based on a pharmaceutical cost-containment program enhances patient safety through minimization of adverse effects and drug interactions, reduction of inappropriate medication prescriptions, controlling resistance of bacterial pathogens to antimicrobial agents, decreasing costs, management of medication supplies, improving the quality of healthcare, and promotion of patient satisfaction [3, 4].
There is relatively limited data on the pharmacists’ role in both promoting adherence to guidelines and improving clinical and/or economic outcomes [5,6,7]. This study aimed to assess the impact of interventions by pharmaceutical care units on the direct cost and use pattern of three high-cost medications in a referral hospital in Southwest of Iran.
Methods
Study setting
This prospective, interventional study (Iranian Registry of Clinical Trial ID: IRCT20161010030246N2) was performed during 6 months from May 2015 to October 2015 in all clinical wards of Namazi Hospital. This period was considered as the post-intervention phase, while the period from April 2014 to September 2014 was identified as the pre-intervention phase. Namazi Hospital, affiliated to Shiraz University of Medical Sciences (Shiraz, Iran) is a general tertiary referral center with 50 wards and nearly 1000 beds. No limitations were considered in selecting 50 clinical wards during the post-intervention phase. The study was approved by the hospital Medical Ethics Committee and Institutional Review Board, and written informed consents were obtained from patients or their family members.
High-cost medications
The database of the automated Hospital Information System regarding prescribed medications in 2014 was analyzed via ABC analysis described elsewhere [1]. Considering the total number of medicines consumed and their relative cost, albumin, intravenous (IV) pantoprazole, and IV immunoglobulin (IVIG) were the top three medications, accounting for the largest proportion of the hospital budget. Overall, 21.3%, 19.5%, and 13.7% of the pharmaceutical budget of the hospital in 2014 were spent for albumin, IVIG, and IV pantoprazole, respectively. The costs were converted to United States dollars at the real-time conversion rate.
Indication checklist
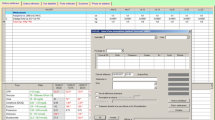
The clinical pharmacists designed an indication checklist draft for the selected drugs by exploiting relevant references and textbooks, such as online UptoDate, as well as Micromedex, American Society of Health System Pharmacists guidelines [8], and Applied Therapeutics: The Clinical Use of Drugs by Koda-Kimble and Young [9]. Subsequently, checklists were reviewed by the hospital medical team, consisting of medical experts with different relevant specialties and sub-specialties (including internists, rheumatologists, nephrologists, gastroenterologist, hematologists, cardiologists, surgeons, and neurologists), and their comments were implemented. The final version of indication checklist consisted of the following items: 1) demographics and related clinical and paraclinical data of the patient, 2) brief description of each indication, 3) medication order, 4) physician and pharmacist comments, and 5) final decision (approved or disapproved). Indication checklists for albumin, IVIG, and IV pantoprazole are provided in Additional file 1.
Guideline implementation
During the pre-intervention phase, wards were allowed to receive their requested medications from the hospital pharmacy according to physician team prescriptions without any limitations. Prescriptions in this period were generally based on both clinical and paraclinical conditions of patients and also physicians’ preferences with no data regarding the rate of inappropriate use.
After coordination with the head of different hospital departments, the physicians’ teams (including attending, fellowships, or residents) were requested to complete the indication checklist form when ordering albumin, IV pantoprazole, and IVIG. The checklists were examined by one of the five general pharmacists based on the clinical conditions and paraclinical data of patients during morning and afternoon shifts on working days (Saturday to Thursday).
General pharmacists were authorized to either approve or disapprove the indication checklist forms under the supervision of clinical pharmacists. Controversial or complicated cases (n = 124) were discussed in regular joint meetings of senior physicians and clinical pharmacists. In other words, the early acceptance rate of guideline implementation in our hospital by the physicians’ team was 83.04%. Possible inter- and intra-individual variations between general pharmacists were minimized by the direct and regular supervision of a senior clinical pharmacist. No specific educational course regarding the appropriate utilization of studied medications was designed or implemented for the physicians’ team in the pre- and post-intervention periods.
Daily lists of names of patients, whose medications were approved, were prepared by pharmacists and given to both the hospital pharmacy and wards. The wards were permitted to take their medications in emergency conditions (e.g., IV pantoprazole for active upper GI bleeding) without the approval of the pharmaceutical care unit during public holidays. In such conditions, the indication checklist forms were checked by the pharmacists at the earliest possible time after the night or holiday shifts. The flow chart of pharmaceutical care unit guidelines is depicted in Fig. 1.
Data collection
Demographic data (including age and sex), admission ward, prescription number and direct cost of each studied medication, and general indices of clinical outcomes (hospital length of stay [LOS] and all-cause in-hospital mortality) were extracted for all patients in both pre- and post-intervention periods from the Hospital Information System. It is worth noting that individuals who received any of the studied medications in the pre-intervention period were considered eligible for inclusion. The cause of disapproved requests for each medication in the post-intervention period was also recorded. In order to eliminate the probable effect of price changes between pre- and post-intervention periods, costs of studied medications in April 2014 (price stability) were considered for all relevant calculations.
Statistical analysis
Categorical variables are expressed as percentage. The normal distribution of continuous variables was examined using Kolmogorov-Smirnov test. Continuous data with and without a normal distribution are expressed as mean ± SD and median (interquartile range), respectively. To determine the relationship between categorical variables, Chi square or Fisher’s exact test was performed (if more than 25% of the categories have frequencies below five). Also, for evaluating parametric and non-parametric continuous variables, independent t test and Mann-Whitney test were applied, respectively.
Univariate logistic regression analysis was used to separately assess the plausible associations between each independent variable (age, sex, admission ward, and intervention) and in-hospital mortality as the dependent variable. Independent variables with P < 0.05 were entered in the multivariate logistic regression model. P < 0.05 was considered significant for the analytical tests. All analyses were performed in SPSS version 20 (IBM Company, New York, NY, USA).
Results
Patient characteristics
In the pre- and post-intervention phases, 4946 and 4895 patients were included, respectively. The patients’ characteristics in the pre- and post-intervention periods are listed in Table 1. More than half (58%) and three-fifth (60.29%) of individuals were males in the pre- and post-intervention phases, respectively. Sex distribution was comparable between the two groups. In contrast, the median interquartile range (IQR) of age was significantly higher (P = 0.002) in the pre-intervention period [50 (38)] than the post-intervention phase [48 (46)]. Except for pediatrics (P < 0.0001) and plastic surgery wards (P = 0.001), distribution of patients in all wards was comparable between the pre- and post-intervention periods. The hospital bed occupancy rate in the pre- and post-intervention phases (92.8% and 92%, respectively) did not differ significantly (P = 0.639).
Pharmaceutical unit reduction
A total number of 13,821 medications were used in the pre-intervention period. After guideline implementation, this rate decreased to 6539. The reduction in requests (50.76%) was statistically significant (P < 0.001). In line with this, the total number of administered albumin and IV pantoprazole decreased significantly after the intervention. In contrast, the number of administered IVIG as well as its direct cost was significantly higher in the post-intervention compared to pre-intervention phase (Table 2).
Pharmaceutical cost reduction
Compared to the pre-intervention period, the direct costs of albumin and IV pantoprazole significantly reduced by 55.8% and 83.92%, respectively in the post-intervention period. In contrast, the mean ± SD of direct cost of IVIG per month in the post-intervention phase ($97,565 ± 19,164) was significantly higher (P = 0.022) than the pre-intervention period ($69,234 ± 17,182) (Table 2). The mean ± SD of total net direct cost of all studied medications per month decreased from $139,102 ± 91,342 in the pre-intervention period to $61,382 ± 49,514 in the post-intervention period. In other words, the monthly direct cost of these three medications reduced by more than half (55.88% equal to $77,720) after guideline implementation, which was statistically significant (P < 0.0001). The annual net cost-saving for albumin, IV pantoprazole, and IVIG was estimated to be approximately $932,640.
Alternative medication costs
In order to assess the changes in cost associated with switching from the studied medications to other agents after guideline implementation, the direct costs of Amino Acid 5% and 10% (as alternatives to albumin in case of parenteral nutrition), oral pantoprazole, oral omeprazole and intravenous ranitidine (as alternatives for IV pantoprazole in case of stress-related mucosal damage prophylaxis) and parenteral methylprednisolone sodium succinate, methylprednisolone acetate, dexamethasone, betamethasone, and rituximab (as alternatives to IVIG in case of autoimmune diseases) were compared between pre- and post- intervention periods.
The mean ± SE monthly direct cost of Amino Acid 5 and 10% was comparable between the pre- and post- intervention periods ($9331 ± 5252 versus $9730 ± 5994; P = 0.962). Similarly, the median total direct cost per month for oral pantoprazole, oral omeprazole, and intravenous ranitidine did not differ significantly (P = 0.827) between the pre-intervention ($3782) and post-intervention ($4314) phases. In contrast, the median direct cost per month for parenteral corticosteroids and rituximab in the post-intervention phase ($924.92 and $18,280.26, respectively) was significantly higher (P < 0.001 for both medication classes) than the pre-intervention phase ($797.47 and $10,189.86, respectively).
Causes of medication request disapproval
Table 3 lists the causes of disapproved requests for albumin and IV pantoprazole in the post-intervention period. Management of edema in patients with serum albumin level above 2 g/dL (24.58%) was the most common inappropriate use of albumin in the post-intervention period, followed by administration as a component of parenteral nutrition (19.87%). Regarding IV pantoprazole, the three most frequent causes of disapproval were the ability to tolerate oral medications (53.02%), presence of only one minor risk factor for prophylaxis of stress-related mucosal damage (24.78%), and acute pancreatitis treatment (6.25%). IVIG requests were not approved by the pharmaceutical care unit in only five cases, including non-refractory sepsis (n = 2), Devic’s disease (n = 2), and idiopathic thrombocytopenic purpura in a patient with platelet count above 30,000/mm3 without active bleeding or planning for an emergent procedure (n = 1).
Clinical outcomes
The median IQR for LOS, as well as all-cause in-hospital mortality rate, was significantly higher in the post-intervention phase than the pre-intervention phase (P < 0.001 and P = 0.043, respectively). For each medication separately, the mortality rate of patients who were prescribed pantoprazole IV was significantly higher (P = 0.033) in the pre-intervention (9.5%) than the post-intervention phase (9.2%) (Table 4).
The results of univariate and multivariate logistic regression analyses regarding mortality rate in all patients (sum of pre- and post-intervention phases) are demonstrated in Table 5. According to the univariate analysis, type of ward (P < 0.001), age (P < 0.001), and intervention (P = 0.043) were significantly associated with mortality. Similarly, these variables remained statistically significant in the multivariate logistic regression model.
Discussion
Pharmaceutical expense reduction
The intervention by the pharmaceutical care unit via implementing clinical guidelines in a referral hospital in Southwest of Iran significantly decreased the direct cost of albumin and IV pantoprazole, but not IVIG. Although evidence-based medicine supports clinical effectiveness and theoretical as well as pharmacological benefits of albumin, IV pantoprazole, and IVIG in certain conditions, they can be overused or their usage pattern may be inappropriate.
In the past three decades, clinical studies from different countries have indicated that at least 50% to more than 90% of albumin prescriptions are inappropriate [10,11,12,13]. Overuse of albumin can be challenging for healthcare systems due to its high cost, limited availability, and potential risk of pathogen transmission [10]. Regarding the costs, a report by the Iranian Food and Drug Organization of Health Ministry indicated that 472,089 vials of albumin 20% have been used within the first 9 months of 2008, which amounts to $21,600,000 (13). By implementing clinical guidelines in our center, the number of administered vials of albumin and its direct cost significantly reduced by 50.83% and 55.8%, respectively. In line with these data, use of albumin guidelines in a surgical intensive care unit (ICU) of a tertiary teaching hospital in the Unites States resulted in the significant reduction of albumin use (54%) and substantial cost-saving (56%) [14].
IV pantoprazole overuse, besides its high cost, can be associated with life-threatening side effects (e.g., Clostridium difficile diarrhea) and drug interactions (e.g., clopidogrel) [15]. Batuwitage et al. reported that proton pump inhibitor (PPI) use was inappropriate in 54% of its recipients in general medical wards of the UK [16]. Although the rate of inappropriate use of IV pantoprazole was unknown in the pre-intervention period in our study, clinical guideline implementation was associated with a significant reduction in the number of administrations by 60.29% and direct cost by 83.92%.
Reduction in PPI use through implementation of appropriate guidelines has been also reported by other researchers. For example, Van Vliet et al. in the Netherlands demonstrated that guideline implementation for PPI prescription was associated with significantly fewer patients starting on PPIs during their hospitalization in two pulmonary medicine wards, compared to the control group (13% versus 21%) [17]. A recent study on implementation of pharmaceutical practice guidelines for three costly medications at a tertiary hospital in Iran resulted in a significant reduction in prescriptions of albumin (36%) and IV pantoprazole (40%) [18].
The comparable direct cost of possible alternative medications for albumin (Amino Acid 5% and 10%) and IV pantoprazole (oral omeprazole, oral pantoprazole, and IV ranitidine) between the pre-intervention and post-intervention phases demonstrated that our intervention and switch in use from the studied medications to the alternatives did not result in an increase in the costs. However, in the post-intervention period, the monthly direct costs of parenteral corticosteroids and rituximab, as IVIG alternatives, were significantly higher than the pre-intervention phase. This may be mostly related to the ability of pharmacies to provide these drugs, and subsequently, the increase in their demand during the post-intervention phase. In this regard, higher consumption and direct cost of IVIG in the post-intervention period is another reason to confirm that switch in use from IVIG to its alternatives is not the cause of increase in their cost within the post-intervention phase.
Despite its high cost, global limited sources, several potential adverse effects (e.g., acute kidney injury and hypersensitivity reactions), and documented cases of hepatitis C transmission [19], the list of possible indications and amount of consumed IVIG have grown rapidly worldwide [20]. The annual global demand for IVIG has shown an increase from 7.4 to 55.0 metric tons from 1984 to 2004 [20]. To the best of our knowledge, there is no official national report or published literature on the consumption rate or cost of IVIG in Iran. Our current intervention failed to result in a significant reduction in the administration rate or direct cost of IVIG. This may be due to two major reasons:
First, completing the indication forms by physicians cannot be the sole effective approach for improving the pattern of IVIG use. More than 50 known off-label indications, patients’ critical clinical conditions, pharmacists’ insufficient knowledge regarding patients’ conditions, and their reliance on diagnosis notes in the patients’ medical charts may explain this result. In this regard, Frayha et al. demonstrated that concurrent action plans, including guideline dissemination to all healthcare teams along with indication form use, were associated with the improved utilization pattern, as well as a 14% decrease ($41,000) in the expenditure of IVIG [21]. In keeping with these data, use of IVIG utilization management tools, including distribution of IVIG guidelines among specialists, development of preprinted IVIG order forms, and IVIG dose adjustments based on trough IgG levels for physicians resulted in a total cost saving of $3,038,056 in 2 years in four Canadian Atlantic Provinces [22].
Second, there was a relative shortage of IVIG formulation in the pre-intervention phase, compared to the post-intervention period. In addition, financial resources for providing IVIG were higher and more available during the post-intervention phase in our center due to the start of the Health Revolution Program since May 2013 in Iran. This issue may have resulted in the increased demand for IVIG in the later phase of the study.
Clinical outcomes
Although our pharmacist-based intervention significantly decreased the total direct cost of the studied medications, it was associated with an elevated all-cause in-hospital mortality rate (14.9% versus 16.4% in the pre- versus post-intervention periods), which was statistically significant. This remained statistically significant even after adjusting for confounding factors, including clinical and demographic characteristics of the population. Besides our intervention, advanced age and internal ward admission were also significantly associated with all-cause in-hospital mortality.
The sub-group analysis revealed that the in-hospital mortality rates were higher in patients receiving albumin and IVIG during the post-intervention phase in comparison to the pre-intervention phase (1.4% and 0.5%, respectively). However, these differences were not statistically significant; also, these differences had no clinical relevance. In line with our data, a two-year evidence-based sequential multifaceted intervention was done on the use of albumin in eight ICUs in the USA. The intervention was associated with the estimated total cost saving of $2.5 million without any significant difference in ICU and in-hospital mortality rates between the baseline and post-intervention [23].
The case appears to be somewhat different for IV pantoprazole, compared to albumin and IVIG in our cohort. The sub-analysis implicated that mortality rate was slightly higher (0.3%) in the pre-intervention period, compared to the post-intervention period. Although this rate was statistically significant, it was unlikely to be therapeutically important. Since stress-related mucosal damage prophylaxis is one of the major indications of IV pantoprazole, it seems crucial to determine the incidence of upper GI bleeding episodes in both pre- and post-intervention periods as a more direct and relevant clinical outcome index. However, extracting these data from the medical records of patients or the Hospital Information System in our center was not feasible during this study.
At least two studies by Van Vliet et al. [17] and Mahmoudi et al. [18] demonstrated that guideline implementation for PPI prescription did not increase the risk of upper GI disorders and GI bleeding, respectively. Finally, a systematic review of 20 randomized clinical trials in adult ICU patients (n = 1971) regarding stress ulcer prophylaxis showed no significant difference between stress ulcer prophylaxis and placebo (no prophylaxis) in terms of mortality and GI bleeding [24].
The average LOS in hospital, as another studied clinical outcome in our investigation, was one day longer in the post-intervention period in comparison with the pre-intervention phase. Although the difference was statistically significant, this should not be essentially interpreted as the direct effect of our intervention on prolonging LOS in hospital. In this regard, Freitas et al. examined variables related to high LOS outliers in nearly nine million inpatient episodes in the Portuguese National Health System. They demonstrated that different variables, such as teaching hospitals (versus non-teaching hospitals), increased age, emergent surgeries (versus planned or elective surgeries), and number of comorbidities, were significantly associated with increased LOS [25].
Data regarding the number of comorbidities and type of surgery were not available in our population to evaluate their possible confounding effects on LOS. Furthermore, patients in the pre- and post-intervention periods were not matched in terms of age and hospital wards. In contrast to our findings, the mean LOS was comparable before and after guideline implementation for three costly medications (albumin, IV pantoprazole, and enoxaparin) in a teaching hospital in Iran [18]. Differences in the complexity of both hospitals and patients, as major determinants of LOS, can partially explain LOS disparities in our study and the study by Mahmoudi and colleagues.
Inappropriate use
The two most common inappropriate uses of albumin in the post-intervention period were management of edema in patients without severe hypoalbuminemia (24.58%) and a component of parenteral nutrition (19.87%). Similarly, Jahangard-Rafsanjani et al. reported that 46.6% of albumin administrations in a healthcare setting in Tehran were for nutritional support [13]. Tanzi et al. in a study on 1672 patients from 53 different healthcare facilities in the USA showed that all 142 indications of albumin for individuals with serum albumin levels below 2 g/dL were inappropriate [12]. It has been demonstrated that enteral and/or parenteral nutrition with amino acids, along with adequate calorie intake rather than albumin, can improve serum albumin level in malnourished patients [10].
Regarding IV pantoprazole, oral tolerance in the absence of enteral tube and lack of indications for prophylaxis of stress-related mucosal damage are the two leading causes of disapproving the medication requests in the intervention phase of our study. Accordingly, there is a misconception by a number of physicians that parenteral PPIs may be more efficacious than their oral formulations. However, no head-to-head or comparative clinical studies have confirmed this idea. Nevertheless, since PPIs degrade in acidic environments and are formulated in a delayed-release formulation, they should not be crushed for administration through the enteral feeding tube [26]. In the Netherlands, preventing medication-associated complications in two pulmonary medicine wards, including NSAIDs, corticosteroids, and antibiotics, was the most common reason for PPI use [27].
In contrast to albumin and IV pantoprazole, inappropriate use of IVIG in our cohort in the post-intervention period was only limited to five cases. Similarly, Constantine et al. demonstrated that after implementing IVIG guidelines and feedback reports in the Atlantic Canada, IVIG utilization for labeled indications remained unchanged (37.1% and 36.1% in the baseline and post-intervention, respectively). The rate of unlabeled but potentially indicated use of IVIG increased from 52.4% at baseline to 58.1% in the implementation phase [22].
A four-year experience in an academic center in Italy implicated that the majority of IVIG uses in neurological and neuromuscular disorders were identified to be either recommended (60.4%) or reasonable (25.6%) [28]. Therefore, according to the findings of our cohort and other relevant studies, as well as suggestions by Pendergrast et al., guideline implementation seems unlikely to have significant decreasing effects on the total amount of IVIG consumption in academic and teaching environments [20].
Conducting clinical trials regarding IVIG indications, which have been only proposed in case reports and uncontrolled case series, using effective and cheaper treatments rather than IVIG, and developing a multispecialty team along with multiple-level surveillance for evaluating and approving IVIG indications can be taken into account as effective approaches to improve the usage pattern of this highly popular and commonly prescribed, but limited-source and costly medication.
Conclusion
The current study demonstrated that a pharmacist-based guideline implementation during a six-month period on 4896 patients significantly reduced the monthly direct cost of albumin, IV pantoprazole, and IVIG by $77,720 in a referral clinical setting in Iran. However, this reduction was along with a minor and clinically negligible, but statistically significant increase in all-cause in-hospital mortality and hospital LOS. Furthermore, our intervention including switching the studied medications to their alternatives did not result in an increase in pharmaceutical costs. In contrast to albumin and IV pantoprazole, our intervention failed to significantly reduce the administration rate or direct cost of IVIG. Multidisciplinary strategies, such as guideline dissemination, educating physicians regarding proper indications of drugs, and clinical guideline implementation supervised by pharmaceutical care units appear to be more effective in improving the usage pattern and reducing pharmaceutical costs for medications with several labeled and off-label uses, such as IVIG.
Abbreviations
- ARDS:
-
Acute Respiratory Distress Syndrome
- GI:
-
Gastrointestinal
- ICU:
-
Intensive Care Units
- IQR:
-
Interquartile Range
- IV:
-
Intravenous
- IVIG:
-
Intravenous Immune Globulin
- LOS:
-
Length of Stay
- PPI:
-
Proton Pump Inhibitors
References
Holloway K, Green T. Drug and therapeutics committees: a practical guide: World Health Organization; 2003. Available at: http://apps.who.int/medicinedocs/en/d/Js4882e/. Accessed 27 March 2017
Maiti R, Bhatia V, Padhy BM, Hota D. Essential Medicines: An Indian Perspective. Indian J Community Med. 2015;40:223–32. https://doi.org/10.4103/0970-0218.164382.
Rosenberg W, Donald A. Evidence based medicine; an approach to clinical problem-solving. BMJ. 1995;310:1122–6. https://doi.org/10.1136/bmj.310.6987.1122.
Schifalacqua MM, Shepard A, Kelley W. Evidence based practice: cost benefit of large sys implementation. Qual Manag Health Care. 2012;21:74–80. https://doi.org/10.1097/QMH.0b013e31824d196f.
Marshall J, Finn CA, Theodore AC. Impact of a clinical pharmacist-enforced intensive care unit sedation protocol on duration of mechanical ventilation and hospital stay. Crit Care Med. 2008;36:427–33. https://doi.org/10.1097/01.CCM.0000300275.63811.B3.
Buckley MS, Kane-Gill SL, Patel SA. Clinical and economic evaluation of an evidence-based institutional epoetin-utilization management program. Clin Ther. 2013;35:294–302. https://doi.org/10.1016/j.clinthera.2013.02.002.
Khalili H, Karimzadeh I, Mirzabeigi P, Dashti-Khavidaki S. Evaluation of clinical pharmacist's interventions in an infectious diseases ward and impact on patient's direct medication cost. Eur J Intern Med. 2013;24:227–33. https://doi.org/10.1016/j.ejim.2012.11.014.
Armstrong TA, Coursin DB, Devlin J, Scott Duke J, Fish D, Gonzalez ER, et al. ASHP therapeutic guidelines on stress ulcer prophylaxis. Am J Health Syst Pharm. 1999;56:347–79.
Alldredge BK, Corelli RL, Ernst ME, Guglielmo BJ, Jacobson PA, Kradjan WA, et al (editors). Koda-Kimble and Young’s applied therapeutics: the clinical use of drugs, 10th edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2013.
Shafiee E, Rezaee H, Entezari T, Hamishehkar H. The evaluation of albumin use in an Iranian university hospital. Pharm Sci. 2016;22:186–9. https://doi.org/10.15171/PS.2016.29.
Vargas E, de Miguel V, Portolés A, Avendaño C, Ambit MI, Torralba A, et al. Use of albumin in two Spanish university hospitals. Eur J ClinPharmacol. 1997;52:465–70.
Tanzi M, Gardner M, Megellas M, Lucio S, Restino M. Evaluation of the appropriate use of albumin in adult and pediatric patients. Am J Health Syst Pharm. 2003;60:1330–5.
Jahangard-Rafsanjani Z, Javadi MR, Torkamandi H, Alahyari S, HajhosseinTalasaz A, Gholami K. The evaluation of albumin utilization in a teaching university hospital in Iran. Iran J Pharm Res. 2011;10:385–90.
Charles A, Purtill M, Dickinson S, Kraft M, Pleva M, Meldrum C, et al. Albumin use guidelines and outcome in a surgical intensive care unit. Arch Surg. 2008;143:935–9. https://doi.org/10.1001/archsurg.143.10.935.
Berardi RR, Fugit RV. Peptic Ulcer Disease. In: JT DP, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: a pathophysiologic approach. 8th ed. China: McGraw-Hili Companies, Inc; 2011. p. 576–7.
Batuwitage BT, Kingham JG, Morgan NE, Bartlett RL. Inappropriate prescribing of proton pump inhibitors in primary care. Postgrad Med J. 2007;83:66–8. https://doi.org/10.1136/pgmj.2006.051151.
van Vliet EP, Steyerberg EW, Otten HJ, Rudolphus A, Knoester PD, Hoogsteden HC, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29:213–21. https://doi.org/10.1111/j.1365-2036.2008.03875.x.
Mahmoudi L, Karamikhah R, Mahdavinia A, Samiei H, Petramfar P, Niknam R. Implementation of Pharmaceutical Practice Guidelines by a Project Model Based: Clinical and Economic Impact. Medicine (Baltimore). 2015;94:e1744. https://doi.org/10.1097/MD.0000000000001744.
Stiehm ER. Adverse effects of human IVIG therapy. Transfus Med Rev. 2013;27:171–8. https://doi.org/10.1016/j.tmrv.2013.05.004.
Pendergrast JM, Sher GD, Callum JL. Changes in intravenous IVIG prescribing patterns during a period of severe product shortages, 1995-2000. Vox Sang. 2005;89:150–60. https://doi.org/10.1111/j.1423-0410.2005.00670.x.
Frayha HH, Nuessle SJ, Arishi H, Rayes H, Qunibi WY, Bazarbashi MS. Improving utilization of intravenous immune globulin through concurrent use of an indication form. Eur J ClinPharmacol. 1997;52:255–60.
Constantine MM, Thomas W, Whitman L, Kahwash E, Dolan S, Smith S, et al. Intravenous IVIG utilization in the Canadian Atlantic provinces: a report of the Atlantic collaborative intravenous immune globulin utilization working group. Transfusion. 2007;47:2072–80. https://doi.org/10.1111/j.1537-2995.2007.01400.x.
Lyu PF, Hockenberry JM, Gaydos LM, Howard DH, Buchman TG, Murphy DJ. Impact of a sequential intervention on albumin utilization in critical care. Crit Care Med. 2016;44:1307–13. https://doi.org/10.1097/CCM.0000000000001638.
Krag M, Perner A, Wetterslev J, Wise MP, HylanderMøller M. Stress ulcer prophylaxis versus placebo or no prophylaxis in critically ill patients. A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Intensive Care Med. 2014;40:11–22. https://doi.org/10.1007/s00134-013-3125-3.
Freitas A, Silva-Costa T, Lopes F, Garcia-Lema I, Teixeira-Pinto A, Brazdil P, et al. Factors influencing hospital high length of stay outliers. BMC Health Serv Res. 2012;12:265. https://doi.org/10.1186/1472-6963-12-265.
Williams DB, Schade RR. Gastroesophageal Reflux Disease. In: JT DP, Talbert RL, Yee GC, et al., editors. Pharmacotherapy: a pathophysiologic approach. 8th ed. China: McGraw-Hili Companies, Inc; 2011. p. 557.
van Vliet EP, Otten HJ, Rudolphus A, Knoester PD, Hoogsteden HC, Kuipers EJ, et al. Inappropriate prescription of proton pump inhibitors on two pulmonary medicine wards. Eur J Gastroenterol Hepatol. 2008;20:608–12. https://doi.org/10.1097/MEG.0b013e3282f52f95.
Sarti L, Falai T, Pinto F, Tendi E, Matà S. Intravenous immune globulin usage for neurological and neuromuscular disorders: an academic centre, 4 years experience. Neurol Sci. 2009;30:213–8. https://doi.org/10.1007/s10072-009-0043-9.
Acknowledgements
The authors would like to thank all pharmacists and staffs of Namazi hospital central pharmacy for their great cooperation in performing the intervention phase of the study.
Funding
No funding support.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request. Identifying/confidential patient data would not be shared.
Author information
Authors and Affiliations
Contributions
IK contributed to study design, statistical analyses, and critical review of the manuscript. AV contributed to study design and critical review of the manuscript. RK contributed to statistical analyses and manuscript drafting. ZO, SM, MK, FR, ES, and AZS contributed to guideline implementation, data collection, and manuscript drafting. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board and the Medical Ethics Committee of the Shiraz University of Medical Sciences approved the study and written informed consent form was received from patients or their family members to participate into the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Indication checklists for albumin, IVIG, and iv pantoprazole. (ZIP 65 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Vazin, A., Karimzadeh, I., Karamikhah, R. et al. Clinical and economical impacts of guideline implementation by the pharmaceutical care unit for high cost medications in a referral teaching hospital. BMC Health Serv Res 18, 815 (2018). https://doi.org/10.1186/s12913-018-3627-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12913-018-3627-3