Abstract
Background
The SI-CURA project (Soluzioni Innovative per la gestione del paziente e il follow up terapeutico della Colite UlceRosA) is an Italian initiative aimed at the development of artificial intelligence solutions to discriminate pathologies of different nature, including inflammatory bowel disease (IBD), namely Ulcerative Colitis (UC) and Crohn’s disease (CD), based on endoscopic imaging of patients (P) and healthy controls (N).
Methods
In this study we develop a deep learning (DL) prototype to identify disease patterns through three binary classification tasks, namely (1) discriminating positive (pathological) samples from negative (healthy) samples (P vs N); (2) discrimination between Ulcerative Colitis and Crohn’s Disease samples (UC vs CD) and, (3) discrimination between Ulcerative Colitis and negative (healthy) samples (UC vs N).
Results
The model derived from our approach achieves a high performance of Matthews correlation coefficient (MCC) > 0.9 on the test set for P versus N and UC versus N, and MCC > 0.6 on the test set for UC versus CD.
Conclusion
Our DL model effectively discriminates between pathological and negative samples, as well as between IBD subgroups, providing further evidence of its potential as a decision support tool for endoscopy-based diagnosis.
Similar content being viewed by others
Introduction
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and Ulcerative colitis (UC), are chronic and recurrent diseases. CD patients have healthy parts of the intestine mixed in between inflamed areas, while UC induces a continuous inflammation of the colon. Further, CD may occur in all the layers of the bowel walls, while UC only affects the innermost lining of the colon. Although they both have an undetermined etiology, research advances have outlined some of the pathways leading to their insurgence: (1) genetic predisposition associated with the environment induces disruption of the intestinal microbial flora; (2) the structure of the epithelial cells and of the immune system of the intestine determine the risk of developing the disease. IBD is diagnosed using a combination of endoscopy (for CD) or colonoscopy (for UC) and imaging studies, such as contrast radiography, magnetic resonance imaging, or computed tomography. Physicians may also check stool samples to make sure symptoms are not being caused by an infection or run blood tests to help confirm the diagnosis. Still, a definite diagnosis of IBD remains a challenging task [1], often affected by subjective judgement [2]. Several automated approaches have been published in the recent literature [2, 3] attempting to provide computational support to improve the diagnostic task, with machine learning (ML) models playing a major role, however several challenges remain [4, 5]. In this line Stidham et al. [6] found deep learning model performance to be similar to an experienced clinician in evaluating severity of UC, while Takenaka et al. [7] derived a high performance model in identifying UC patients with endoscopic remission. Klein et al. [8] focused on analysis of colonic biopsies of CD patients to predict post-biopsy clinical phenotypes and outcomes, similar to Waljee et al. [9, 10] developing a model to predict IBD-related hospital admission and disease flare.
As a consequence, the emergence of Artificial Intelligence (AI) solutions based on Deep Learning (DL) models comes as no surprise. The models exploit the potential of artificial neural networks to automatically process different types of data [11, 12], including endoscopic imaging [13]. Our work naturally embeds in this research line, combining recent DL architectures with more classical ML strategies such as ensemble learning to further enhance the predictive performance. The promising results obtained can support the clinicians in providing a more objective and reliable diagnosis, thus reducing the risk of misidentification of CD and UC, an important aspect considering different treatment options and follow-ups of the two conditions [13].
Methods
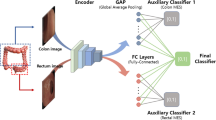
We tackle the problem of IBD detection from endoscopic imaging with an ensemble learning approach based on a fine-tuned ResNet architecture. An overview of the general workflow is presented in Fig. 1. Briefly, the input images first undergo a random partitioning into train and test sets: the train set is used for model development in a k-fold cross-validation (CV) schema, while the test set is kept apart to obtain predictions from the trained model. Then, after a preprocessing phase aimed at preparing the images for the predictive modeling, three pre-trained Residual Network models [14] of increasing complexity are fine-tuned on the preprocessed images in a transfer learning setting. Finally, the models are combined in a single meta-model with a stacking ensemble method.
Experimental workflow. After an upstream processing of the input images (blocks “Data splitting”, “Class balancing”, “Transform”), for each classification task three pre-trained ResNet variants of increasing complexity (“Model i”, \(i = 1,2,3\)) are used as weak learners and fine-tuned in 5-fold cross-validation on the input data (see Methods). A meta-model is then built by stacking ensemble of the three weak learners, evaluating the performance on the external test set in terms of different classification metrics (see Methods). N–P, negative versus positive; UC–CD, Ulcerative Colitis versus Crohn’s Disease; UC–N, Ulcerative Colitis versus negative; MCC, Matthews Correlation Coefficient (MCC); TNR, true negative rate; TPR, true positive rate; NPV, negative predictive value; PPV, positive predictive value
Outcome definition
In this work, we investigate three major outcomes, namely: (1) discrimination between negative (healthy) samples and positive (pathological) samples (N–P), (2) discrimination between Ulcerative Colitis and Crohn’s Disease (UC–CD) samples and, (3) discrimination between negative samples and Ulcerative Colitis (UC–N).
Dataset description
The SI-CURA dataset includes 14,226 three-channel RGB (red, green, blue) endoscopic images of different sizes split between “Positive” patients (P, n = 11,404) and “Negative” healthy controls (N, n = 2822). In this work, we consider a single image to be a sample for the classification task. Positive (pathological) samples are further labeled as Ulcerative Colitis (UC), Crohn’s disease (CD), and Inflammatory bowel disease (IBD). Table 1 illustrates the sample stratification for each class and Fig. 2 shows an example of positive and negative images.
In developing and evaluating the model, the overall dataset was split into two main subsets: training (90% of total sample size) and test (10%). The training set was further split for model development according to a 5-fold cross-validation schema. Since the P data is 3\(\times\) the N data, under- and over-sampling were used to balance the class distributions using the Imbalanced-dataset-sampler Python library,Footnote 1 which automatically estimates the sampling weights and mitigates overfitting when used in conjunction with data augmentation techniques.
Data preprocessing
An image editor was used to remove undesired artifacts, such as signs, writings, medical instruments, black, white, and corrupted images. Further, all images were converted to JPEG with lossy compression to have a consistent format across the dataset. Table 1 details the number of elements in each class after preprocessing.
Data augmentation is a technique commonly used to increase the amount of relevant data in the original dataset, thus providing additional examples to the neural network. Augmentation exploits the fact that convolutional neural networks are invariant to transformations such as translation, size, viewpoint, rotation. The following techniques were used: RGB to HSV (hue, saturation, value) colour model conversion, random horizontal flip, and random vertical flip, all of which had an equal probability of being applied.
Several experiments were performed with different batch and image sizes. The best results were obtained with a batch size of 32, 64, and 128, depending on the model’s depth. To speed up the training time, we rescaled all images to 700\(\times\)700 pixels, equal to the average image dimension.
Deep learning architecture
We tackled the tasks through a transfer learning approach using the following variants of Residual Networks (ResNet) [14]: ResNet18, ResNet34, ResNet50, ResNet101, and ResNet152, all of which were imported pre-trained on ImageNet from the PyTorch Hub.Footnote 2 The choice of using pre-trained models leverages the information already learned by the network after an extensive training on the large ImageNet dataset. The fully-connected head of the ResNet networks was swapped with one performing binary classification: an untrained sequential module ending with a linear transformation and two outputs. Moreover, the rectified linear unit function (ReLU) [15] and dropout technique (with \(p = 0.5\) probability) were used in this module. ReLU activation has been widely used in deep neural networks due to its advantages over other functions (e.g. sigmoid): most importantly, it overcomes the vanishing gradient problem [16] that has been afflicting neural networks for several years. We adopted dropout to increase regularisation, preventing the coadaptation of the neurons. The weights of the layers were frozen except for the last two, which were updated with a small variation of the learning rate. In a typical transfer learning approach, the layers of the network are frozen to the trained weights except for the last (“higher”, or “top”) layers (farther to the input). This is because the first-layer features are more general and the last-layer features are more specific [17]. There are different strategies for fine-tuning the higher layers: typically, the final fully-connected classification part of the network is trained, leaving the convolutional layers frozen. However, it is also possible to unfreeze one or more convolutional layers as well to fine-tune the performance. We chose to unfreeze the last two convolutional layers as a compromise between achieving a good performance and avoiding overfitting, following recommendations in the literature [18]. The optimiser used for the training was Adaptive Movement Estimation (Adam) [19], a replacement for Stochastic Gradient Descent that combines the advantages of the Adaptive Gradient (AdaGrad) and Root Mean Square Propagation (RMSProp) algorithms, making it a popular choice in recent studies [20]: AdaGrad maintains a per-parameter learning rate that improves performance on problems with sparse gradients, and RMSProp also maintains per-parameter learning rates using a decaying average of recent partial gradients. This means that Adam improves parameter optimisation, particularly in online and non-stationary (e.g., noisy) tasks. The actual number of epochs was determined by early stopping based on the validation loss, with a patience of 30 epochs. The maximum number of epochs was set to 200, a value high enough to allow the net to learn as much as possible until the early stopping technique takes place.
Differential learning rates
The learning rate is one of the most important hyperparameters that can be tuned to improve optimisation convergence. It is usually chosen by time-consuming techniques such as grid search that exhaustively experiments with different values, picking the one that works best in terms of performance on the validation set. A more convenient approach, which was adopted in this study, is the learning rate finder [21]. During each epoch, the algorithm makes the learning rate cyclically vary between a lower and an upper bound. The loss corresponding to each learning rate is computed, and the learning rate yielding the steepest drop in the loss is chosen.
The learning rate finder method was complemented by the use of different learning rates during the training, an approach commonly referred to as “differential learning rates”, which is particularly useful in a transfer learning setting. In fact, only the layers immediately preceding the fully-connected classifier head were unfrozen and trained. Since the ResNet model is pre-trained on the large ImageNet dataset, these layers only need a small amount of fine-tuning to achieve good prediction performance. In particular, the learning rate values for the ResNet’s layer4 (just before the classifier) and layer3 were reduced by a third and by a ninth, respectively.
Ensemble learning
Ensemble learning is a machine learning paradigm where multiple models (“weak learners”) are trained to solve the same problem and finally combined to achieve better results. Two ensemble methods were used in this study: averaging prediction and stacking. While the former keeps the models independent from each other and averages their predictions, the latter combines the predictions of the weak learners through a final classifier, resulting in a meta-model. We combined three weak learners in a stacking ensemble meta-model: ResNet34, ResNet50, and ResNet101 were used as weak learners in the N–P and UC–N cases, and ResNet34, ResNet50, and ResNet152 in the UC–CD case. Other combinations were tried, but they performed worse. The ensemble meta-model has six input features (two for each weak learner) and two outputs features, and it was further trained for several epochs by using the early stopping criterion.
Performance evaluation metrics
The metrics used for assessing the model performance in both training and evaluation stages are Matthews Correlation Coefficient (MCC), true positive rate (TPR), true negative rate (TNR), positive predictive value (PPV), negative predictive value (NPV), and the confusion matrices. The MCC was used as the main metric because it is particularly suitable for binary and multiclass classification [22], and it is generally regarded as a balanced performance measure that can be used even if the classes are of very different sizes. MCC is computed from the values of the confusion matrix, true positives (TP), true negatives (TN), false positives (FP), and false negatives (FN), as in the following:
The values of MCC range between −1 and 1, where 1 means perfect classification, −1 perfect misclassification (inverse prediction), and 0 random guess or, generally speaking, absence of correlation between the predictions and the ground truth.
We also computed the training and validation losses and the 95% studentized bootstrap confidence intervals for the training MCC.
Model interpretation
In an attempt to better understand which input features are deemed important by the trained deep learning models, we used two explainable AI (XAI) algorithms implemented in the Captum library,Footnote 3 namely Saliency and Guided backpropagation. Saliency [23] is one of the most straightforward approaches for estimating input attribution: it computes the gradient of the model output with respect to the input pixels of a given image. While a simple approach to deep model interpretation, Saliency maps cannot capture the input feature interactions and tend to be noisy. Guided backpropagation [24] (GBP) is an extension of the Saliency algorithm aiming to alleviate these issues: GBP also computes the gradient of the output with respect to the input; in addition, it overrides ReLU backpropagation, resulting in backpropagation of non-negative gradients only. Each method computes an attribution value (the higher, the more important) for each input pixel: the resulting attribution maps are then qualitatively assessed by overlaying them on the original image.
Implementation
The following frameworks and libraries were used: PyTorch v1.5.0 with torchvision v0.6.0a0, Captum v0.4.1, CUDA 10.2, and Jupyter v6.1.4. Color model conversion was performed by the PIL library. The packages were installed in a Anaconda virtual environment for improved reproducibility. The analyses were run on a ppc64le server, provided by GPI S.p.A., with 128 CPUs (max 4023 MHz, min 2394 MHz) and 4 NVIDIA Tesla P100 SXM2 16GB GPUs. The training of neural networks was performed on multiple GPUs using PyTorch’s DataParallel.
Results
Classification
Figures 3, 4, 5 show the cross-validation and test set performance of the weak learners and the ensemble meta-models for all classification tasks. The test set confusion matrices are reported in Fig. 6 for the best-performing weak learner of each task. Table 2 reports the results in terms of average cross-validation MCC with 95% confidence intervals and the test set MCC, TPR, TNR, PPV, and NPV. The rows of Table 2 are ranked task-wise by decreasing test set MCC. The average-prediction ensemble models performed consistently worse for all tasks and are not shown. According to Table 2 and Fig. 3, the top three nets (or weak learners) for N–P are ResNet50, ResNet34, and ResNet101. While the first one is trained with an unbalanced training data loader, the latter two nets are trained using a balanced data loader.
As for the UC–CD task, the best results were obtained by ResNet34, ResNet50, and ResNet152 (Table 2, Fig. 4). The first two nets were trained with a balanced data loader and the last one with an unbalanced data loader. In this task, the classes are not as imbalanced as in N–P (Table 1): however, the dataset balancing technique helped maintain balanced the confusion matrices in the training and evaluation phase. As expected, the meta-model slightly improves over the weak learners on the test set (Table 2).
The best-performing networks for the UC–N task are a ResNet34 model with a balanced data loader and an ensemble model using ResNet50, ResNet34, and ResNet101 as weak learners trained with unbalanced, balanced, and unbalanced data loaders, respectively. The best metrics are shown in Table 2. In this case, the performance improvement of the ensemble model over the single best-performing weak learner ResNet34 is limited.
The performance on the test set is reasonably comparable to that in cross-validation for all tasks and models, except for the UC–CD ensemble model which exhibits higher CV performance (MCC = 0.906 vs. 0.688, Fig. 4, Table 2).
Interpretability
We applied the chosen model interpretation methods to a random subsample of test set N–P images using a ResNet50 model, which was the best performing weak learner on the task N–P (Table 2). For two representative images (1 Positive, 1 Negative), Fig. 7 visualizes the Saliency and Guided Backpropagation (GuidedBackProp) attribution maps overlaid on the original image, which is also shown separately for comparison. We observe that GuidedBackProp attributions are less noisy than Saliency ones, as expected. Moreover, typical endoscopic features of IBD such as mucosal erythemas appear to have higher attribution values according to GuidedBackProp (Fig. 7, bottom). Using a gradient-based XAI method such as Saliency and Guided backpropagation is particularly appealing because it allows a straightforward visual interpretation of the input features deemed important by the underlying model. However, the identified features should be the object of further analyses since they may be affected by different kinds of bias, such as illumination (Fig. 7).
Confusion matrices. Test set confusion matrices for the best-performing weak learners of each classification task (see Table 2)
Model interpretability. Qualitative visualization of attribution maps by Saliency and Guided backpropagation (GuidedBackProp) algorithms for a Negative (A, top row) and Positive (B, bottom row) input image. The original image is also shown for comparison. The attribution maps are visualized as heatmaps, with darker shades representing more important features according to the algorithm
Discussion
In this work, we developed and evaluated a prototype DL framework based on ResNet architectures merged by ensemble learning, able to identify disease patterns from endoscopic images of different IBDs, namely Ulcerative Colitis and Crohn’s Disease, and distinguish negative (healthy) samples. The DL models achieve a test set performance MCC = 0.940 for the classification of healthy controls versus IBD patients, MCC = 0.688 for Ulcerative Colitis versus Crohn’s Disease, and MCC = 0.931 for Ulcerative Colitis versus healthy individuals. On the UC–CD task, we observed a lower, yet relatively good, predictive performance (MCC = 0.688). This result might be due to the intrinsic difficulty in distinguishing between these two clinical subtypes of IBD, using solely colonoscopy-based diagnosis, whereas additional methods such as endoscopic ultrasonography may be more effective in differentiating UC from CD [25]. It should be noted that the results of the ensemble model demonstrate that there is only a marginal increase in predictive performance compared to using a single ResNet, possibly because it is difficult to further improve over already high-performance results. Overall, the obtained results in all the three classification tasks indicate a very good to excellent predictive performance, highlighting the potential of the framework to evolve into a valuable tool for the clinicians in their diagnostic tasks. Given the several subtleties of endoscopic imaging, linked to both the intrinsic disease features and to potential artifacts (for instance due to light effects), disagreement on the diagnosis is not uncommon among clinicians dealing with this task: the automated system proposed here can provide an additional view on the problem, clarifying the issues hampering the correct assessment of the pathology. Nonetheless, despite the encouraging results, the current study should be considered a proof of concept rather than a consolidated pipeline, which we have already planned to improve in future development. In this regard, additional model architectures other than ResNet could be evaluated, and different loss functions can be used to overcome the data limitation involving unbalanced classes. Moreover, the neural networks could be trained for more epochs, with additional combinations of hyperparameters, including automated approaches to data preprocessing and artefact removal, such as traditional image segmentation [26]. Additionally, alternative ensemble models can be tested, trained with images in different colour spaces. The model outcome can also be improved by enhancing the training data, even through augmenting techniques creating synthetic data: for example, generative adversarial networks. Finally, strengthening the resampling strategy will further improve the overall reproducibility of the study, while the analysis of the data trajectories across the DL layers can provide a valuable direction regarding the model interpretability.
Conclusions
The results presented in this study demonstrate the vast potential of deep neural networks in discriminating between pathological and negative samples as well as discriminating between IBD subgroups, namely Ulcerative Colitis and Crohn’s Disease. Further development of this work and other studies in this area in general have the potential to become powerful tools in the hands of clinicians, aiding diagnosis and the clinical decision process. However, additional studies would be required to evaluate the utility of machine-learning aided diagnosis of IBD in clinical practice.
Availability of data and materials
The data generated and analysed during the current study are not publicly available due to privacy restrictions and commercial confidentiality, however they can be made available in a de-identified manner upon reasonable request and an appropriate agreement, contacting the corresponding author.
Abbreviations
- IBD:
-
Inflammatory bowel diseases
- CD:
-
Crohn’s disease
- UC:
-
Ulcerative colitis
- AI:
-
Artificial intelligence
- XAI:
-
Explainable AI
- DL:
-
Deep learning
- ML:
-
Machine learning
- RGB:
-
Red, green, blue
- HSV:
-
Hue, saturation, value
- ReLU:
-
Rectified linear unit
- ResNet:
-
Residual neural network
- MCC:
-
Matthew’s correlation coefficient
- TPR:
-
True positive rate
- TNR:
-
True negative rate
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
References
Negreanu L, Voiosu T, State M, Voiosu A, Bengus A, Mateescu BR. Endoscopy in inflammatory bowel disease: from guidelines to real life. Ther Adv Gastroenterol. 2019;12:1–10.
Chen G, Shen J. Artificial intelligence enhances studies on inflammatory bowel disease. Front Bioeng Biotechnol. 2021;9:570.
Tontini GE, Rimondi A, Vernero M, Neumann H, Vecchi M, Bezzio C, Cavallaro F. Artificial intelligence in gastrointestinal endoscopy for inflammatory bowel disease: a systematic review and new horizons. Ther Adv Gastroenterol. 2021;14:1–12.
Semmler G, Wernly S, Wernly B, Mamandipoor B, Bachmayer S, Semmler L, Aigner E, Datz C, Osmani V. Machine learning models cannot replace screening colonoscopy for the prediction of advanced colorectal adenoma. J Pers Med. 2021;11:10. https://doi.org/10.3390/jpm11100981.
Semmler G, Wernly S, Wernly B, Mamandipoor B, Bachmayer S, Semmler L, Aigner E, Datz C, Osmani V. Gastroenterologist against the machine - opportunities and limitations of machine learning models for prediction of advanced adenoma. In: Zeitschrift Für Gastroenterologie, vol. 59, 2021. https://doi.org/10.1055/s-0041-1734270
Stidham RW, Liu W, Bishu S, Rice MD, Higgins PD, Zhu J, Nallamothu BK, Waljee AK. Performance of a deep learning model vs human reviewers in grading endoscopic disease severity of patients with ulcerative colitis. JAMA Netw Open. 2019;2(5):193963–193963.
Takenaka K, Ohtsuka K, Fujii T, Negi M, Suzuki K, Shimizu H, Oshima S, Akiyama S, Motobayashi M, Nagahori M, et al. Development and validation of a deep neural network for accurate evaluation of endoscopic images from patients with ulcerative colitis. Gastroenterology. 2020;158(8):2150–7.
Klein A, Mazor Y, Karban A, Ben-Itzhak O, Chowers Y, Sabo E. Early histological findings may predict the clinical phenotype in Crohn’s colitis. United Eur Gastroenterol J. 2017;5(5):694–701.
Waljee AK, Wallace BI, Cohen-Mekelburg S, Liu Y, Liu B, Sauder K, Stidham RW, Zhu J, Higgins PD. Development and validation of machine learning models in prediction of remission in patients with moderate to severe crohn disease. JAMA Netw Open. 2019;2(5):193721–193721.
Waljee AK, Lipson R, Wiitala WL, Zhang Y, Liu B, Zhu J, Wallace B, Govani SM, Stidham RW, Hayward R, et al. Predicting hospitalization and outpatient corticosteroid use in inflammatory bowel disease patients using machine learning. Inflamm Bowel Dis. 2018;24(1):45–53.
Cohen-Mekelburg S, Berry S, Stidham RW, Zhu J, Waljee AK. Clinical applications of artificial intelligence and machine learning-based methods in inflammatory bowel disease. J Gastroenterol Hepatol. 2021;36(2):279–85.
Cinaglia P, Caroprese L, Cascini GL, Dattola F, Iaquinta P, Iusi M, Veltri P, Zumpano E. Bioinformatics solutions for image data processing. In: Koprowski, R. (ed.) Medical and Biological Image Analysis. IntechOpen, Rijeka. 2018. Chap. 4. https://doi.org/10.5772/intechopen.76459.
Sundaram S, Choden T, Mattar MC, Desai S, Desai M. Artificial intelligence in inflammatory bowel disease endoscopy: current landscape and the road ahead. Ther Adv Gastrointest Endosc. 2021;14:1–8.
He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. In: Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), pp. 770–778. IEEE, 2016.
Xu B, Wang N, Chen T, Li M. Empirical evaluation of rectified activations in convolutional network. arXiv:1505.00853 2015.
Bengio Y, Simard P, Frasconi P. Learning long-term dependencies with gradient descent is difficult. IEEE Trans Neural Netw. 1994;5(2):157–66.
Yosinski J, Clune J, Bengio Y, Lipson H. How transferable are features in deep neural networks? Adv Neural Inf Process Syst. 2014;27:3320–8.
Kim HE, Cosa-Linan A, Santhanam N, Jannesari M, Maros ME, Ganslandt T. Transfer learning for medical image classification: a literature review. BMC Med Imaging. 2022;22(1):1–13.
Kingma DP, Ba J. Adam: A Method for Stochastic Optimization. arXiv:1412.6980, In: Proceedings of the 3rd International Conference for Learning Representations (ICLR). 2015
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–44.
Smith LN. Cyclical Learning Rates for Training Neural Networks. In: Proceedings of the IEEE Winter Conference on Applications of Computer Vision (WACV), pp. 464–472. IEEE, 2017.
Chicco D, Jurman G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics. 2020;21:6.
Simonyan K, Vedaldi A, Zisserman A. Deep inside convolutional networks: Visualising image classification models and saliency maps. arXiv:1312.6034 2013.
Springenberg JT, Dosovitskiy A, Brox T, Riedmiller M. Striving for simplicity: the all convolutional net. arXiv:1412.6806 2014.
Ellrichmann M, Wietzke-Braun P, Dhar S, Nikolaus S, Arlt A, Bethge J, Kuehbacher T, Wintermeyer L, Balschun K, Klapper W, et al. Endoscopic ultrasound of the colon for the differentiation of crohn’s disease and ulcerative colitis in comparison with healthy controls. Aliment Pharmacol Ther. 2014;39(8):823–33.
Cinaglia P, Tradigo G, Cascini GL, Zumpano E, Veltri P. A framework for the decomposition and features extraction from lung dicom images. In: Proceedings of the 22nd International Database Engineering & Applications Symposium. IDEAS 2018, pp. 31–36. Association for Computing Machinery, New York, NY, USA (2018). https://doi.org/10.1145/3216122.3216127
Acknowledgements
A shorter version of this work has been previously presented online during the CIBB 2021 conference https://davidechicco.github.io/cibb2021/papers.html..
About this supplement
This article has been published as part of BMC Medical Informatics and Decision Making Volume 22 Supplement 6, 2022 Selected articles from the 17th International Conference on Computational Intelligence Methods for Bioinformatics and Biostatistics (CIBB 2021). The full contents of the supplement are available online at https://bmcmedinformdecismak.biomedcentral.com/articles/supplements/volume-22-supplement-6.
Funding
This work was supported by GPI S.p.A. through the Contract 210202009864 between GPI S.p.A. and FBK within the framework project “SI-CURA - Soluzioni Innovative per la gestione del paziente e il follow up terapeutico della Colite UlceRosA” funded by Regione Puglia with the POR Puglia FESR-FSE 2014–2020 Innonetwork. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
MC performed the XAI analysis, prepared figures and tables, and coordinated the revision of the paper after reviewing. NP preprocessed the data, wrote the original scripts, ran the predictive modeling, and contributed to an earlier version of the manuscript. MC, VO, GJ coordinated the project, interpreted the results, and wrote the manuscript. MP implemented the k-fold cross-validation, ran the predictive modeling, and contributed to XAI analysis. AC1, MDD, AC2 provided access to the endoscopic imaging data and interpreted the results. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study that produced the data used for these analyses was conducted in accordance with the Declaration of Helsinki and approved by a local ethics committee in Italy, namely the Istituto Tumori Bari “Giovanni Paolo II” - IRCCS, with the protocol number n. 333 / C.E. of 31/07/2019. Informed consent to participate was obtained from the study participants. The team acquired the permission to access and analyze the clinical patient data by GPI S.p.A. through the Contract 210202009864 between GPI S.p.A. and FBK.
Competing interests
GPI S.p.A. took part in the writing of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chierici, M., Puica, N., Pozzi, M. et al. Automatically detecting Crohn’s disease and Ulcerative Colitis from endoscopic imaging. BMC Med Inform Decis Mak 22 (Suppl 6), 300 (2023). https://doi.org/10.1186/s12911-022-02043-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12911-022-02043-w