Abstract
Background
The European Union (EU) aims to optimize patient protection and efficiency of health-care research by harmonizing procedures across Member States. Nonetheless, further improvements are required to increase multicenter research efficiency. We investigated IRB procedures in a large prospective European multicenter study on traumatic brain injury (TBI), aiming to inform and stimulate initiatives to improve efficiency.
Methods
We reviewed relevant documents regarding IRB submission and IRB approval from European neurotrauma centers participating in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI). Documents included detailed information on IRB procedures and the duration from IRB submission until approval(s). They were translated and analyzed to determine the level of harmonization of IRB procedures within Europe.
Results
From 18 countries, 66 centers provided the requested documents. The primary IRB review was conducted centrally (N = 11, 61%) or locally (N = 7, 39%) and primary IRB approval was obtained after one (N = 8, 44%), two (N = 6, 33%) or three (N = 4, 23%) review rounds with a median duration of respectively 50 and 98 days until primary IRB approval. Additional IRB approval was required in 55% of countries and could increase duration to 535 days. Total duration from submission until required IRB approval was obtained was 114 days (IQR 75–224) and appeared to be shorter after submission to local IRBs compared to central IRBs (50 vs. 138 days, p = 0.0074).
Conclusion
We found variation in IRB procedures between and within European countries. There were differences in submission and approval requirements, number of review rounds and total duration. Research collaborations could benefit from the implementation of more uniform legislation and regulation while acknowledging local cultural habits and moral values between countries.
Similar content being viewed by others
Background
A Research Ethics Committee or Institutional Review Board (collectively referred to as IRB in the remainder of this manuscript) is appointed to review research protocols to ensure their compliance with ethical standards and national laws. IRBs have an essential role in (clinical) research to protect the dignity, fundamental rights, safety, and well-being of research participants and their formal approval is compulsory before a clinical study can start [1]. Although several international models exist to improve the harmonization of ethical principles, the functioning of IRBs are subject to national legislation and regulation, which refine their structure and function to better serve local needs and cultural preferences [2, 3]. Approval of research protocols submitted to IRBs is subject to these differences, which may complicate the conduct of international research.
Managing variations in IRB procedures is important because of the increasing number of research initiatives which involve multiple European Union (EU) Member States [4,5,6]. Variation could be improved by harmonization of European law, which is the process of creating uniformity in laws, regulations and practices between countries. Regarding research and IRB procedures, lack of procedural harmonization ‘leads to a complex and uncertain framework for ethical review and for participant information consent, resulting in numerous inefficiencies in observational studies’ [7]. Greater procedural harmonization is generally considered desirable, because it could improve quality and efficiency of healthcare research by decreasing costs, increasing statistical validity, [8,9,10] optimizing data management, [10] allowing choice of relevant and generalizable outcome variables, [9] promoting uniform product safety regulations [8] and minimizing waste of resources due to inefficiencies [8].
Although most IRBs have websites that describe the local submission process and provide access to submission guidelines and forms, up to date systematic information on IRB procedures and their level of harmonization in European health-care research is scarce. We are aware of only one previous meta-analysis on IRB procedures across European countries from 2005 to 2007 that was also related to research involving acutely mentally incapacitated individuals [6]. The Collaborative European Neurotrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study is a large observational study conducted in many countries across Europe that provides a unique opportunity to assess European IRB policies and procedures [11].
This study aims to improve the efficiency of future research initiatives by quantifying the differences in IRB procedures through analyzing the procedural details, problems and challenges that researchers encountered in obtaining IRB approval for the general research protocol of the CENTER-TBI study.
Methods
Study setting
The Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI, www.center-tbi.eu) Core study is a prospective observational study on traumatic brain injury (TBI), which was conducted between December 2014 and December 2017 in 63 neurotrauma centers across Europe and Israel [11, 12]. The study included patients with TBI of all severities, and aims to improve characterization of TBI, in order to facilitate the development of precision medicine approaches and to identify best practices by using a comparative effectiveness research (CER) approach [11,12,13,14]. In the context of the project high-quality Personal Health related Data (PHD) were collected with repositories for neuro-imaging, DNA, and serum biomarkers. Prior to the study start and collection of clinical data, a uniform CENTER-TBI research protocol including all relevant documents was sent to all responsible IRBs to ensure its legal, ethical and statistical soundness and to obtain IRB approval.
A total of 68 centers from 19 countries initially submitted applications for IRB approval. Because this article focuses on IRB approval in Europe, two centers from Israel were excluded from our analysis. The 66 center that participated in this present study are from Austria (N = 2), Belgium (N = 5), Denmark (N = 2), Finland (N = 2), France (N = 7), Germany (N = 4), Hungary (N = 3), Italy (N = 8), Latvia (N = 3), Lithuania (N = 2), the Netherlands (N = 7), Norway (N = 3), Romania (N = 1), Serbia (N = 1), Spain (N = 4), Sweden (N = 2), Switzerland (N = 1), and the United Kingdom (UK), (N = 9). Sixty-one European centers were initiated and actively enrolled patients in the study.
Data collection and administration
All IRB submission documents, communication records and approval documents were collated per center by the Contract Research Organization, ICON plc (ICON), directly after final approval of IRBs [15]. ICON is a global company operating in the healthcare industry that was responsible for the clinical monitoring of CENTER-TBI data. The received IRB documents were obtained in 15 different languages (Danish, Dutch, English, Finnish, French, German, Hungarian, Italian, Latvian, Lithuanian, Norwegian, Romanian, Serbian, Spanish, and Swedish) and were partly translated before analysis. The authors contacted the principle investigators to obtain additional information to minimize the amount of unclear or missing data. Identifiable information was deleted to protect the privacy of stakeholders. This resulted in a final set of documents, that was analyzed for this study.
Analyses
We assessed the IRB review procedures by using the final set of documents and aimed to answer the following research questions in order to evaluate differences in obtaining IRB approval (1) Was the study considered to be observational or interventional? (2) Was the research protocol to be submitted to a central IRB or local IRB for primary IRB review and primary IRB approval? (3) Was additional IRB review required after primary IRB approval had already been obtained? If yes, to what extent? (4) How many review rounds were conducted before primary IRB approval was obtained? What were the reasons? (5) What was the time between protocol submission and obtaining the required IRB approval to start the study? The use of ‘primary’ in this context should be interpreted as first in an order and ‘additional’ as second in an order, without including a statement on importance.
To elaborate on the fifth question, we reconstructed six timeframes regarding the primary IRB review procedure: (1) time between protocol submission and primary IRB approval or first IRB reaction, (2) time between first IRB reaction and first reaction of researcher, (3) time between first reaction of researcher and primary IRB approval or second IRB reaction, (4) time between second IRB reaction and second reaction researcher, (5) time between second reaction researcher and primary IRB approval, and (6) total time between protocol submission and primary IRB approval. The existence of these timeframes naturally depended on the actual procedure. Data on any additional IRB review focused only on the duration of this particular review until the required IRB approval was obtained.
In order to assess regional variation, countries were grouped into six regions based on the United Nation geo-scheme: Baltic States (Latvia, and Lithuania), Eastern Europe (Hungary, Romania, and Serbia), Northern Europe (Denmark, Finland, Norway, and Sweden), Southern Europe (Italy, and Spain), the United Kingdom (UK), and Western Europe (Austria, Belgium, France, Germany, the Netherlands, Switzerland) [16]. Incomplete data was marked ‘Missing’ (M) and all timeframes were reported in days.
To determine significant differences between the time from submission till approval of the research protocol between primary local IRBs and primary central IRBs, we performed a Mann-Whitney U test (continuous). Analyses were performed using R version 3.6.0. Finally, a descriptive analysis of questions, comments and answers from both IRB and researcher during the IRB review procedure was performed to summarize the problems and challenges that researchers encountered in obtaining IRB approval. IRB reactions were categorized and reported by their appearance: (1) Procedure, (2) Blood collection and biomarkers, (3) MRI, (4) Privacy and data security, (5) Other.
Results
A total of 66 neurotrauma centers from 18 countries were included in this analysis. Most centers were located in Western Europe (N = 26, 39%) and least in Eastern Europe (N = 5, 8%) and the Baltic States (N = 5, 8%). Most participating centers were from the UK (N = 9), followed by Italy (N = 8), The Netherlands and France (N = 7) (Table 1). In all countries the local principal investigators were responsible to submit the general CENTER-TBI research protocol for IRB review and IRB approval.
Observational or interventional
The majority of countries (N = 14, 78%) considered the study to be observational, while others judged it to be observational with diagnostic interventions (The Netherlands), interventional (France, Hungary) and observational and interventional (Serbia) (Table 1).
Primary central or primary local IRB review
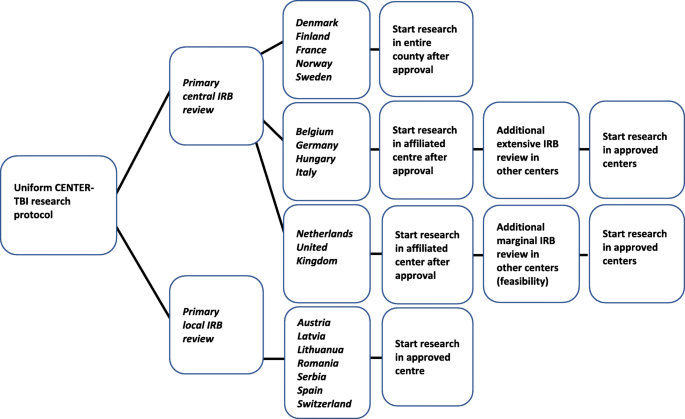
Primary IRB review started directly after protocol submission and was considered ‘central’ when submitted to a central institution or an institution that was part of a national network (N = 11, 61%). There were three options: (1) Primary central IRB approval had a national impact and applied to all participating centers within a country, without the need for additional IRB review (N = 5; Denmark, Finland, France, Norway, Sweden). (2) Primary central IRB approval only allowed study start in the research centers associated with the approving IRB. Other participating centers in the country required approval after an additional extensive local IRB review. This involved the re-evaluation of the entire protocol and applicable ethics (N = 4; Belgium, Germany, Hungary, Italy). (3) Primary central IRB approval only allowed study start in the research centers associated with the approving IRB. Other participating centers required additional approval after marginal local IRB review, mainly assessing local feasibility (N = 2; UK, The Netherlands) (Fig. 1).
Primary IRB review was considered ‘local’ when the protocol was submitted to an independent ‘local’ IRB. Obtained primary local IRB approvals only applied to the associated research centers and allowed study start without any additional requirements (N = 7; Austria, Switzerland, Spain, Lithuania, Latvia, Romania, Serbia). Primary local IRB review could be performed simultaneously in each independent IRB (Fig. 1).
For every protocol submission, there were two outcome options after IRB review: (1) the required (primary or additional) IRB approval had been obtained and the study could start, or (2) researchers were asked to answer questions or make protocol changes, which was followed by an extra IRB review round. This process varied between IRBs and was repeated until the required IRB approval was eventually obtained. None of the submissions in this study were rejected.
IRB review rounds
Eight countries (44%), including all countries from Eastern Europe and the Baltic State, obtained primary IRB approval in the first round after submission, while six countries (Austria, Belgium, France, Finland, Spain and UK) required one extra review round and four countries (Denmark, Germany, Norway and Sweden) required two extra review rounds (Fig. 2). Extra review rounds were found in 73% of centers after primary central IRB submission and in 20% after primary local IRB submission.
Detailed overview of primary IRB review rounds and duration. This figure provides a detailed overview of the number of primary local and central IRB review rounds and their duration in days. *The number of review rounds was only reported for the initial center of each country. **Information on the first review round was missing. ***Only the total number of days was available
Several IRBs commented on different aspects of the protocol: selection criteria (n = 3, 38%), patient/proxy consent (n = 4, 50%), and information forms (n = 3, 38%). Also, specific questions were asked on possible non-standard care factors in particular MRI scans (N = 4), blood sample collection (N = 4). Four questions were asked about privacy and data security, mainly related to the period after study completion. All relevant information can be found in the supplementary files.
Duration from protocol submission to IRB approval
The median time from protocol submission until the required IRB approval was obtained to start the study was 114 days (IQR 75–224). The fastest required IRB approval was obtained after one day in Serbia and Romania, whereas the longest time was found in a center in the UK (535 days). Obtaining central IRB approval (138 days, IQR: 91–229) took significantly longer (p = 0.0074) than obtaining local IRB approval (50 days, IQR: 29–102) (Table 2).
In Norway and Denmark, the majority of time from submission to primary central IRB approval was spent by researchers (67 and 69%, respectively), while in France (95%) and Hungary (71%) most time was consumed by IRBs. Regarding primary local IRB submissions, researchers only accounted for 12% of time in Spain and 21% in Austria (Fig. 2).
Additional IRB review rounds after primary central IRB review were required in 55% of countries. An additional marginal (feasibility) review had a median duration of 104 days (IQR: 62–224), whereas an additional extensive IRB review took 189 days (IQR: 140–270) (Table 3).
Variation between centers within countries was least in Lithuania (31 to 47 days), Germany (288 to 312 days), Belgium (131 to 155 days), and Hungary (177 to 204 days), compared to Spain (69 to 349 days), the Netherlands (27 to 224 days), the UK (58 to 535 days), and Italy (65 to 288 days) (Table 3).
Discussion
This study shows variation in IRB procedures between and within European countries, indicating a lack of uniform legislation and regulation, or inconsistencies in how such legislation or regulation were implemented. In some countries, a primary central IRB approval was sufficient for study initiation, while others required an additional IRB review at the participating site. Also, the number of review rounds, duration until IRB approval, and the nature of questions and comments from the IRBs varied. Not all IRBs considered the study to be observational, demonstrating a different way of understanding the study. The apparent lack of integration and harmonization in this context suggests that the efficiency of European research collaborations could benefit from improving knowledge on the existing variation in procedures, inefficiencies and differences in value systems between and within countries.
The duration from protocol submission to required IRB approval was highly variable and ranged from one day up to nearly one year. In literature, differences between IRB procedures were also reported and IRB review durations varied from weeks to several months [6, 17]. The difference in total duration between primary central and primary local IRB approval could respectively be overestimated and underestimated by the short primary IRB review times in Serbia and Romania and the missing data of the first review round for the UK. The difference is not necessarily related to the number of review rounds, but might be more explained by the reason and nature (primary central/local review or extensive/marginal additional local review) of the extra review round(s), the accompanying amount of work and the working speed of both IRB and research team. The influence of the latter was substantiated by our data as responding to questions from the IRB seemed to account for an important part of time in several countries (e.g. Denmark and Norway), while the majority of time in other countries (e.g. Belgium, Spain and France) was accounted for by the time taken in primary evaluation by IRBs. The exact reasons for these ‘delays’ could however not be derived from our data and deserves further study. They might be caused by the difficulty of requirements or questions, although, according to the communication records, IRBs mainly requested extra explanation of research procedures. Based on the IRB information requests in this study, special attention should be given to the description of inclusion criteria, informed consent procedures, patient information forms, non-standard care procedures, privacy and data security. A quick response by investigators and agreeing on a maximal turnover time of 1 month to 2 months for IRBs could already minimize substantial delay. This is also in correspondence with literature, where IRB turnover time targets range from 30 to 60 days [17, 18].
The question whether CENTER-TBI was an observational or an interventional study did not appear to be a clear explanation for differences in number and duration of review rounds. Interventional studies are generally subject to a more extensive review process, where observational study reviews may be more marginal. Nonetheless, duration was short in France and long in the UK. CENTER-TBI is registered as an observational study, in which ‘the investigator is not acting upon study participants, but instead observing natural relationships between factors and outcomes’ [19]. Two IRBs considered the study to be purely interventional. Interventional studies are studies ‘where the researcher intercedes as part of the study design’ [19]. An explanation for this opposing classification is that the IRBs did and did not consider the following procedures to be standard-of-care: (1) Different amounts of additional blood draws at presentation and follow-up. (2) Neuropsychological assessments and outcome questionnaires up to a 24-month follow-up. (3) Additional MRIs at sites participating in the MRI sub-study.
Extra work without clear benefits delays projects and should be avoided when possible. An additional IRB review after primary central IRB approval is usually double work and could result in an extra delay of weeks to more than a year, without always having clear benefits over the already obtained primary approval [17]. Cancelling potentially unnecessary (extensive) additional IRB review procedures could not only reduce turnover time, but also reduce costs. The exact costs of European IRB review procedures are unfortunately unknown, but the direct costs of an IRB review and approval in the US have been calculated to be $107.544 ($82.610 in IRB fees and $24.934 in labor) [20].
Delays in obtaining IRB approval not only adversely affect study initiation, but are also associated with several other risks. Long procedures with many feedback rounds will delay study start, frustrate researchers and might even endanger meeting subsidiary demands. Researchers might attempt to speed up the process by changing the protocol or submitting the protocol to IRBs that are considered to be less strict but able to process the submission the quickest. This does not necessarily serve primary research objectives and might even hamper quality and generalizability of study results.
Optimization of IRB review procedures is urgently needed as multinational collaborations in healthcare research are increasing and even promoted by multiple European research grant [4, 5, 21]. Harmonization and adequate implementation of regulatory and ethical standards between European countries could improve the present situation [7, 22]. The EU already aims to freely cooperate across borders by defining common standards and removing legal obstacles, but true harmonization of Member State laws in a research context has clearly not been established yet [21,22,23,24]. For example, the General Data Protection Regulation (GDPR) aimed to ensure a fair and transparent processing of personal data and aimed to improve patients’ control over their own data [25]. The implementation and use of the GDPR however showed the difficulty of harmonization in the protection of the EU citizens in this context. This was especially caused by the possibility for European countries to use their own national legislation in addition to the GDPR, which does not improve the desired harmonization.
Harmonization remains a highly complex process due to variation of national regulations that are based on national customs, culture, ethics, religion and other beliefs [6]. Harmonization of laws is designed to incorporate different legal systems under a basic framework. To overcome the highly complex process of harmonization in the area of research, it has been suggested to combine similarities between legislations and regulations of countries under a basic framework like a European research directive. A framework should acknowledge these local cultural or religious beliefs, as disregarding them is neither feasible nor desirable. While the desirable goal of harmonizing regulation will certainly benefit research in the future, both IRBs and researchers will have to put in efforts until that time. IRBs can accelerate the turnover by only requiring central IRB approval and researchers should respond quicker and more comprehensively to questions from IRBs, preventing the repetition of questions.
Strengths and limitations
The CENTER-TBI study provides a unique opportunity to provide comprehensive insight in the procedural differences between European IRBs. The study benefits from its large size and because the data acquisition process increased the quality and completeness of documents. Despite the quality of the documents, results were still dependent on the recorded information. Therefore, we could not always identify causal factors for variation, which is something to look for in future initiatives. The data on IRB review procedures in an observational study conducted with mentally incapacitated patients in neurotrauma centers might not be generalizable for other research settings.
Conclusions
This study shows variation between IRB procedures across Europe, which pose major challenges to large European research collaborations. Differences are likely caused by the lack of harmonization, integration and implementation of national legislations and regulations. To optimize efficiency for multinational European studies in context of obtaining IRB approval, the encountered differences and inefficiencies should be studied further and policymakers should evaluate the opportunities to optimize regulatory harmonization, while acknowledging the boundaries of national sovereignty and local cultural preferences.
Availability of data and materials
There are legal constraints that prohibit us from making all data publicly available. Data could be identifiable because the limited number of centres per country that were included in this study. Readers may contact Dr. Erwin J. O. Kompanje (erwinkompanje@me.com) for reasonable requests for the data.
Abbreviations
- EU:
-
European Union
- CENTER-TBI:
-
Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury
- CER:
-
Comparative Effectiveness Research
- GDPR:
-
General Data Protection Regulation
- ICON:
-
ICON plc
- IRB:
-
Institutional Review Board
- M:
-
Missing
- PHD:
-
Personal Health related Data
- TBI:
-
Traumatic Brain Injury
- UK:
-
United Kingdom
References
Steering Commitee on Bioethics, Council of Europe. Guide for Research Ethics Committee Members, revised version 3 December 2010. https://www.coe.int/t/dg3/healthbioethic/activities/02_biomedical_research_en/Guide/Guide_EN.pdf. Accessed 3 Sept 2019.
Emanuel E, Crouch R, Lie R, et al. The Oxford textbook of clinical research ethics. Oxford: Oxford University Press, Reprint edition; 2011.
World Health Organization. Standards and operational guidance for ethics review of health-related research with human participants. 2011. https://apps.who.int/iris/bitstream/handle/10665/44783/9789241502948_eng.pdf;jsessionid=0CC3C3EA5BABF39889211B2E3B4AA76B?sequence=1. Accessed 3 Sept 2019.
Innovative Medicine Innitiative, IMI mission and objectives. https://www.imi.europa.eu/about-imi/mission-objectives. Accessed August 3, 2019.
European Commission, Horizon 2020 - The Framework Programme for Research and Innovation. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52011DC0808&from=EN. Published 2011. Accessed September 3, 2019.
Tridente A, Holloway P, Hutton P, et al. Methodological challenges in European ethics approvals for a genetic epidemiology study in critically ill patients: the GenOSept experience. BMC Med Ethics. 2019;20:30.
Urushihara H, Parmenter L, Tashiro S, et al. Bridge the gap: the need for harmonized regulatory and ethical standards for postmarketing observational studies. Pharmacoepidemiol Drug Saf. 2017;26:1299–306.
Aledort L. Harmonization of clinical trial guidelines for assessing the risk of inhibitor development in hemophilia a treatment. J Thromb Haemost. 2011;9(3):423–7.
Oliver DJ. Harmonisation of research outcomes for meaningful translation to practice: the role of Core outcome sets and the CROWN initiative. Aust N Z J Obs Gynaecol. 2018;58:15–6.
Bowles K, Potashnik S, Ratcliffe S, et al. Conducting research using the electronic health record across multi-hospital systems: semantic harmonization implications for administrators. J Nurs Adm. 2013;43:355–60.
Maas A, Menon D, Steyerberg E, et al. Collaborative European NeuroTrauma effectiveness research in traumatic brain injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery. 2015;76:67–80.
Steyerberg E, Wiegers E, Sewalt C, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18:923–34.
Maas A, Menon D, Adelson P, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048.
Cnossen M, Polinder S, Lingsma H, et al. Variation in structure and process of care in traumatic brain injury: provider profiles of European Neurotrauma centers participating in the CENTER-TBI study. PLoS One. 2016;11(8):e0161367.
ICON plc. http://www.iconplc.com. Accessed August 8, 2019.
United Nations, Standard country or area codes for statistical use (M49). https://unstats.un.org/unsd/methodology/m49/. Published 1999. Accessed September 5, 2019.
Mascette A, Bernard G, Dimichele D, et al. Are central institutional review boards the solution? The National Heart, Lung, and Blood Institute working Group’s report on optimizing the IRB process. Acad Med. 2012;87:1710–4.
Adams P, Kaewkungwal J, Limphattharacharoen C, et al. Is your ethics committee efficient? Using “IRB Metrics” as a self-assessment tool for continuous improvement at the Faculty of Tropical Medicine, Mahidol University, Thailand. PLoS ONE. 2014;9(11):e113356.
Thiese M. Observational and interventional study design types; an overview. Biochem Med. 2014;24(2):199–210.
Ravina B, Deuel L, Siderowf A, et al. Local institutional review board (IRB) review of a multicenter trial: local costs without local context. Ann Neurol. 2010;67(2):258–60.
European Union. Consolidated version of the treaty on the functioning of the european union. Official Journal of the European Union C 326/47. 2012. https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=OJ%3AC%3A2012%3A326%3ATOC. Accessed 9 Sept 2019.
Henshall C, Mardhani-Bayne L, Fronsdal KB, et al. Interactions between health technology assessment, coverage, and regulatory processes: emerging issues, goals, and opportunities. Int J Technol Assess Heal Care. 2011;27(3):253–60.
European Union. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General data Protection Regulation). Official Journal of the European Union L 119/1, version 4 May 2016. http://data.europa.eu/eli/reg/2016/679/oj. Accessed 9 Sept 2019.
European Commission. The European Union. What it is and what it does. Luxembourg: Publications Office of the European Union 2018. doi:https://doi.org/10.2775/665897.
Timmers M, Van Veen E-B, Maas A, et al. Will the Eu data protection regulation 2016/679 inhibit critical care research? Med Law Rev. 2019;27:59–78.
Acknowledgements
Collaborating authors:
The CENTER-TBI participants and investigators:
Cecilia Åkerlund1, Krisztina Amrein2, Nada Andelic3, Lasse Andreassen4, Audny Anke5, Anna Antoni6, Gérard Audibert7, Philippe Azouvi8, Maria Luisa Azzolini9, Ronald Bartels10, Pál Barzó11, Romuald Beauvais12, Ronny Beer13, Bo-Michael Bellander14, Antonio Belli15, Habib Benali16, Maurizio Berardino17, Luigi Beretta9, Morten Blaabjerg18, Peter Bragge19, Alexandra Brazinova20, Vibeke Brinck21, Joanne Brooker22, Camilla Brorsson23, Andras Buki24, Monika Bullinger25, Manuel Cabeleira26, Alessio Caccioppola27, Emiliana Calappi 27, Maria Rosa Calvi9, Peter Cameron28, Guillermo Carbayo Lozano29, Marco Carbonara27, Simona Cavallo17, Giorgio Chevallard30, Arturo Chieregato30, Giuseppe Citerio31, 32, Iris Ceyisakar33, Mark Coburn34, Jonathan Coles35, Jamie D. Cooper36, Marta Correia37, Amra Čović 38, Nicola Curry39, Endre Czeiter24, Marek Czosnyka26, Claire Dahyot-Fizelier40, Paul Dark41, Helen Dawes42, Véronique De Keyser43, Vincent Degos16, Francesco Della Corte44, Hugo den Boogert10, Bart Depreitere45, Đula Đilvesi 46, Abhishek Dixit47, Emma Donoghue22, Jens Dreier48, Guy-Loup Dulière49, Ari Ercole47, Patrick Esser42, Erzsébet Ezer50, Martin Fabricius51, Valery L. Feigin52, Kelly Foks53, Shirin Frisvold54, Alex Furmanov55, Pablo Gagliardo56, Damien Galanaud16, Dashiell Gantner28, Guoyi Gao57, Pradeep George58, Alexandre Ghuysen59, Lelde Giga60, Ben Glocker61, Jagoš Golubovic46, Pedro A. Gomez 62, Johannes Gratz63, Benjamin Gravesteijn33, Francesca Grossi44, Russell L. Gruen64, Deepak Gupta65, Juanita A. Haagsma33, Iain Haitsma66, Raimund Helbok13, Eirik Helseth67, Lindsay Horton 68, Jilske Huijben33, Peter J. Hutchinson69, Bram Jacobs70, Stefan Jankowski71, Mike Jarrett21, Ji-yao Jiang57, Faye Johnson72, Kelly Jones52, Mladen Karan46, Angelos G. Kolias69, Erwin Kompanje73, Daniel Kondziella51, Evgenios Koraropoulos47, Lars-Owe Koskinen74, Noémi Kovács75, Ana Kowark34, Alfonso Lagares62, Linda Lanyon58, Steven Laureys76, Fiona Lecky77, 78, Didier Ledoux76, Rolf Lefering79, Valerie Legrand80, Aurelie Lejeune81, Leon Levi82, Roger Lightfoot83, Hester Lingsma33, Andrew I.R. Maas43, Ana M. Castaño-León62, Marc Maegele84, Marek Majdan20, Alex Manara85, Geoffrey Manley86, Costanza Martino87, Hugues Maréchal49, Julia Mattern88, Catherine McMahon89, Béla Melegh90, David Menon47, Tomas Menovsky43, Benoit Misset76, Davide Mulazzi27, Visakh Muraleedharan58, Lynnette Murray28, Ancuta Negru91, David Nelson1, Virginia Newcombe47, Daan Nieboer33, József Nyirádi2, Otesile Olubukola77, Matej Oresic92, Fabrizio Ortolano27, Aarno Palotie93, 94, 95, Paul M. Parizel96, Jean-François Payen97, Natascha Perera12, Vincent Perlbarg16, Paolo Persona98, Wilco Peul99, Anna Piippo-Karjalainen100, Matti Pirinen93, Horia Ples91, Suzanne Polinder33, Inigo Pomposo29, Jussi P. Posti 101, Louis Puybasset102, Andreea Radoi 103, Arminas Ragauskas104, Rahul Raj100, Malinka Rambadagalla105, Jonathan Rhodes106, Sylvia Richardson107, Sophie Richter47, Samuli Ripatti93, Saulius Rocka104, Cecilie Roe108, Olav Roise109,110, Jonathan Rosand111, Jeffrey V. Rosenfeld112, Christina Rosenlund113, Guy Rosenthal55, Rolf Rossaint34, Sandra Rossi98, Daniel Rueckert61, Martin Rusnák114, Juan Sahuquillo103, Oliver Sakowitz88, 115, Renan Sanchez-Porras115, Janos Sandor116, Nadine Schäfer79, Silke Schmidt117, Herbert Schoechl118, Guus Schoonman119, Rico Frederik Schou120, Elisabeth Schwendenwein6, Charlie Sewalt33, Toril Skandsen121, 122, Peter Smielewski26, Abayomi Sorinola123, Emmanuel Stamatakis47, Simon Stanworth39, Robert Stevens124, William Stewart125, Ewout W. Steyerberg33, 126, Nino Stocchetti127, Nina Sundström128, Anneliese Synnot22, 129, Riikka Takala130, Viktória Tamás123, Tomas Tamosuitis131, Mark Steven Taylor20, Braden Te Ao52, Olli Tenovuo101, Alice Theadom52, Matt Thomas85, Dick Tibboel132, Marjolein Timmers73, Christos Tolias133, Tony Trapani28, Cristina Maria Tudora91, Peter Vajkoczy 134, Shirley Vallance28, Egils Valeinis60, Zoltán Vámos50, Gregory Van der Steen43, Joukje van der Naalt70, Jeroen T.J.M. van Dijck 99, Thomas A. van Essen99, Wim Van Hecke135, Caroline van Heugten136, Dominique Van Praag137, Thijs Vande Vyvere135, Roel P. J. van Wijk99, Alessia Vargiolu32, Emmanuel Vega81, Kimberley Velt33, Jan Verheyden135, Paul M. Vespa138, Anne Vik120, 139, Rimantas Vilcinis131, Victor Volovici66, Nicole von Steinbüchel38, Daphne Voormolen33, Petar Vulekovic46, Kevin K.W. Wang140, Eveline Wiegers33, Guy Williams47, Lindsay Wilson68, Stefan Winzeck47, Stefan Wolf141, Zhihui Yang140, Peter Ylén142, Alexander Younsi88, Frederick A. Zeiler47,143, Veronika Zelinkova20, Agate Ziverte60, Tommaso Zoerle27.
1 Department of Physiology and Pharmacology, Section of Perioperative Medicine and Intensive Care, Karolinska Institutet, Stockholm, Sweden.
2 János Szentágothai Research Centre, University of Pécs, Pécs, Hungary.
3 Division of Surgery and Clinical Neuroscience, Department of Physical Medicine and Rehabilitation, Oslo University Hospital and University of Oslo, Oslo, Norway.
4 Department of Neurosurgery, University Hospital Northern Norway, Tromso, Norway.
5 Department of Physical Medicine and Rehabilitation, University Hospital Northern Norway, Tromso, Norway.
6 Trauma Surgery, Medical University Vienna, Vienna, Austria.
7 Department of Anesthesiology & Intensive Care, University Hospital Nancy, Nancy, France.
8 Raymond Poincare hospital, Assistance Publique – Hopitaux de Paris, Paris, France.
9 Department of Anesthesiology & Intensive Care, S Raffaele University Hospital, Milan, Italy.
10 Department of Neurosurgery, Radboud University Medical Center, Nijmegen, The Netherlands.
11 Department of Neurosurgery, University of Szeged, Szeged, Hungary.
12 International Projects Management, ARTTIC, Munchen, Germany.
13 Department of Neurology, Neurological Intensive Care Unit, Medical University of Innsbruck, Innsbruck, Austria.
14 Department of Neurosurgery & Anesthesia & intensive care medicine, Karolinska University Hospital, Stockholm, Sweden.
15 NIHR Surgical Reconstruction and Microbiology Research Centre, Birmingham, UK.
16 Anesthesie-Réanimation, Assistance Publique – Hopitaux de Paris, Paris, France.
17 Department of Anesthesia & ICU, AOU Città della Salute e della Scienza di Torino - Orthopedic and Trauma Center, Torino, Italy.
18 Department of Neurology, Odense University Hospital, Odense, Denmark.
19 BehaviourWorks Australia, Monash Sustainability Institute, Monash University, Victoria, Australia.
20 Department of Public Health, Faculty of Health Sciences and Social Work, Trnava University, Trnava, Slovakia.
21 Quesgen Systems Inc., Burlingame, California, USA.
22 Australian & New Zealand Intensive Care Research Centre, Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia.
23 Department of Surgery and Perioperative Science, Umeå University, Umeå, Sweden.
24 Department of Neurosurgery, Medical School, University of Pécs, Hungary and Neurotrauma Research Group, János Szentágothai Research Centre, University of Pécs, Hungary.
25 Department of Medical Psychology, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany.
26 Brain Physics Lab, Division of Neurosurgery, Dept of Clinical Neurosciences, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
27 Neuro ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy.
28 ANZIC Research Centre, Monash University, Department of Epidemiology and Preventive Medicine, Melbourne, Victoria, Australia.
29 Department of Neurosurgery, Hospital of Cruces, Bilbao, Spain.
30 NeuroIntensive Care, Niguarda Hospital, Milan, Italy.
31 School of Medicine and Surgery, Università Milano Bicocca, Milano, Italy.
32 NeuroIntensive Care, ASST di Monza, Monza, Italy.
33 Department of Public Health, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands.
34 Department of Anaesthesiology, University Hospital of Aachen, Aachen, Germany.
35 Department of Anesthesia & Neurointensive Care, Cambridge University Hospital NHS Foundation Trust, Cambridge, UK.
36 School of Public Health & PM, Monash University and The Alfred Hospital, Melbourne, Victoria, Australia.
37 Radiology/MRI department, MRC Cognition and Brain Sciences Unit, Cambridge, UK.
38 Institute of Medical Psychology and Medical Sociology, Universitätsmedizin Göttingen, Göttingen, Germany.
39 Oxford University Hospitals NHS Trust, Oxford, UK.
40 Intensive Care Unit, CHU Poitiers, Potiers, France.
41 University of Manchester NIHR Biomedical Research Centre, Critical Care Directorate, Salford Royal Hospital NHS Foundation Trust, Salford, UK.
42 Movement Science Group, Faculty of Health and Life Sciences, Oxford Brookes University, Oxford, UK.
43 Department of Neurosurgery, Antwerp University Hospital and University of Antwerp, Edegem, Belgium.
44 Department of Anesthesia & Intensive Care, Maggiore Della Carità Hospital, Novara, Italy.
45 Department of Neurosurgery, University Hospitals Leuven, Leuven, Belgium.
46 Department of Neurosurgery, Clinical centre of Vojvodina, Faculty of Medicine, University of Novi Sad, Novi Sad, Serbia.
47 Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK.
48 Center for Stroke Research Berlin, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
49 Intensive Care Unit, CHR Citadelle, Liège, Belgium.
50 Department of Anaesthesiology and Intensive Therapy, University of Pécs, Pécs, Hungary.
51 Departments of Neurology, Clinical Neurophysiology and Neuroanesthesiology, Region Hovedstaden Rigshospitalet, Copenhagen, Denmark.
52 National Institute for Stroke and Applied Neurosciences, Faculty of Health and Environmental Studies, Auckland University of Technology, Auckland, New Zealand.
53 Department of Neurology, Erasmus MC, Rotterdam, the Netherlands.
54 Department of Anesthesiology and Intensive care, University Hospital Northern Norway, Tromso, Norway.
55 Department of Neurosurgery, Hadassah-hebrew University Medical center, Jerusalem, Israel.
56 Fundación Instituto Valenciano de Neurorrehabilitación (FIVAN), Valencia, Spain.
57 Department of Neurosurgery, Shanghai Renji hospital, Shanghai Jiaotong University/school of medicine, Shanghai, China.
58 Karolinska Institutet, INCF International Neuroinformatics Coordinating Facility, Stockholm, Sweden.
59 Emergency Department, CHU, Liège, Belgium.
60 Neurosurgery clinic, Pauls Stradins Clinical University Hospital, Riga, Latvia.
61 Department of Computing, Imperial College London, London, UK.
62 Department of Neurosurgery, Hospital Universitario 12 de Octubre, Madrid, Spain.
63 Department of Anesthesia, Critical Care and Pain Medicine, Medical University of Vienna, Austria.
64 College of Health and Medicine, Australian National University, Canberra, Australia.
65 Department of Neurosurgery, Neurosciences Centre & JPN Apex trauma centre, All India Institute of Medical Sciences, New Delhi-110029, India.
66 Department of Neurosurgery, Erasmus MC, Rotterdam, the Netherlands.
67 Department of Neurosurgery, Oslo University Hospital, Oslo, Norway.
68 Division of Psychology, University of Stirling, Stirling, UK.
69 Division of Neurosurgery, Department of Clinical Neurosciences, Addenbrooke’s Hospital & University of Cambridge, Cambridge, UK.
70 Department of Neurology, University of Groningen, University Medical Center Groningen, Groningen, Netherlands.
71 Neurointensive Care, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK.
72 Salford Royal Hospital NHS Foundation Trust Acute Research Delivery Team, Salford, UK.
73 Department of Intensive Care and Department of Ethics and Philosophy of Medicine, Erasmus Medical Center, Rotterdam, The Netherlands.
74 Department of Clinical Neuroscience, Neurosurgery, Umeå University, Umeå, Sweden.
75 Hungarian Brain Research Program - Grant No. KTIA_13_NAP-A-II/8, University of Pécs, Pécs, Hungary.
76 Cyclotron Research Center, University of Liège, Liège, Belgium.
77 Centre for Urgent and Emergency Care Research (CURE), Health Services Research Section, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK.
78 Emergency Department, Salford Royal Hospital, Salford UK.
79 Institute of Research in Operative Medicine (IFOM), Witten/Herdecke University, Cologne, Germany.
80 VP Global Project Management CNS, ICON, Paris, France.
81 Department of Anesthesiology-Intensive Care, Lille University Hospital, Lille, France.
82 Department of Neurosurgery, Rambam Medical Center, Haifa, Israel.
83 Department of Anesthesiology & Intensive Care, University Hospitals Southhampton NHS Trust, Southhampton, UK.
84 Cologne-Merheim Medical Center (CMMC), Department of Traumatology, Orthopedic Surgery and Sportmedicine, Witten/Herdecke University, Cologne, Germany.
85 Intensive Care Unit, Southmead Hospital, Bristol, Bristol, UK.
86 Department of Neurological Surgery, University of California, San Francisco, California, USA.
87 Department of Anesthesia & Intensive Care,M. Bufalini Hospital, Cesena, Italy.
88 Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany.
89 Department of Neurosurgery, The Walton centre NHS Foundation Trust, Liverpool, UK.
90 Department of Medical Genetics, University of Pécs, Pécs, Hungary.
91 Department of Neurosurgery, Emergency County Hospital Timisoara, Timisoara, Romania.
92 School of Medical Sciences, Örebro University, Örebro, Sweden.
93 Institute for Molecular Medicine Finland, University of Helsinki, Helsinki, Finland.
94 Analytic and Translational Genetics Unit, Department of Medicine; Psychiatric & Neurodevelopmental Genetics Unit, Department of Psychiatry; Department of Neurology, Massachusetts General Hospital, Boston, MA, USA.
95 Program in Medical and Population Genetics; The Stanley Center for Psychiatric Research, The Broad Institute of MIT and Harvard, Cambridge, MA, USA.
96 Department of Radiology, University of Antwerp, Edegem, Belgium.
97 Department of Anesthesiology & Intensive Care, University Hospital of Grenoble, Grenoble, France.
98 Department of Anesthesia & Intensive Care, Azienda Ospedaliera Università di Padova, Padova, Italy.
99 Dept. of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands and Dept. of Neurosurgery, Medical Center Haaglanden, The Hague, The Netherlands.
100 Department of Neurosurgery, Helsinki University Central Hospital.
101 Division of Clinical Neurosciences, Department of Neurosurgery and Turku Brain Injury Centre, Turku University Hospital and University of Turku, Turku, Finland.
102 Department of Anesthesiology and Critical Care, Pitié -Salpêtrière Teaching Hospital, Assistance Publique, Hôpitaux de Paris and University Pierre et Marie Curie, Paris, France.
103 Neurotraumatology and Neurosurgery Research Unit (UNINN), Vall d’Hebron Research Institute, Barcelona, Spain.
104 Department of Neurosurgery, Kaunas University of technology and Vilnius University, Vilnius, Lithuania.
105 Department of Neurosurgery, Rezekne Hospital, Latvia.
106 Department of Anaesthesia, Critical Care & Pain Medicine NHS Lothian & University of Edinburg, Edinburgh, UK.
107 Director, MRC Biostatistics Unit, Cambridge Institute of Public Health, Cambridge, UK.
108 Department of Physical Medicine and Rehabilitation, Oslo University Hospital/University of Oslo, Oslo, Norway.
109 Division of Orthopedics, Oslo University Hospital, Oslo, Norway.
110 Institue of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway.
111 Broad Institute, Cambridge MA Harvard Medical School, Boston MA, Massachusetts General Hospital, Boston MA, USA.
112 National Trauma Research Institute, The Alfred Hospital, Monash University, Melbourne, Victoria, Australia.
113 Department of Neurosurgery, Odense University Hospital, Odense, Denmark.
114 International Neurotrauma Research Organisation, Vienna, Austria.
115 Klinik für Neurochirurgie, Klinikum Ludwigsburg, Ludwigsburg, Germany.
116 Division of Biostatistics and Epidemiology, Department of Preventive Medicine, University of Debrecen, Debrecen, Hungary.
117 Department Health and Prevention, University Greifswald, Greifswald, Germany.
118 Department of Anaesthesiology and Intensive Care, AUVA Trauma Hospital, Salzburg, Austria.
119 Department of Neurology, Elisabeth-TweeSteden Ziekenhuis, Tilburg, the Netherlands.
120 Department of Neuroanesthesia and Neurointensive Care, Odense University Hospital, Odense, Denmark.
121 Department of Neuromedicine and Movement Science, Norwegian University of Science and Technology, NTNU, Trondheim, Norway.
122 Department of Physical Medicine and Rehabilitation, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway.
123 Department of Neurosurgery, University of Pécs, Pécs, Hungary.
124 Division of Neuroscience Critical Care, John Hopkins University School of Medicine, Baltimore, USA.
125 Department of Neuropathology, Queen Elizabeth University Hospital and University of Glasgow, Glasgow, UK.
126 Dept. of Department of Biomedical Data Sciences, Leiden University Medical Center, Leiden, The Netherlands.
127 Department of Pathophysiology and Transplantation, Milan University, and Neuroscience ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano, Italy.
128 Department of Radiation Sciences, Biomedical Engineering, Umeå University, Umeå, Sweden.
129 Cochrane Consumers and Communication Review Group, Centre for Health Communication and Participation, School of Psychology and Public Health, La Trobe University, Melbourne, Australia.
130 Perioperative Services, Intensive Care Medicine and Pain Management, Turku University Hospital and University of Turku, Turku, Finland.
131 Department of Neurosurgery, Kaunas University of Health Sciences, Kaunas, Lithuania.
132 Intensive Care and Department of Pediatric Surgery, Erasmus Medical Center, Sophia Children’s Hospital, Rotterdam, The Netherlands.
133 Department of Neurosurgery, Kings college London, London, UK.
134 Neurologie, Neurochirurgie und Psychiatrie, Charité – Universitätsmedizin Berlin, Berlin, Germany.
135 icoMetrix NV, Leuven, Belgium.
136 Movement Science Group, Faculty of Health and Life Sciences, Oxford Brookes University, Oxford, UK.
137 Psychology Department, Antwerp University Hospital, Edegem, Belgium.
138 Director of Neurocritical Care, University of California, Los Angeles, USA.
139 Department of Neurosurgery, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway.
140 Department of Emergency Medicine, University of Florida, Gainesville, Florida, USA.
141 Department of Neurosurgery, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
142 VTT Technical Research Centre, Tampere, Finland.
143 Section of Neurosurgery, Department of Surgery, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada.
Åkerlund | Cecilia | |
|---|---|---|
Amrein | Krisztina | |
Andelic | Nada | |
Andreassen | Lasse | |
Anke | Audny | |
Antoni | Anna | |
Audibert | Gérard | |
Azouvi | Philippe | |
Azzolini | Maria Luisa | |
Bartels | Ronald | |
Barzó | Pál | |
Beauvais | Romuald | |
Beer | Ronny | |
Bellander | Bo-Michael | |
Belli | Antonio | |
Benali | Habib | |
Berardino | Maurizio | |
Beretta | Luigi | |
Blaabjerg | Morten | |
Bragge | Peter | |
Brazinova | Alexandra | |
Brinck | Vibeke | |
Brooker | Joanne | |
Brorsson | Camilla | |
Buki | Andras | |
Bullinger | Monika | |
Cabeleira | Manuel | |
Caccioppola | Alessio | |
Calappi | Emiliana | |
Calvi | Maria Rosa | |
Cameron | Peter | |
Carbayo Lozano | Guillermo | |
Carbonara | Marco | |
Castaño-León | Ana M. | |
Cavallo | Simona | |
Chevallard | Giorgio | |
Chieregato | Arturo | |
Citerio | Giuseppe | |
Ceyisakar | Iris | |
Coburn | Mark Steven | |
Coles | Jonathan | |
Cooper | Jamie D. | |
Correia | Marta | |
Čović | Amra | |
Curry | Nicola | |
Czeiter | Endre | |
Czosnyka | Marek | |
Dahyot-Fizelier | Claire | |
Dark | Paul | |
Dawes | Helen | |
De Keyser | Véronique | |
Degos | Vincent | |
Della Corte | Francesco | |
den Boogert | Hugo | |
Depreitere | Bart | |
Đilvesi | Đula | |
Dixit | Abhishek | |
Donoghue | Emma | |
Dreier | Jens | |
Dulière | Guy-Loup | |
Ercole | Ari | |
Esser | Patrick | |
Ezer | Erzsébet | |
Fabricius | Martin | |
Feigin | Valery L. | |
Foks | Kelly | |
Frisvold | Shirin | |
Furmanov | Alex | |
Gagliardo | Pablo | |
Galanaud | Damien | |
Gantner | Dashiell | |
Gao | Guoyi | |
George | Pradeep | |
Ghuysen | Alexandre | |
Giga | Lelde | |
Glocker | Ben | |
Golubović | Jagoš | |
Gomez | Pedro A. | |
Gratz | Johannes | |
Gravesteijn | Benjamin | |
Grossi | Francesca | |
Gruen | Russell L. | |
Gupta | Deepak | |
Haagsma | Juanita A. | |
Haitsma | Iain | |
Helbok | Raimund | |
Helseth | Eirik | |
Horton | Lindsay | |
Huijben | Jilske | |
Hutchinson | Peter J. | |
Jacobs | Bram | |
Jankowski | Stefan | |
Jarrett | Mike | |
Jiang | Ji-yao | |
Johnson | Faye | |
Jones | Kelly | |
Karan | Mladen | |
Kolias | Angelos G. | |
Kompanje | Erwin | |
Kondziella | Daniel | |
Koraropoulos | Evgenios | |
Koskinen | Lars-Owe | |
Kovács | Noémi | |
Lagares | Alfonso | |
Lanyon | Linda | |
Laureys | Steven | |
Lecky | Fiona | |
Ledoux | Didier | |
Lefering | Rolf | |
Legrand | Valerie | |
Lejeune | Aurelie | |
Levi | Leon | |
Lightfoot | Roger | |
Lingsma | Hester | |
Maas | Andrew I.R. | |
Maegele | Marc | |
Majdan | Marek | |
Manara | Alex | |
Manley | Geoffrey | |
Maréchal | Hugues | |
Martino | Costanza | |
Mattern | Julia | |
McMahon | Catherine | |
Melegh | Béla | |
Menon | David | |
Menovsky | Tomas | |
Misset | Benoit | |
Mulazzi | Davide | |
Muraleedharan | Visakh | |
Murray | Lynnette | |
Nair | Nandesh | |
Negru | Ancuta | |
Nelson | David | |
Newcombe | Virginia | |
Nieboer | Daan | |
Nyirádi | József | |
Oresic | Matej | |
Ortolano | Fabrizio | |
Otesile | Olubukola | |
Palotie | Aarno | |
Parizel | Paul M. | |
Payen | Jean-François | |
Perera | Natascha | |
Perlbarg | Vincent | |
Persona | Paolo | |
Peul | Wilco | |
Piippo-Karjalainen | Anna | |
Pirinen | Matti | |
Ples | Horia | |
Polinder | Suzanne | |
Pomposo | Inigo | |
Posti | Jussi P. | |
Puybasset | Louis | |
Rădoi | Andreea | |
Ragauskas | Arminas | |
Raj | Rahul | |
Rambadagalla | Malinka | |
Rehorčíková | Veronika | |
Rhodes | Jonathan | |
Richardson | Sylvia | |
Richter | Sophie | |
Ripatti | Samuli | |
Rocka | Saulius | |
Roe | Cecilie | |
Roise | Olav | |
Rosand | Jonathan | |
Rosenfeld | Jeffrey | |
Rosenlund | Christina | |
Rosenthal | Guy | |
Rossaint | Rolf | |
Rossi | Sandra | |
Rueckert | Daniel | |
Rusnák | Martin | |
Sahuquillo | Juan | |
Sakowitz | Oliver | |
Sanchez-Porras | Renan | |
Sandor | Janos | |
Schäfer | Nadine | |
Schmidt | Silke | |
Schoechl | Herbert | |
Schoonman | Guus | |
Schou | Rico Frederik | |
Schwendenwein | Elisabeth | |
Sewalt | Charlie | |
Skandsen | Toril | |
Smielewski | Peter | |
Sorinola | Abayomi | |
Stamatakis | Emmanuel | |
Stanworth | Simon | |
Kowark | Ana | |
Stevens | Robert | |
Stewart | William | |
Steyerberg | Ewout W. | |
Stocchetti | Nino | |
Sundström | Nina | |
Synnot | Anneliese | |
Takala | Riikka | |
Tamás | Viktória | |
Tamosuitis | Tomas | |
Taylor | Mark Steven | |
Te Ao | Braden | |
Tenovuo | Olli | |
Theadom | Alice | |
Thomas | Matt | |
Tibboel | Dick | |
Timmers | Marjolein | |
Tolias | Christos | |
Trapani | Tony | |
Tudora | Cristina Maria | |
Vajkoczy | Peter | |
Valeinis | Egils | |
Vallance | Shirley | |
Vámos | Zoltán | |
van der Naalt | Joukje | |
Van der Steen | Gregory | |
van Dijck | Jeroen T.J.M. | |
van Essen | Thomas A. | |
Van Hecke | Wim | |
van Heugten | Caroline | |
Van Praag | Dominique | |
van Wijk | Roel | |
Vande Vyvere | Thijs | |
Vargiolu | Alessia | |
Vega | Emmanuel | |
Velt | Kimberley | |
Verheyden | Jan | |
Vespa | Paul M. | |
Vik | Anne | |
Vilcinis | Rimantas | |
Volovici | Victor | |
von Steinbüchel | Nicole | |
Voormolen | Daphne | |
Vulekovic | Petar | |
Wang | Kevin K.W. | |
Wiegers | Eveline | |
Williams | Guy | |
Wilson | Lindsay | |
Winzeck | Stefan | |
Wolf | Stefan | |
Yang | Zhihui | |
Ylén | Peter | |
Younsi | Alexander | |
Zeiler | Frederick A. | |
Ziverte | Agate | |
Zoerle | Tommaso |
Funding
CENTER-TBI was supported by the European Union 7th Framework program (EC grant 602150). Additional funding was obtained from the Hannelore Kohl Stiftung (Germany), from OneMind (USA) and from Integra LifeSciences Corporation (USA). David K. Menon was supported by a Senior Investigator Award from the National Institute for Health Research (UK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
MT, JD, RW, VL, EV, AM, DM, GC, NS, EK participated in the conceptualization of the manuscript. MT and JD contributed equally, collected the data with VL and drafted the manuscript and the supplementary files. MT, JD, EV and RW analyzed the data. MT, JD, RW, VL, EV, AM, DM, GC, NS, EK had a role in data interpretation and provided feedback on the manuscript. EK supervised the project. MT, JD, RW, VL, EV, AM, DM, GC, NS, EK approved the submitted final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All IRBs approved the CENTER-TBI research protocol and the assessment of IRB data. A complete list can be found on https://www.center-tbi.eu/project/ethical-approval.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Timmers, M., van Dijck, J.T.J.M., van Wijk, R.P.J. et al. How do 66 European institutional review boards approve one protocol for an international prospective observational study on traumatic brain injury? Experiences from the CENTER-TBI study. BMC Med Ethics 21, 36 (2020). https://doi.org/10.1186/s12910-020-00480-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12910-020-00480-8