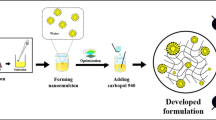
Abstract
Background
As the largest organ, the skin has been frequently affected by trauma, chemical materials, toxins, bacterial pathogens, and free radicals. Recently, many attempts have been made to develop natural nanogels that, besides hydrating the skin, could also be used as antioxidant or antibacterial agents.
Methods
In this study, the chemical composition of the Mentha spicata essential oil was first investigated using GC–MS analysis. Its nanoemulsion-based nanogel was then investigated; successful loading of the essential oil in the nanogel was confirmed using FTIR analysis. Besides, nanogel’s antioxidative, anticancer, and antibacterial activities were investigated.
Results
Carvone (37.1%), limonene (28.5%), borneol (3.9%), β-pinene (3.3%), and pulegone (3.3%) were identified as five major compounds in the essential oil. By adding carboxymethylcellulose (3.5% w/v) to the optimal nanoemulsion containing the essential oil (droplet size of 196 ± 8 nm), it was gelified. The viscosity was fully fitted with a common non-Newtonian viscosity regression, the Carreau-Yasuda model. The antioxidant effect of the nanogel was significantly more potent than the essential oil (P < 0.001) at all examined concentrations (62.5–1000 µg/mL). Furthermore, the potency of the nanogel with an IC50 value of 55.0 µg/mL was substantially more (P < 0.001) than the essential oil (997.4 µg/mL). Also, the growth of Staphylococcus aureus and Escherichia coli after treatment with 1000 µg/mL nanogel was about 50% decreased compared to the control group. Besides, the prepared electrospun polycaprolactone-hydroxypropyl methylcellulose nanofibers mat with no cytotoxic, antioxidant, or antibacterial effects was proposed as lesion dressing after treatment with the nanogel. High potency, natural ingredients, and straightforward preparation are advantages of the prepared nanogel. Therefore, it could be considered for further consideration in vivo studies.
Similar content being viewed by others
Introduction
Skin, the body's largest organ, has been affected by mechanical, thermal, and physical injury, hazardous substances, damaging UV sunlight, and many pathogens [1]. For instance, skin cancer is the fifth most common cancer worldwide. It is divided into melanoma and non-melanoma [2]. Melanoma is the most aggressive type of skin cancer and accounts for 90% of all skin cancer mortality [3, 4]. Besides, Escherichia coli and Staphylococcus aureus are two bacterial pathogens that can cause life-threatening infections [5]. S. aureus is a gram-positive bacterium mainly responsible for post-operative wound infection, toxic shock syndrome, and food poisoning. E. coli is a gram-negative bacterium that lives in the human intestine, and it is the cause of urinary tract disease and wound infections [6, 7].
Drug resistance and side effects of chemical medicine have led to many attempts to develop natural drugs, especially using essential oils (EOs) [8, 9]. For instance, Mentha spicata EO possesses many biological effects, including cholinesterase inhibitors, pancreatic lipase inhibitors, antimicrobial, and antiproliferative agents [10,11,12]. However, for practical application, the potencies of EOs should be improved and prepared in the appropriate dosage form. Preparing nanoemulsions containing EOs is a promising approach to meeting the challenges [13, 14]. Nanoemulsions are uniform dispersion of at least two immiscible liquids together by emulsifier(s) in the nanoscale [15]. Nanoemulsion as a drug delivery system increases the EO's effectiveness and delivery [16]. Furthermore, if the nanoemulsion is gelified, its topical application is facilitated as the viscosity increases [17, 18].
On the other hand, the lesions should be covered after topically administrated dosage forms such as nanogel. Electrospun nanofibers scaffold could be used as a wound dressing; they possess many characteristics such as high surface area, flexibility, and mechanical performance [19]. Besides, Their pores are so small that they prevent the entry of environmental pathogens but do not prevent air exchange with the lesion [20].
To the authors' best knowledge, nanogel containing M. spicata EO was not reported. Therefore, in this study, the chemical compositions of the M. spicata EO were first investigated. Biological effects, including antioxidant, anticancer, and antibacterial, were then investigated. After that, an attempt was made to improve its efficacy by preparing nanoemulsion-based nanogel. Finally, an electrospun nanofibers scaffold was proposed for after-treatment with the nanogel.
Materials and methods
Materials
M. spicata EO was purchased from Pharmaceutical Company Essential Oil Dr. Soleimani, Gorgan (36.8418° N, 54.4334° E), Iran. The EO was extracted from bark using the hydro distillation process. Human melanoma cells A-375 (ATCC CRL-1619), S. aureus (ATCC 25,923), and E. coli (ATCC 25,922) were provided by the Pasteur Institute of Iran. 3-(4,5-dimethyl-thiazol-2-yl)-2,5- diphenyl tetrazolium bromide (MTT) and phosphate-buffered saline (PBS) tablets and, Polycaprolactone (PCL) were provided by Sigma-Aldrich (USA). Hydroxypropyl methyl (HPMC) cellulose was prepared from Nikita, India. Penicillin–streptomycin, trypsin, Dimethyl Sulfoxide (DMSO), and Dulbecco's Modified Eagle's Media (DMEM) cell culture medium were supplied by Shellmax (China). Gibco (USA) produced fetal bovine serum (FBS).
GC–MS analysis
M. spicata EO was analyzed by a gas chromatography–mass spectrometry device (GC–MS) (Alginate 6890, USA) with an HP-5MS silica fused column that connected to a mass spectrometer (Alginate 5973, USA) as described in our previous report [21].
Preparation and characterization of nanoemulsion-based nanogel
A fixed amount of M. spicata EO (10 µL) was mixed with different amounts of tween 20 (10–30 µL) for 5 min at 2000 rpm, room temperature. The PBS solution as an aqueous phase was then added dropwise up to the final volume (5000 µL) and stirred for 40 min. Using a scatteroscope instrument (K-one LTD, Korea) dynamic light scattering (DLS) technique, the nanoemulsion's mean droplet size and distribution were measured as D50 and SPAN. SPAN was calculated by D90-D10/D50, where D is the diameter of droplets, and D10, D50, and D90 are the percentile of droplets with a diameter lower than these values. Finally, an optimal nanoemulsion with proper size characteristics, including mean droplet size < 200 nm and SPAN < 1 [22], was selected for further gelation.
The optimum nanoemulsion was gelified by adding carboxymethylcellulose (3.5% w/v); the mixture was stirred overnight in a mild condition (200 rpm, room temperature). The viscosity of nanogel was investigated utilizing a Rheometer machine (MCR-302 model, Anton Pear Co, Austria) in shear rates of 0.1 to 100 S−1. Noteworthy, a blank gel was also prepared similarly, only without EO. Furthermore, the nanogel was stored at 4 °C and room temperature for six months and checked for any biphasic or creaming.
Evaluation of antioxidant activity
Antioxidative activities of M. spicata EO and the nanogel were studied by the DPPH test; their serial dilutions (62.5–1000 µg/mL) were prepared using PBS solution containing 0.5% DMSO as the solvent. The assay was performed as follows; 150 μL of DPPH solution (0.3 mM) was first added to each well, and 50 μL of serial dilution was then added. The treated plates were incubated in darkness for 30 min to complete the reaction. The wells' optical density (OD) was read at 517 nm using a plate reader device (Synergy HTX multi-mode, USA). The percentage of antioxidant activity was calculated using Eq. 1.
In-vitro cell viability studies
MTT assay was performed to investigate the anticancer activities of the EO and nanogel against A-375 melanoma cells, as described in our previous study [23]. Serial dilutions of the EO and nanogel were prepared in PBS solution containing 0.5% DMSO. Fifty μL cell (1 × 104) was added to each well and incubated for 24 h (37 °C and 5% carbon dioxide) for attached cells and reached a confluence of ~ 80%. The liquid content of each well was then replaced with 50 and 50 μL of fresh complete media culture (RPMI containing 15% FBS and 1% penicillin/streptomycin) and serial dilution and incubated for 24 h. After that, the liquid content of each well was replaced with 50 μL of 0.5 g/ml MTT solution and incubated for 4 h. The formazan crystals were dissolved by adding 50 μL of DMSO to each well, and the OD of wells was read at 570 nm using the plate reader. The cell viability at each concentration was calculated using Eq. (2). Six well/plates were considered control groups filled with PBS solution containing 0.5% DMSO instead of serial dilution.
Evaluation of antibacterial activity
The antibacterial activity of the EO and the nanogel was investigated using the 96-well microdilution test on E. coli and S. aureus [24]. Serial dilutions (62.5–1000 µg/mL) were prepared using PBS solution containing 0.5% DMSO. New cultured bacterial (24 h) were suspended in the Muller Hinton broth to reach 0.5 McFarland turbidity (1.5 × 108 CFU/ml). Fifty and fifty µL of the bacterial suspensions and PBS solution containing 0.5% DMSO were added to each well. After that, treated plates were incubated at 37 ºC for 24 h, and the OD of wells was read at 630 nm using the plate reader. The percentage of bacteria growth was calculated using Eq. 3. Six/well plates were considered control groups; they were filled with 50 and 50 µL of the bacteria suspension and PBS solution containing 0.5% DMSO.
Preparation and characterization of nanofibers
PCL-HPMC nanofibers as dressing after treatment with the nanogel were prepared using the electrospinning technique. PCL (12%) and HPMC (3%) polymers were dissolved in HFIP; stirred at 2000 rpm overnight at room temperature. The polymer solution was filled into a 10 mL syringe (with an 18 gauge needle) and placed in an electrospinning machine (Fanavaran Nano-Meghyas, FNM Co, ltd, Iran). Nanofibers were made by optimizing the injection rate (0.6–1.2 mL/h), applied voltage (15–20 kV), and the distance between the needle and the collector (100 rpm, 70–100 mm). A thin layer of aluminum was wrapped around the collector for easy separation of the nanofiber, and it was rotated at 100 rpm. The prepared nanofibers were cut in 0.5 cm and used as an independent sample in the mentioned assays. The wells were filled with 50 µL of bacteria (or cells in MTT tets) suspension, 50 µL of PBS solution containing 0.5% DMSO, and a piece of nanofiber.
A scanning electronic microscopy instrument (SEM) (TESCAN-Vega model, TESCAN Co, Czech Republic) was used to characterize the morphology and size of the nanofibers. Nanofibers mat was cut 0.5 cm and covered with gold vapors by sputtering coating (Q15R-ES model, Quorum Technologies Co, England) before subjecting to SEM devise. Besides, the nanofibers' functional groups and molecular interactions were evaluated by Fourier Transform Infrared (FTIR). For this purpose, PCL, HPMC polymers, and PCL-HPMC nanofibers spectra were obtained using FTIR spectroscopy (Tensor II model, Bruker Co, Germany). Also, the nanofibers' surface hydrophobicity was studied using a contact angle apparatus (CA 500 A model, Sharif Solar Co, Iran). Seven µL of deionized water was dropped on the surface of the nanofibers mat, and its angle with the surface was measured using the apparatus’ software.
Statistical analysis
All tests were done in triplicates, and data have been reported as mean ± SD; excel software (Microsoft Office, v 2010, USA) was used to calculate means and standard deviations. The independent sample T-test was used to compare different groups with a minimum significance of 0.05 (STATA 16 app, USA). The calculation of IC50 was performed using CalcuSyn; v 2011 software (Biosoft England, UK). The lack of overlap between the upper and lower limits of IC50 was considered a significant sign.
Results
GC–MS analysis of M. spicata EO
The identified components with higher portions than 1% in M. spicata EO with GC–MS are listed in Table 1. The five major compounds with 37.1, 28.5, 3.9, 3.3, and 3.3% portions were carvone, limonene, borneol, β-pinene, and pulegone.
Physicochemical properties of the nanofibers
The preparation steps for obtaining PCL-HPMC electrospun nanofibers are summarized in Table 2. The nanofibers were obtained at an injection rate of 1.2 mL/h, a distance of 80 mm between the needle and collector, and a voltage of 15 kV. The nanofibers were smooth and randomly oriented with no bead (Fig. 1A). The mean diameter of the nanofibers was 464 ± 45 nm. Moreover, the contact angle of the water with its mat surface was 123 ± 5º (Fig. 1B). It is confirmed to have a hydrophobic surface.
FTIR spectra of the PCL and HPMC powders and electrospun PCL-HPMC nanofibers are shown in Fig. 2. In the spectrum of the HPMC, the small peak at 2896 cm−1 is attributed to the stretching vibration of C-H. The bands that appeared at 1051 cm−1 and 3418 cm−1 are related to the stretching vibration of C-O, and O–H groups, respectively. Besides, the FTIR spectrum of PCL powder showed an absorption peak at 1722 cm−1, which is assigned to the stretching vibration of C = O. The peaks that appeared at 2943 and 2865 cm−1 are attributed to the stretching vibration of -CH2- [25]. The peak at 1292 cm−1 is related to C–C stretching vibration. Another characteristic band at 1236 and 1164 cm−1 is assigned to C–O–C symmetric and asymmetric stretching vibrations [26]. In the FTIR spectra of electrospun PCL-HPMC nanofibers, the changes in shape, intensity, and wavelength of some absorption peaks were observed, corresponding to the interaction between PCL and HPMC. For instance, the absorption peak intensity at 1722 cm−1 attributed to C = O in PCL powder was changed and shifted to 1702 cm−1 in the spectrum of prepared nanofibers. Moreover, when PCL was blended with HPMC, the characteristic peaks of both polymers appeared in the FTIR spectra of PCL-HPMC nanofibers, thus indicating the presence of both PCL and HPMC in the structure of obtained nanofibers [27].
Characteristics of the nanoemulsions and the nanogel
Components and size measurements of the prepared nanoemulsions are listed in Table 3. Only NS2 with a mean droplet size of 196 nm and SPAN 0.96 possess proper characteristics; its DLS diagram is depicted in Fig. 3. The nanoemulsion was then gelified; its viscosity in different shear rates is fully fitted with the Carreau-Yasuda model (Fig. 4). It is well-known for non-Newtonian fluids such as polymeric solutions [28]. The viscosity of non-Newtonian decreases with increasing the shear rates and vice versa [29, 30]. Furthermore, no biphasic and creaming was observed in the nanoegel after six months of storage at 4 °C and room temperature.
Antibacterial properties
Figure 5 shows the antibacterial activities of different concentrations of the EO and the nanogel on E. coli. The efficacy of the nanogel was significantly more potent than the EO at 500 and 1000 µg/mL; p-values = 0.0432 and 0.0156). Interestingly, nanofiber and blank gel did not affect bacterial growth.
The antibacterial effects of the EO nanogel and blank gel on S. aureus are depicted in Fig. 6. Their efficacies are dose-dependent, and there is a significant difference (p-value < 0.001) observed between them at all examined concentrations (62.5–1000 µg/mL). The nanofiber and blank gel did not affect the growth of S. aureus.
Antioxidant properties
Figure 7 shows the antioxidant activities of the EO and the nanogel; the antioxidant effect increases with the increasing concentration. Also, the potency of the nanogel was significantly more potent than the EO (p-value < 0.001).
Anticancer properties
The cytotoxicity effect of the EO and the nanogel A-375 melanoma cells are shown in Fig. 8. The IC50 value of the nanogel (55.0 µg/mL) about 18 folds was more potent than the EO with an IC50 value of 997.4 µg/mL. Besides, the potency of the nanogel at all concentrations was substantially more than the EO (p-value < 0.001). Moreover, the viability of cells 8% was reduced after treatment with blank gel, and nanofiber did not affect the viability of the cells.
Discussions
In this study, chemical compounds of M. spicata EO as a natural medicine were investigated by GC–MS analysis; carvone (37.1%) was the major compound. Previous reports indicated that carvone has antimicrobial, anticonvulsant, antioxidant, and antitumor potentials [31]. The preparation of EO-based nanoformulations as a promising approach for improving the efficacy and designing the appropriate dosage forms (topical, systemic, or inhalation) has recently been more considered [32, 33]. High-energy and low-energy (spontaneous) methods are common in nanoemulsions preparation [34]. This study used the low-energy method to prepare the nanoemulsion to prevent the evaporation of EO’s volatile components. Besides, as the low viscosity of nanoemulsions is a challenge for topical applications, the optimum nanoemulsion was thus gelified in the current study. Interestingly, nanogel containing M. spicata EO was not reported so far. Therefore, this study investigated the biological properties (antibacterial, antioxidant, and anticancer properties) of the nanoemulsion-based nanogel containing M. spicata EO.
The efficacy of the nanogel was significantly more potent than the EO against E. coli and S. aureus. Besides, M. spicata EO and nanogel were found to have antioxidant activity; the nanogel was significantly more potent than unformulated EOs. Free radicals are involved in melanoma development, causing adverse skin effects such as aging and inflammation [35, 36].
The cell wall of Gram-negative bacteria is more complex than Gram-positive bacteria and generally is more resistant [37]. However, the efficacy of the EO against E. coli was more potent than S. aureus in the current study. It is accepted that EO has selective effects on gram-negative or positive bacteria [38, 39]. For instance, Myrtus communis EO with IC50 4547 µg/mL on E. coli was significantly more potent against S. aureus (IC50 394 µg/mL) [33]. Besides, Mentha piperita EO with IC50 27 mg/mL on S. aureus was significantly more potent against E. coli, IC50 18 mg/mL [34].
Furthermore, some reports on nanogels containing EOs from other species in the Mentha family have been published. For instance, the growth of S. aureus after treatment with 1250 µg/mL nanogel containing M. piperita EO 30% decreased [40]. Besides, no significant effect (< 10%) on the growth of P. aeruginosa and S. aureus was reported after treatment with 2500 µg/mL nanogel containing M. longifolia EO [20].
In the current study, the efficacy of the nanogel was 18 folds more potent than the EO against A-375 melanoma cells; IC50 values were obtained at 55.0 and 997.4 µg/mL. Cancer cells' membranes are wider than normal cells for obtaining nutrient molecules, and they have downregulated gap junctions and become ready for metastasis [41, 42]. The weak lymphatic system with large gaps are two important factors in inactive nano-drug delivery systems; nanostructures easily enter cancer cells and are not allowed to exit, improving their efficacy [43, 44]. Moreover, large amounts of EO droplets could be loaded into one nanostructure like nanogel, improving efficacy [38, 45].
After topical treatment of nanogel, a dressing is required to cover the lesions. It should prevent pathogens from entering the body and, at the same time, allow air exchange [46]. Nanofibers with a high surface area, small pore size, and high porosity have attracted huge interest in wound dressing in the last decades [47]. Electrospinning is still a promising technique for preparing nanofibers [48]. PCL is an excellent dressing with promising properties, including biocompatibility and slow degradation rate [49,50,51].
Furthermore, solid surfaces are classified into five categories according to the angle (θ) at which the water drop forms; 0–30° strongly wettable, 30–75° moderately wettable, 75–105° neutrally wettable, 105– < 150° hydrophobe, 150–180° super hydrophobe [20]. The angle of water with the PCL nanofibers was reported at 150° [46]. To improve its hydrophobicity, a hydrophilic polymer is commonly added. For instance, polycaprolactone-alginate electrospun nanofibers with 188 nm diameter and a contact angle of 144° for antibacterial dressing was introduced [20]. Polycaprolactone-chitosan with 200 nm diameter and contact angle of 109° for dressing cutaneous leishmaniasis was proposed [52]. The current study reduced the angle to 123° by adding HPMC. The prepared PCL-HPMC nanofiber (with no-cytotoxic, antibacterial, and antioxidant effects) is proposed as a dressing to cover the treated area.
Conclusions
This study aimed to improve the biological activities of the M. spicata EO by preparation of the nanogel containing the EO. Interestingly, the antibacterial, antioxidant, and anticancer effects of the nanogel were significantly more potent than the EO. Besides, the nanogel could thus be considered for further consideration in vivo studies.
Availability of data and materials
All data is available from corresponding authors on reasonable request.
References
Fischer M, Gemende I, Marsch WC, Fischer P. Skin function and skin disorders in Parkinson’s disease. J Neural Transm. 2001;108(2):205–13. https://doi.org/10.1007/s007020170088s.
Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978–86. https://doi.org/10.1111/J.1365-4632.2010.04474.X.
Ali Z, Yousaf N, Larkin J. Melanoma epidemiology, biology and prognosis. EJC Suppl. 2013;11:81. https://doi.org/10.1016/J.EJCSUP.2013.07.012.
Aubuchon M, Bolt L, Janssen-Heijnen M, Verleisdonk-Bolhaar S, van Marion A, van Berlo C. Epidemiology, management and survival outcomes of primary cutaneous melanoma: a ten-year overview. Acta Chir Belg. 2017;117(1):29–35. https://doi.org/10.1080/00015458.2016.1242214.
Lestari ES, Severin JA, Filius PM, Kuntaman K, Duerink DO, Hadi U, Wahjono H, Verbrugh HA, Antimicrobial Resistance in Indonesia P, Prevention. Antimicrobial resistance among commensal isolates of Escherichia coli and Staphylococcus aureus in the Indonesian population inside and outside hospitals. Eur J Clin Microbiol Infect Dis. 2008;27(1):45–51. https://doi.org/10.1007/s10096-007-0396-z.
Jose B, Reddy LJ. Evaluation of antibacterial activity of the leaf and flower essential oils of Gliricidia sepium from south India. Int J Appl Pharm. 2010;2(2):20–2.
Yousefpoor Y, Bolouri B, Bayati M, Shakeri A, Eskandari Y. The combined effects of Aloe vera gel and silver nanoparticles on wound healing in rats. Nanomed J. 2016;3(1):57–64. https://doi.org/10.7508/NMJ.2016.01.007.
Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53(1):615–27. https://doi.org/10.1146/annurev.med.53.082901.103929.
Noorpisheh Ghadimi S, Sharifi N, Osanloo M. The leishmanicidal activity of essential oils: a systematic review. J HerbMed Pharmacol. 2020;9(4):300–8. https://doi.org/10.34172/jhp.2020.38.
Ali-Shtayeh MS, Jamous RM, Abu-Zaitoun SY, Khasati AI, Kalbouneh SR. Biological properties and bioactive components of Mentha spicata L. essential Oil: focus on potential benefits in the treatment of obesity, Alzheimer’s Disease, Dermatophytosis, and Drug-Resistant Infections. Evid Based Complement Alternat Med. 2019;2019. https://doi.org/10.1155/2019/3834265
Kanatt SR, Chander R, Sharma A. Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chem. 2007;100(2):451–8. https://doi.org/10.1016/j.foodchem.2005.09.066.
Ani KAAA. Primary phytochemical identification and some biochemical parameters study of ethanolic extract of mentha spicata leaves in Mice. J Chem Pharm Res. 2016;8(7):818–22.
Prakash A, Baskaran R, Paramasivam N, Vadivel V. Essential oil based nanoemulsions to improve the microbial quality of minimally processed fruits and vegetables: a review. Food Res Int. 2018;111:509–23. https://doi.org/10.1016/j.foodres.2018.05.066.
Donsì F, Ferrari G. Essential oil nanoemulsions as antimicrobial agents in food. J Biotechnol. 2016;233:106–20. https://doi.org/10.1016/j.jbiotec.2016.07.005.
Valizadeh A, Shirzad M, Pourmand MR, Farahmandfar M, Sereshti H, Amani A. Levofloxacin nanoemulsion gel has a powerful healing effect on infected wound in streptozotocin-induced diabetic rats. Drug Deliv Transl Res. 2021;11(1):292–304. https://doi.org/10.1007/s13346-020-00794-5.
Abbasifard M, Yousefpoor Y, Amani A, Arababadi MK. Topical Bee Venom Nano-emulsion Ameliorates Serum Level of Endothelin-1 in Collagen-Induced Rheumatoid Arthritis Model. BioNanoScience. 2021:1–6. https://doi.org/10.1007/s12668-021-00871-0
Ghanbariasad A, Azadi S, Agholi M, Osanloo M. The nanoemulsion-based nanogel of Artemisia dracunculus essential oil with proper activity against Leishmania tropica and Leishmania major. Nanomed Res J. 2021;6(1):89–95. https://doi.org/10.22034/NMRJ.2021.01.010.
Khurana S, Jain N, Bedi P. Nanoemulsion based gel for transdermal delivery of meloxicam: physico-chemical, mechanistic investigation. Life Sci. 2013;92(6–7):383–92. https://doi.org/10.1016/j.lfs.2013.01.005.
Asghari F, Faradonbeh DR, Malekshahi ZV, Nekounam H, Ghaemi B, Yousefpoor Y, Ghanbari H, Faridi-Majidi R. Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydr Polym. 2021:118926. https://doi.org/10.1016/j.carbpol.2021.118926
Qasemi H, Fereidouni Z, Karimi J, Abdollahi A, Zarenezhad E, Rasti F, Osanloo M. Promising antibacterial effect of impregnated nanofiber mats with a green nanogel against clinical and standard strains of Pseudomonas aeruginosa and Staphylococcus aureus. J Drug Deliv Sci Technol. 2021:102844. https://doi.org/10.1016/j.jddst.2021.102844
Alipanah H, Farjam M, Zarenezhad E, Roozitalab G, Osanloo M. Chitosan nanoparticles containing limonene and limonene-rich essential oils: potential phytotherapy agents for the treatment of melanoma and breast cancers. BMC complement med ther. 2021;21(1):1–10. https://doi.org/10.1186/s12906-021-03362-7.
Osanloo M, Firooziyan S, Abdollahi A, Hatami S, Nematollahi A, Elahi N, Zarenezhad E. Nanoemulsion and nanogel containing Artemisia dracunculus essential oil; larvicidal effect and antibacterial activity. BMC Res Notes. 2022;15(1):276. https://doi.org/10.1186/s13104-022-06135-8.
Valizadeh A, Khaleghi AA, Roozitalab G, Osanloo M. High anticancer efficacy of solid lipid nanoparticles containing Zataria multiflora essential oil against breast cancer and melanoma cell lines. BMC Pharmacol Toxicol. 2021;22(1):52. https://doi.org/10.1186/s40360-021-00523-9.
Osanloo M, Ghaznavi G, Abdollahi A. Surveying the chemical composition and antibacterial activity of essential oils from selected medicinal plants against human pathogens. Iran J Microbiol. 2020;12(6):577–58. https://doi.org/10.18502/ijm.v12i6.5032.
Ramalingam R, Dhand C, Leung CM, Ezhilarasu H, Prasannan P, Ong ST, Subramanian S, Kamruddin M, Lakshminarayanan R, Ramakrishna S. Poly-ε-caprolactone/gelatin hybrid electrospun composite nanofibrous mats containing ultrasound assisted herbal extract: Antimicrobial and cell proliferation study. Nanomaterials. 2019;9(3):462. https://doi.org/10.3390/nano9030462.
Eldurini S, Abd El-Hady BM, Shafaa MW, Gad AAM, Tolba E. A multicompartment vascular implant of electrospun wintergreen oil/ polycaprolactone fibers coated with poly(ethylene oxide). Biomed J. 2020;44(5):589–97. https://doi.org/10.1016/j.bj.2020.04.008.
Eskitoros-Togay ŞM, Bulbul YE, Tort S, Korkmaz FD, Acartürk F, Dilsiz N. Fabrication of doxycycline-loaded electrospun PCL/PEO membranes for a potential drug delivery system. Int J Pharm. 2019;565:83–94. https://doi.org/10.1016/j.ijpharm.2019.04.073.
Avazmohammadi R, Castañeda PP. The rheology of non-dilute dispersions of highly deformable viscoelastic particles in Newtonian fluids. J Fluid Mech. 2015;763:386–432. https://doi.org/10.1017/jfm.2014.687.
Zare Y, Park SP, Rhee KY. Analysis of complex viscosity and shear thinning behavior in poly (lactic acid)/poly (ethylene oxide)/carbon nanotubes biosensor based on Carreau-Yasuda model. Results Phys. 2019;13: 102245. https://doi.org/10.1016/j.rinp.2019.102245.
Kwack J, Masud A. A stabilized mixed finite element method for shear-rate dependent non-Newtonian fluids: 3D benchmark problems and application to blood flow in bifurcating arteries. Comput Mech. 2014;53(4):751–76. https://doi.org/10.1007/s00466-013-0928-6.
Bicas J, Neri-Numa I, Ruiz A, De Carvalho J, Pastore G. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem Toxicol. 2011;49(7):1610–5. https://doi.org/10.1016/j.fct.2011.04.012.
Alipanah H, Abdollahi A, Firooziyan S, Zarenezhad E, Jafari M, Osanloo M. Nanoemulsion and Nanogel Containing Eucalyptus globulus Essential Oil; Larvicidal Activity and Antibacterial Properties. Interdiscip Perspect Infect Dis. 2022;2022:1616149. https://doi.org/10.1155/2022/1616149.
Roozitalab G, Yousefpoor Y, Abdollahi A, Safari M, Rasti F, Osanloo M. Antioxidative, anticancer, and antibacterial activities of a nanoemulsion-based gel containing Myrtus communis L. essential oil. Chem Pap. 2022. https://doi.org/10.1007/s11696-022-02185-1
Osanloo M, Abdollahi A, Valizadeh A, Abedinpour N. Antibacterial potential of essential oils of Zataria multiflora and Mentha piperita, micro-and nano-formulated forms. Iran J Microbiol. 2020;12(1):43. https://doi.org/10.18502/ijm.v12i1.2517.
Dakah A, Zaid S, Suleiman M, Abbas S, Wink M. In vitro propagation of the medicinal plant Ziziphora tenuior L. and evaluation of its antioxidant activity. Saudi J Biol Sci. 2014;21(4):317–23. https://doi.org/10.1016/j.sjbs.2013.12.002.
Yoo KM, Lee CH, Lee H, Moon B, Lee CY. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008;106(3):929–36. https://doi.org/10.1016/j.foodchem.2007.07.006.
Trombetta D, Castelli F, Sarpietro MG, Venuti V, Cristani M, Daniele C, Saija A, Mazzanti G, Bisignano G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother. 2005;49(6):2474–8. https://doi.org/10.1128/AAC.49.6.2474-2478.2005.
Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals (Basel). 2013;6(12):1451–74. https://doi.org/10.3390/ph6121451.
Castillejos L, Calsamiglia S, Ferret A, Losa R. Effects of a specific blend of essential oil compounds and the type of diet on rumen microbial fermentation and nutrient flow from a continuous culture system. Anim Feed Sci Technol. 2005;119(1–2):29–41. https://doi.org/10.1016/j.anifeedsci.2004.12.008.
Sanei-Dehkordi A, Abdollahi A, Safari M, Karami F, Ghaznavi G, Osanloo M. Nanogels Containing Foeniculum vulgare Mill. and Mentha piperita L. Essential oils: mosquitoes’ repellent activity and antibacterial effect. Interdiscip Perspect Infect Dis. 2022;2022:4510182. https://doi.org/10.1155/2022/4510182.
Leithe E, Sirnes S, Omori Y, Rivedal E. Downregulation of gap junctions in cancer cells. Crit Rev Oncog. 2006;12(3–4):225–56. https://doi.org/10.1615/critrevoncog.v12.i3-4.30.
Ruch R. Gap junctions and connexins in cancer formation, progression, and therapy. Cancers (Basel). 2020;12(11). https://doi.org/10.3390/cancers12113307
Adepu S, Ramakrishna S. Controlled drug delivery systems: current status and future directions. Molecules (Basel, Switzerland). 2021;26(19):5905. https://doi.org/10.3390/molecules26195905.
Zhang X-Y, Lu W-Y. Recent advances in lymphatic targeted drug delivery system for tumor metastasis. Cancer Biol Med. 2014;11(4):247–54. https://doi.org/10.7497/j.issn.2095-3941.2014.04.003.
de Matos SP, Lucca LG, Koester LS. Essential oils in nanostructured systems: Challenges in preparation and analytical methods. Talanta. 2019;195:204–14. https://doi.org/10.1016/j.talanta.2018.11.029.
Abdollahi A, Zarenezhad E, Osanloo M, Ghaznavi G, Khalili Pour M. Promising antibacterial activity of a mat of polycaprolactone nanofibers impregnated with a green nanogel. Nanomed Res J. 2020;5(2):192–201. https://doi.org/10.22034/NMRJ.2020.02.010.
Yalcinkaya F, Komarek M, Lubasova D, Sanetrnik F, Maryska J. Preparation of antibacterial nanofibre/nanoparticle covered composite yarns. J Nanomater. 2016;2016. https://doi.org/10.1155/2016/7565972
Lee S, Obendorf SK. Use of electrospun nanofiber web for protective textile materials as barriers to liquid penetration. Text Res J. 2007;77(9):696–702. https://doi.org/10.1177/0040517507080284.
Janani I, Lakra R, Kiran MS, Korrapati PS. Selectivity and sensitivity of molybdenum oxide-polycaprolactone nanofiber composites on skin cancer: preliminary in-vitro and in-vivo implications. J Trace Elem Med Biol. 2018;49:60–71. https://doi.org/10.1016/j.jtemb.2018.04.033.
Ko Y-M, Choi D-Y, Jung S-C, Kim B-H. Characteristics of plasma treated electrospun polycaprolactone (PCL) nanofiber scaffold for bone tissue engineering. J Nanosci Nanotechnol. 2015;15(1):192–5. https://doi.org/10.1166/jnn.2015.8372.
Yew CHT, Azari P, Choi JR, Muhamad F, Pingguan-Murphy B. Electrospun polycaprolactone nanofibers as a reaction membrane for lateral flow assay. Polymers. 2018;10(12):1387. https://doi.org/10.3390/polym10121387.
Ghanbariasad A, Amoozegar F, Rahmani M, Zarenezhad E, Osanloo M. Impregnated Nanofibrous Mat with Nanogel of Citrus sinensis Essential Oil as a New Type of Dressing in Cutaneous Leishmaniasis. Biointerface Res Appl Chem. 2021;11(4):11066–76. https://doi.org/10.33263/BRIAC114.1106611076.
Acknowledgements
Not applicable
Funding
Fasa University of Medical Sciences supported this study, grant No. 401135.
Author information
Authors and Affiliations
Contributions
FR prepared the nanogel and nanofibers and performed MTT and DPPH assays. FR and YY wrote the MS. AA performed the antibacterial tests. MS interpreted ATR-FTIR. GhR contributed to the preparation of nanogel and MTT assay. MO design of the study, statistical analysis, and revised the MS. All authors contributed to drafting MS and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Fasa University of Medical Sciences ethics committee has ethically approved this study. Besides, all methods were performed in accordance with the relevant inter/national guidelines and regulations. Moreover, as the research did not involve human study; thus, no constant form was used.
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rasti, F., Yousefpoor, Y., Abdollahi, A. et al. Antioxidative, anticancer, and antibacterial activities of a nanogel containing Mentha spicata L. essential oil and electrospun nanofibers of polycaprolactone-hydroxypropyl methylcellulose. BMC Complement Med Ther 22, 261 (2022). https://doi.org/10.1186/s12906-022-03741-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-022-03741-8