Abstract
Background
Lycium barbarum polysaccharide (LBP), the most abundant functional component of wolfberry, is considered a potent antioxidant and an anti-ageing substance. This review aims to outline the hallmarks of ageing in the pathogenesis of osteoarthritis (OA), followed by the current understanding of the senolytic effect of LBP and its potential use in the prevention and treatment of OA. This will be discussed through the lens of molecular biology and herbal medicine.
Methods
A literature search was performed from inception to March 2020 using following keywords: “Lycium barbarum polysaccharide”, “DNA damage”, antioxidant, anti-apoptosis, anti-inflammation, anti-ageing, osteoarthritis, chondrocytes, fibroblasts, osteoblasts, osteoclasts, and “bone mesenchymal stem cell”. The initial search yielded 2287 papers, from which 35 studies were selected for final analysis after screening for topic relevancy by the authors.
Results
In literature different in vitro and in vivo ageing models are used to demonstrate LBP’s ability to reduce oxidative stress, restore mitochondrial function, mitigate DNA damage, and prevent cellular senescence. All the evidence hints that LBP theoretically attenuates senescent cell accumulation and suppresses the senescence-associated secretory phenotype as observed by the reduction in pro-inflammatory cytokines, like interleukin-1beta, and matrix-degrading enzymes, such as MMP-1 and MMP-13. However, there remains a lack of evidence on the disease-modifying effect of LBP in OA, although its chondroprotective, osteoprotective and anti-inflammatory effects were reported.
Conclusion
Our findings strongly support further investigations into the senolytic effect of LBP in the context of age-related OA.
Similar content being viewed by others
Background
Osteoarthritis (OA) is one of the fastest growing disabilities worldwide and is typically associated with irregular chronic pain which affects patients’ quality of life. OA has attracted many scientists throughout the centuries to explore its underlying mechanisms. However, to date there is still no disease-modifying drug available [1]. Consequently, identifying risk factors and selecting the right targets remain a great and unmet challenge in the OA field [2]. Today, OA research has confirmed that among others, hypertension, obesity and joint injury are associated with the induction of OA [2,3,4,5].
Evidence showed that the prevalence of OA increases with age. Different studies found that the incidence of OA dramatically increased with age both in women and men from all regions of the world [6, 7]. It affects an estimated 10% of men and 18% of women over 60 years old [6]. At present, with the rapid ageing population, the global incidence of OA is also rising steadily and it is estimated that 67 million people in the United States will be affected by 2030 [8]. All the evidence confirms OA as an age-related disease.
Hence, over the past decade, the role of ageing in the pathogenesis of OA has been intensively investigated [9, 10]. According to Lopez-Otin et al., there are nine hallmarks of ageing: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Most of them are caused by oxidative stress and reactive oxygen species (ROS) whose imbalance is a key feature of ageing [11]. In the pathogenesis of OA, most of these hallmarks, including genomic instability, mitochondrial dysfunction, and cellular senescence, have been studied [12]. For example, rapid increase of ROS leads to mitochondrial DNA (mtDNA) damage and mitochondrial dysfunction, causing premature chondrocyte senescence and apoptosis, which eventually increases the risk of OA [13,14,15]. Therefore, blocking major senescence signalling pathways in OA pathogenesis could be a valid method in delaying the onset of OA meanwhile it also creates the possibility of using anti-ageing reagents as emerging drug candidates for OA treatment.
Lycium barbarum (LB, Gouqi, wolfberry, or Fructus lycii) is a well-known traditional herb with widespread distribution [16]. It is extremely important in China and other Asian countries, not only because it can be used as nutritional supplement in daily life, but also because of its medicinal value [17,18,19]. Especially in recent years, with the in-depth study of LB, it has been highly valued by Chinese and foreign medical scientists and dietetic health experts for its antioxidant and anti-ageing effects [20,21,22,23,24,25,26].
Lycium barbarum polysaccharide (LBP), the most abundant functional component of wolfberry [27], is an important functional additive of dietary supplements and plays an important role in the anti-ageing and antioxidant function of LB. Xue S, et al. reported that a dose of 220 μg/mL and a dose of 440 μg/mL LBP could reverse the H2O2-induced oxidative injury [28]. Another study suggested that LBP could extend the average life span of Drosophila melanogaster due to an increase in antioxidant activity, i.e. an upregulation of the SOD and CAT levels [29]. Moreover, LBP has been studied in the reproductive system [30,31,32,33,34,35,36,37] as well as in the cells and tissues derived from different germ layers [38,39,40,41,42,43,44,45,46,47,48] to evaluate its protective value. Again, LBP performed excellently in scavenging free radicals, maintaining mitochondrial function and showed exquisite antioxidant effects. Recently, numerous mice models also showed the same results [49,50,51,52,53]. Overall, these findings demonstrated that LBP has good ROS-reducing and antioxidant properties in different in vivo and in vitro models. This will restore the oxidative stress balance and finally attenuate ageing to some extent. Based on its properties, we hypothesized that LBP could act as an anti-ageing reagent that may have the potential to prevent age-related OA.
Although the pathological mechanism of ageing-associated OA and LBP anti-ageing effects have been discussed for several years, the direct effects of LBP in the treatment of OA are relatively less studied up to this moment [54,55,56,57]. Therefore, in this review, we provided an overview of the pathogenesis of OA focussing on the role of genomic instability, mitochondrial dysfunction and cellular senescence. Next, we systematically searched the published studies for the LBP anti-ageing effects in different models. Additionally, we attempted to postulate the beneficial effect of LBP on the pathogenesis of OA. Finally, we proposed LBP as a potential treatment against OA and try to provide a new horizon for pharmaceutical scientists.
Methods
The workflow of our systematic review on LBP has been illustrated in Fig. 1. In brief, the search was performed in March 2020 by using the keyword “Lycium barbarum polysaccharide” combined with “DNA damage”, antioxidant, anti-apoptosis, anti-inflammation, anti-ageing, osteoarthritis, chondrocytes, fibroblasts, osteoblasts, osteoclasts, and “Bone Mesenchymal Stem Cell”. A total of 2287 records were identified by different databases. The filter was set to select all articles published in peer-reviewed journals, which were available in full text and written in English or Chinese, excluding duplication articles, resulting in 544 articles. Next, a screening for topic relevance through title and article abstracts was performed. In the end, 35 studies were selected for final analysis, including in vitro studies, animal studies, and clinical studies.
Results
Hallmarks of ageing in the pathogenesis of OA
OA is a degenerative joint disease characterized by cartilage degeneration, synovial hypertrophy and functional ligament damage. The occurrence of OA is a complex biological process in which multiple factors, such as genetic factors, gender, diet, obesity and age, play a role [58]. Among them, advanced age is most closely related to OA [2]. In-depth studies showed that oxidative stress and excessive accumulation of ROS are important factors leading to ageing and that they are involved in almost all hallmarks of the ageing process [11, 59, 60]. In the following, different hallmarks of ageing, including genomic instability (DNA damage and telomere attrition), mitochondrial dysfunction, cellular senescence, and other factors involved in cartilage degradation in the pathogenesis of OA will be discussed.
Genomic instability (telomere attrition and DNA damage)
Genomic instability is the most direct hallmark of ageing in OA. In the classic replicative senescence hypothesis, telomere attrition is one of the major markers of ageing. As the cell undergoes mitosis, the telomere length gradually shortens until it reaches the minimum length required for replication, eventually leading to cell cycle stagnation [61]. As early as the beginning of the twenty-first century, telomere erosion has been confirmed in isolated human cartilage, and it has become more serious with age [7]. The research findings supported that there was a partial association between OA and replicative senescence [7]. However, not all elderly suffers from OA, which means that in addition to telomere erosion caused by replicative senescence, there are other forms of telomere damage, which may be the main cause of the onset of OA [62,63,64,65].
Fortunately, more and more researchers have paid attention to this problem in recent years, and a large amount of evidence has emerged to prove that exogenously induced cellular senescence, also known as stress-induced senescence, is associated with OA [59, 65,66,67,68]. There is evidence showing that a high concentration of oxygen leads to premature senescence of human articular chondrocytes through increased telomere erosion and mtDNA damage [66]. Telomere shortening or DNA damage activates tumour protein p53 which later promotes the expression of p21 and p16, ultimately leading to cellular senescence [3]. Subsequently, another experiment explained that ROS accelerates the senescence of human chondrocytes by inducing telomere instability which is responsible for the occurrence of OA [65]. Of note, the level of ROS is a critical factor in ageing. The sensitivity of telomere terminus to ROS plays an essential role in the pathogenesis of OA.
Mitochondrial dysfunction
Mitochondrial dysfunction, another manifestation of ageing, occurs in response to damage, including oxidative and inflammatory damage. It is also one of the important hallmarks of age-related OA.
The major cause of chondrocytes mitochondrial dysfunction is the rapid increase of ROS generation [69,70,71,72]. In OA chondrocytes, it has been observed that boosting ROS changed the adenosine triphosphate (ATP) synthesis and mitochondrial respiratory chain (MRC) activity, which ultimately lead to mitochondrial dysfunction [72]. Recently, it has been reported that mitochondria will rapidly release ROS and superoxide radicals after a single, blunt-impact injury performed to osteochondral explants in vitro, causing acute chondrocytes death and exacerbating OA characterization [70]. It is suggested that mitochondrial dysfunction is highly related to OA. A rabbit model demonstrated that mitochondrial dysfunction caused by advanced oxidation production products (AOPPs) could lead to chondrocytes apoptosis and aggravate the osteoarthritic symptoms of rabbit cartilage [71].
In addition, high expression of inflammatory factors in chondrocytes was another reason for mitochondrial dysfunction. Recent studies showed that upregulation of IL-1β and TNF-α could induce overexpression of NO which damaged mtDNA and reduced mitochondrial transcription [3, 73]. It also revealed that pro-inflammatory factors contribute to the process between mitochondrial damage and apoptosis in chondrocytes [3, 73]. Furthermore, other studies have shown that in the absence of autophagy, mtDNA mutations and reduced mtDNA repair capacity could also lead to mitochondrial dysfunction in chondrocytes, exacerbating the risk of OA [74,75,76].
Mitochondrial dysfunction may induce several pathological processes of OA, including oxidative stress response, chondrocyte apoptosis as well as acute chondrocytes death [77]. Taken together, inhibiting or repairing mitochondrial dysfunction could be an emerging strategy for OA.
Cellular senescence
Cellular senescence is one of the nine recognized hallmarks of ageing. Normally, somatic cells have a Hayflick limit, that is a maximum of ~ 50 divisions. This is followed by growth arrest which is called senescence [78]. The senescent cells (SnCs) remain viable and metabolically active but secrete a variety of pro-inflammatory cytokines, growth factors, chemokines and proteinases known as the senescence-messaging secretome or senescence-associated secretory phenotype (SASP) [79, 80]. Senescence is a protective mechanism in various physiological processes, for example it evokes tumour suppression and limits fibrosis in wound healing [81]. On the other hand, senescence of stem or progenitor cells will impair tissue regeneration, and SASP can damage the surrounding tissue [79]. Over the past decade, senescence has been linked with ageing and age-related pathologies [82, 83], including OA [12, 68].
The number of senescent cells increases gradually with age. Changes in signalling pathways associated with ageing in chondrocytes, the main cell type found in articular cartilage, will raise the risk of OA [7]. To maintain the normal function of joints, chondrocytes will continuously synthesize new matrix molecules throughout their lives. In addition, insulin-like growth factor-1 (IGF-1) and transforming growth factor-β (TGF-β) are indispensable signalling molecules in the synthesis and catabolism of articular cartilage. However, the anabolic response of rat chondrocytes to IGF-1 worsened with age, while the ability of chondrocytes to release IGF-1 binding protein increased [84]. It indicated that chondrocytes senescence would cause a dynamic imbalance in the synthesis and degradation of articular cartilage. Besides, chondrocytes senescence could also affect the TGF-β signalling pathway. An experiment showed that the expression of TGF-β family factor receptors bAlk2, bAlk3, bAlk4, bAlk5, and bBmpr2 decreased significantly with age in bovine articular cartilage, which eventually led to age-related cartilage thinning and collagen loss [85]. Moreover, chondrocyte anabolism is also affected by the NF-κB signalling pathway [86, 87]. Chondrocytes senescence resulted in the accumulation of advanced glycation end products (AGEs) [88], and the expression of AGEs could activate the NF-κB signalling pathway, increase the production of matrix metalloproteinase (MMP) -13, and finally cause degradation of articular cartilage [89]. Together, this demonstrated that changes in the chondrocyte signalling pathways will have a considerable impact on the incidence of OA.
The hallmark of OA is degeneration of articular cartilage and chondrocyte senescence is largely responsible for this phenomenon. In human OA cartilage lesions, SnCs were found near the cluster of chondrocytes [90], which exhibited characteristics of progenitor cells with increased proliferation [91, 92]. Adult articular chondrocytes have limited proliferation capacity. In response to altered mechanical loading [64, 93] or oxidative stress [65], articular chondrocytes underwent premature senescence with shortening of telomeres, which provoked the onset of OA [94]. It has been well documented that OA chondrocytes expressed a variety of the SnCs markers such as telomere attrition [7], activation of senescence-associated beta-galactosidase (SAβGal) [90], overexpression of p16Ink4a [95] as well as MMP-1, 3 and 13 [96]. The percentage of SnCs cells in articular cartilage increased with the severity of knee OA [97]. Moreover, transplantation of SAβGal-positive SnCs into synovial joint led to an OA-like lesion in rodents [13]. Furthermore, ablation of p16Ink4a-positive SnCs using a genetically modified mice model could mitigate OA [94]. All the evidence suggests that the accumulation of SnCs impairs homeostasis of articular cartilage. Thus, removal of SnCs could be a promising therapeutic strategy for OA.
Other hallmarks of ageing in OA
Besides genomic instability, mitochondrial dysfunction and cellular senescence, inflammaging could be the other hallmark of ageing in OA. Inflammaging refers to chronic low-grade inflammation that develops with advanced age. Typically inflammaging is observed both locally and systemically in OA [98]. In addition, synovial inflammation and subchondral bone disturbance were often involved in the initiation of OA [99, 100]. Such inflammation will accelerate cellular senescence and make normal cells secrete SASP [101]. Recent studies have identified p16Ink4a-positive SnCs in inflamed synovium [94], cartilage surface [94] and aged bone microenvironment [102]. Besides, inflammatory mediators, like COX-2, IL-1β and TNF-α, were shown to be upregulated in the OA group when compared to the control group [103]. These results indicate that there is a strong association between inflammaging, senescence and OA.
In fact, inflammation plays a crucial part in OA development. Inflammaging in OA is caused by damage-associated molecular patterns (DAMPs), e.g. high-mobility group box 1 (HMGB1) protein, S100 family and uric acid [101, 104]. The DAMPs are mainly triggered by abnormal ROS accumulation [105]. The up-regulated DAMPs will then promote SASP factors release, i.e. MMPs and inflammatory cytokines, through mitogen-activated protein kinases (MAPK) and NF-κB signalling pathway [104, 105], which will finally provoke the OA progress. It is noteworthy that DAMPs can cause chronic low-grade inflammation in the joint and can accelerate cellular pro-senescence by cell-to-cell communication through NF-κB pathway [106].
Stem cell exhaustion was found to be another hallmark of age-related OA. The expression of p16Ink4a was found to be upregulated in aged mesenchymal stem cells (MSCs) [107]. As bone marrow-derived mesenchymal stem cells (bmMSC) can differentiate into osteoblasts and chondrocytes for repair of aged cartilage, their functional senescence or depletion will contribute to the development of osteoarthritis [108]. An in vivo study has demonstrated that metformin-stimulated adipose tissue-derived human MSCs (Ad-hMSCs) could prevent the degeneration of cartilage effectively and prolong the survival time of MSCs in inflamed joints [109]. Articular cartilage stem cells (ACSCs) were known to repair articular cartilage [110]. However, with the development of OA, the population of ACSCs would gradually diminish [110]. As a whole, preventing stem cell exhaustion would be an effective way to prevent OA.
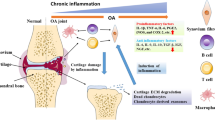
In short, the pathogenesis of OA caused by ageing is characterized by oxidative damage: DNA damage, telomere attrition, mitochondrial dysfunction, cellular senescence, inflammaging, and stem cell exhaustion (Fig. 2). As age-related OA is a multifactorial disease, it is necessary to utilize therapy strategies which can target multiple signalling pathways to treat OA more efficiently.
Schematic illustration of pathogenesis of OA in the literature. ROS (reactive oxygen species); NO (nitric oxide); mtDNA (mitochondrial deoxyribonucleic acid); TNF-α (tumor necrosis factor-α); IL-1β (interleukin-1β); IL-6 (interleukin-6); IL-8 (interleukin-8); IL-1 (interleukin-1); MMP-1 (matrix metalloproteinase-1); MMP-13 (matrix metalloproteinase-13); SA-βgal (Senescence-associated beta-galactosidase); OA (osteoarthritis)
Evidence of LBP anti-ageing effects
Lycium barbarum (LB) is a traditional Chinese herbal medicine and has a complex chemical composition with extremely diverse targets. There are many bioactive substances in LB such as LBP, betaine, carotenoids, zeaxanthin, alkaloids, β-sitosterol, cerebroside, thiamine, riboflavin, flavonoids and phenolics [27, 111]. Among them, the polysaccharide content in LB (dried fruits) could reach as much as 5–8% [112]. LBP, as the most abundant one, has unique advantages: it is easy to extract, and its pharmacological characteristics are ideal for further research. So far, the most common isolation techniques for LBP from LB are through leaching extraction, microwave extraction, and ultrasonic extraction, by using water or ethanol as solvent [113]. Compared to the other active substances of LB, LBP is favoured because of its relatively simple extraction process. Moreover, it shows good anti-ageing properties in different experimental models (Table 1).
In vitro studies
According to literature, LBP could scavenge free radicals e.g., ABTS free radical, DPPH free radical, superoxide anion and hydroxyl radical. This will reduce oxidative stress and plays an important role in delaying ageing [18, 27, 124]. Another study found that increasing concentrations of LBP resulted in a higher free radical scavenging rate. However, when the dose was around 100 to 250 μg/ml, the scavenging rate gradually reached a plateau [124].
LBP has demonstrated antioxidizing and anti-apoptotic effects in vitro [28, 38, 39, 125]. By reducing oxidative stress and other harmful factors related to ageing, LBP can reduce DNA fracture damage, strengthen cell activity, and achieve anti-ageing effects on cells. LBP was able to upregulate the nuclear factor E2-related factor 2 (Nrf2) [38, 39, 125] and induce translocation of cytoplasmic Nrf2 to the nucleus to bind to the antioxidant response element (ARE) [38]. Moreover, LBP could down-regulate the expression of the transcription inhibitor Bach1, reducing the competition with Nrf2 for binding to ARE. In return, it boosted the expressions of antioxidant enzyme genes [28, 38, 125].
The anti-ageing effect of LBP is dose dependent. The effective dose of LBP in vitro was around 200 μg/ml. At this dosage LBP inhibits mitochondria clustering, reduces the level of ROS, and increases the mtDNA copy number and the expression of sirtuin-1 (SIRT1), AMPK, GPX4, SOD1 and Bcl-2 [115]. However, at a concentration of 1600 μg/ml LBP delayed the growth of murine two-cell embryos [115]. It is suggested that the dosage was critical for the anti-ageing effects of LBP.
Animal studies
The anti-ageing effect of LBP in vivo has been widely discussed (Table 1), with mice as most commonly used animal model.
In ageing mouse models, studies found that LBP reduced the level of advanced glycation end products (AGE) and IL-2. It also enhanced the memory and the learning ability of ageing mice [123]. Moreover, LBP could also promote the proliferation of lymphocytes and the activity of SOD [123]. It was suggested that LBP was involved in activating antioxidant enzyme activity in cells [123]. The contents of ROS and MDA were found to be decreased while the activities of antioxidant enzymes like SOD, CAT and GSH-Px were found to be increased in a dose-dependent manner in the aged mice treated with LBP [120, 121]. This strongly suggested that LBP can regulate the level of oxidation products and the antioxidant enzyme activity. Later, some studies revealed that LBP might delay oxidative stress induced animal ageing [117, 119, 126]. LBP might reduce DNA damage and inhibit the micronucleus rates in the mice’s marrow cells in a dose-dependent manner [122]. Moreover, LBP could reduce the G0/G1 ratio of bone marrow mononuclear cells (BMNC) and inhibit apoptosis of BMNC in mice [126].
LBP could delay senescence as well as prolong the lifespan of zebrafish embryos [116, 118]. It inhibited apoptosis of zebrafish embryos during early development and alleviated the ageing of zebrafish by blocking p53 signalling pathway [116, 118]. It increased the expression of murine double minute 2 (Mdm2) while decreasing the expression of Bax, p21, and p53 gene [116, 118]. On the other side, LBP has been observed to enhance the expression of the telomerase reverse transcriptase (TERT) which regulates and catalyses telomerase activity to maintain and extend the telomere structure of the chromosome in zebrafish embryos [116, 118]. This strongly demonstrates that LBP can alleviate telomeres shortening, a widely knowledgeable key factor associated with the ageing phenomenon, to delay cellular senescence.
Although mouse and zebrafish models are prevalent in LBP research, the effects of LBP have recently been investigated in other in vivo models, such as Drosophila melanogaster [29] and C. elegans [114]. All the experiments confirm that LBP has the ability to extend the average lifespan. Apart from increasing the expression level of antioxidant enzymes and reducing the content of MDA, LBP has also been found to regulate the lifespan through sir2.1, daf-12, and daf-16 [114].
Clinical studies
In line with the in vitro and in vivo studies, clinical studies also demonstrated that LBP as an antioxidant reduced DNA damage effects. The DNA repair rate was significantly improved for those who consumed LBP [122]. Relative to the placebo group, serum SOD and GSH-Px were significantly higher, while MDA decreased in the LBP group [18]. It reveals that LBP has the same effect on humans as on animals. This provides strong evidence for future studies on the anti-ageing effect of LBP in humans.
Collectively, LPB, a potent antioxidant, possesses an anti-ageing potential through regulating the level of oxidation products, the antioxidant enzyme activity, DNA damage, mitochondrial function, and cellular senescence (Fig. 3).
Schematic illustration of anti-ageing effects of LBP in the literature. LBP (Lycium barbarum polysaccharide); Nrf2 (nuclear factor E2-related factor 2); Bach1 (BTB domain and CNC homolog 1); Bcl-2 (B-cell lymphoma 2); GSH-Px (glutathione peroxidase); SOD (superoxide dismutase); CAT (catalase); ROS (reactive oxygen species); DPPH (2,2-Diphenyl-1-picrylhydrazyl); MDA (malondialdehyde); DNA (deoxyribonucleic acid); MDM2 (murine double minute 2); TERT (telomerase reverse transcriptase)
Potential anti-OA effect of LBP
In recent years, LBP proved to have a beneficial effect on OA through its anti-inflammatory effects (Table 2). It was reported that LBP significantly reduced the levels of IL-1 β, TNF-α, iNOS and NF-κB p65 in the supernatant of OA chondrocytes [56]. LBP could upregulate miR-124 to reverse IL-1β induced upregulation of Cox-2 and inflammatory cytokines such as IL-6 and IL-8 [54]. This supported the notion that LBP could inhibit NF- κB signalling pathway and IL-1β evoked inflammatory injury in vitro. When LBP was added to palmitate-induced MC3T3-E1 osteoblast cells, the apoptosis was significantly reduced in a dose-dependent manner [55]. It was revealed that LBP could decrease the expression of Caspase-3, Caspase-9, Caspase-12, GRP78 and CHOP [55]. The above results showed that LBP exerted anti-inflammatory effects by inhibiting the JNK and NF-κB signalling pathways. Moreover, LBP could reduce paw thickness and protect bone integrity [57].
To date, LBP treatment has rarely been applied to OA models in vivo. In the murine collagen type II-induced arthritis model, it was found that LBP decreased the expression of inflammatory mediators TNF-α, IL-6 and IL-17 in a dose-dependent manner. Additionally, it could reduce the expression of matrix-degrading enzymes MMP-1 and MMP-3 [57]. Therefore, LBP can protect the skeletal integrity of mice by reducing inflammation, significantly alleviating collagen type II-induced arthritis in mice.
In brief, LBP potentially exerts an anti-inflammatory effect against age-related OA, which is achieved by inhibiting the JNK and NF-κB signalling pathways as well as by down-regulating inflammatory factors (Fig. 4). The LBP treatment was found to be effective in OA.
Schematic illustration of potential anti-OA effects of LBP in the literature. LBP (Lycium barbarum polysaccharide); TNF-α (tumour necrosis factor-α); iNOS (inducible nitric oxide synthase); NF-κBp65 (nuclear factor kappa-B); OA (osteoarthritis); miR-124 (microRNA-124); IL-1β (interleukin-1β); Cox-2 (cyclooxygenase-2); IL-6 (interleukin-6); IL-8 (interleukin-8); MMP-1 (matrix metalloproteinase-1); MMP-3 (matrix metalloproteinase-3)
Discussion
Osteoarthritis is a degenerative disease accompanied by chronic pain which seriously affects patients’ life quality. There are several factors that promote the onset of OA such as joint injury, ageing and obesity. According to the previous research, cellular apoptosis and abnormal autophagy are also factors that mainly respond to OA occurrence [127,128,129]. In addition, many findings have also studied the key role of metabolism in OA progress [130,131,132]. However, the whole pathogenesis of OA development is still largely unknown at this moment. Current treatment of OA relies on pain killers for pain relief, there are no disease-modifying drugs up till today. This review mainly focused on the pathogenesis of OA caused by ageing, including mitochondrial dysfunction, cellular senescence, and inflammaging [12, 101], trying to find a novel trend for OA therapy.
Recently, researchers tried to specifically block ageing-related hallmarks to prevent joint damage, for example, by removing senescent chondrocytes [94]. This approach showed promising results and might be the new trend for OA therapy. Based on this approach, LBP, an anti-ageing component, could be used to mitigate OA progression. With its anti-oxidizing and anti-inflammatory ability, LBP can lower oxidative stress and inflammation both locally and systemically which can improve OA progression [57]. The beneficial effect of LBP on OA could potentially be achieved through a synergistic interaction of multiple pathways. However, more research is needed to further deepen the knowledge on the senolytic pathways of LBP in OA joints.
To date, LBP has been studied extensively and there is a growing body of evidence indicating that LBP exerted anti-ageing properties. It has been largely studied in the treatment of glaucoma, macular degradation, and other age-related disorders in liver, kidney and heart [17], whereas the role of LBP on OA remains poorly understood. Current understanding of LBP in OA treatment is limited to its anti-inflammatory effects [57]. The effects of LBP on other ageing hallmarks in OA have seldomly been explored. In the existing studies, the underlying mechanism has not been explored, nor have appropriate experimental models been selected. Whether LBP can also be used as a potential treatment drug for OA is still unknown and worth further research.
Here we summarise the characteristics of LBP and OA in terms of ageing, propose a hypothesis of how LBP can improve OA and postulate the Chinese herb, i.e., LBP, as a novel disease-modifying drug for OA therapy.
Collectively, all listed works support the hypothesis that LBP could modify age-related OA and after careful study of the available research the potential underlying mechanism could be identified (Fig. 5). LBP can upregulate Nrf2, induce translocation of Nrf2 from cytoplasm to nucleus, and bind with ARE to promote the expression of antioxidant enzyme genes. Due to the presence of a large number of antioxidant enzymes (SOD, GSH-Px, CAT), ROS are eliminated. Consequently, LBP can alleviate the condition of OA by inhibiting the consequences related to oxidative damage i.e., DNA damage, telomere attrition, mitochondrial dysfunction, cellular senescence and inflammaging. First, LBP may block the p53-induced cellular senescence pathway and inhibit the development of OA by improving the DNA repair levels and protecting telomere integrity. Second, LBP may help to maintain the integrity of articular cartilage by protecting the function of mitochondria and reducing the degradation of the cartilage matrix. Third, LBP may inhibit caspase-3 expression, which is involved in chondrocyte apoptosis, to reverse the OA phenomenon. Fourth, LBP can trigger inflammatory protection mechanisms that reduce inflammatory cytokine levels, thereby relieving the symptoms of OA.
Proposed preventive and therapeutic mechanism of LBP in OA Factors related to anti-ageing effect of LBP are shown in pink; factors that are related to OA but not yet proved to be related to LBP is shown in blue; largely overlapped working mechanism of LBP with pathomechanims of OA (in yellow). It points to a direction that LBP is an emerging disease-modifying drug candidate for OA therapy. LBP (Lycium barbarum polysaccharide); Bach1 (BTB domain and CNC homolog 1); Bcl-2 (B-cell lymphoma 2); GSH-Px (glutathione peroxidase); SOD (superoxide dismutase); CAT (catalase); ROS (reactive oxygen species); DPPH (2,2-Diphenyl-1-picrylhydrazyl); MDA (malondialdehyde); Nrf2 (nuclear factor E2-related factor 2); TNF-α (tumor necrosis factor-α); NO (nitric oxide); DNA (deoxyribonucleic acid); MDM2 (murine double minute 2); TERT (telomerase reverse transcriptase); SA-βgal (Senescence-associated beta-galactosidase); MMP-1 (matrix metalloproteinase-1); MMP-3 (matrix metalloproteinase-3); miR-124 (microRNA-124); IL-1β (interleukin-1β); Cox-2 (cyclooxygenase-2); IL-6 (interleukin-6); IL-8 (interleukin-8); iNOS (inducible nitric oxide synthase); NF-κBp65 (nuclear factor kappa-B); OA (osteoarthritis)
Given the above results, LBP theoretically has a positive effect on OA. Therefore, in the future, the focus will be on how to construct suitable experimental models to study the effect of LBP on OA.
Conclusions
In conclusion, this review clarified the potential interaction between the basic pathological mechanism of OA and the anti-ageing effect of LBP. LBP showed a potential advantage in the treatment of OA due to its impact on multiple signalling pathways leading to lowering oxidative stress, restoring mitochondrial function, mitigating DNA damage, and preventing cellular senescence. Hence, we have a strong reason to believe that LBP, not only has well-documented antioxidant and anti-ageing effects but also may exert beneficial effects on OA treatment.
Availability of data and materials
Not applicable.
Abbreviations
- ATP:
-
Adenosine triphosphate
- ACSCs:
-
Articular cartilage stem cells
- Ad-hMSCs:
-
Adipose tissue-derived human MSCs
- AGEs:
-
Advanced glycation end products
- AMPK:
-
AMP-activated protein kinase
- AOPPs:
-
Advanced oxidation production products
- ARE:
-
Antioxidant response element
- Bach1:
-
BTB domain and CNC homolog 1
- Bcl-2:
-
B-cell lymphoma 2
- bmMSC:
-
Bone marrow-derived mesenchymal stem cells
- BMNC:
-
Bone marrow mononuclear cells
- CAT :
-
Catalase
- CHOP:
-
C/−EBP homologous protein
- Cox-2:
-
Cyclooxygenase-2
- DAMPs:
-
Damage-associated molecular patterns
- DNA :
-
Deoxyribonucleic acid
- DPPH :
-
2,2-Diphenyl-1-picrylhydrazyl
- GPX4:
-
Glutathione Peroxidase 4
- GRP78:
-
78-kDa glucose-regulated protein
- GSH-Px :
-
Glutathione peroxidase
- HMGB1:
-
High-mobility group box 1 protein
- IGF-1:
-
Insulin-like growth factor-1
- IL:
-
Interleukin
- IL-1β:
-
Interleukin-1β
- iNOS:
-
Inducible nitric oxide synthase
- JNK:
-
Jun amino-terminal kinases
- LB:
-
Lycium barbarum
- LBP:
-
Lycium barbarum polysaccharide
- MAPK:
-
Mitogen-activated protein kinases
- MDA:
-
Malondialdehyde
- Mdm2:
-
Murine double minute 2
- miR-124:
-
MicroRNA-124
- MMP:
-
Matrix metalloproteinase
- MRC:
-
Mitochondrial respiratory chain
- MSCs:
-
Mesenchymal stem cells
- mtDNA:
-
Mitochondrial DNA
- NF-κB:
-
nuclear factor kappa B
- NO:
-
Nitric oxide
- Nrf2:
-
Nuclear factor E2-related factor 2
- OA:
-
Osteoarthritis
- ROS:
-
Reactive oxygen species
- SASP:
-
Senescence-associated secretory phenotype
- SA-βGal:
-
Senescence-associated beta-galactosidase
- SIRT1:
-
Sirtuin-1
- SnCs:
-
Senescent cells
- SOD:
-
Superoxide dismutase
- TERT:
-
Telomerase reverse transcriptase
- TGF-β:
-
Transforming growth factor-β
- TNF-α:
-
Tumour necrosis factor-α
References
Anandacoomarasamy A, March L. Current evidence for osteoarthritis treatments. Ther Adv Musculoskelet Dis. 2010;2(1):17–28. https://doi.org/10.1177/1759720X09359889.
Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. 2015;386(9991):376–87. https://doi.org/10.1016/S0140-6736(14)60802-3.
Kapoor M. In: Kapoor M, Mahomed NN, editors. Osteoarthritis: pathogenesis, diagnosis, available treatments, drug safety, regenerative and precision medicine. Cham; 2015.
Verdecchia P, Angeli F, Mazzotta G, Martire P, Garofoli M, Gentile G, et al. Treatment strategies for osteoarthritis patients with pain and hypertension. Ther Adv Musculoskelet Dis. 2010;2(4):229–40. https://doi.org/10.1177/1759720X10376120.
Singh G, Miller JD, Lee FH, Pettitt D, Russell MW. Prevalence of cardiovascular disease risk factors among US adults with self-reported osteoarthritis: data from the Third National Health and Nutrition Examination Survey. Population. 2002;7:17.
Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–56.
Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 2001;56(4):B172–9. https://doi.org/10.1093/gerona/56.4.B172.
Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226–9. https://doi.org/10.1002/art.21562.
崔介君, 孙培龙, 马新. 原花青素的研究进展. 食品科技. 2003(2):92–95.
Poulet B, Veronica U, Stone TC, Pead M, Gburcik V, Beier F, et al. Gene array profiling of articular chondrocytes in mice with different susceptibility to natural disease reveals specific gene signatures linked to healthy ageing and spontaneous OA. Osteoarthr Cartil. 2012;20:S59–60. https://doi.org/10.1016/j.joca.2012.02.025.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. https://doi.org/10.1016/j.cell.2013.05.039.
Loeser R, Collins J, Diekman B. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412–20. https://doi.org/10.1038/nrrheum.2016.65.
Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J Gerontol A Biol Sci Med Sci. 2017;72(6):780–5.
Dai SM, Shan ZZ, Nakamura H, Masuko-Hongo K, Kato T, Nishioka K, et al. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006;54(3):818–31. https://doi.org/10.1002/art.21639.
Maneiro E, Lopez-Armada M, De Andres M, Carames B, Martin M, Bonilla A, et al. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann Rheum Dis. 2005;64(3):388–95. https://doi.org/10.1136/ard.2004.022152.
Fukuda T, Yokoyama J, Ohashi H. Phylogeny and biogeography of the genus Lycium (Solanaceae): inferences from chloroplast DNA sequences. Mol Phylogenet Evol. 2001;19(2):246–58. https://doi.org/10.1006/mpev.2001.0921.
Cheng J, Zhou ZW, Sheng HP, He LJ, Fan XW, He ZX, et al. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des Devel Ther. 2015;9:33–78.
Amagase H, Sun B, Borek C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr Res. 2009;29(1):19–25. https://doi.org/10.1016/j.nutres.2008.11.005.
Kwok S, Bu Y, Lo A, So K, Lai J. A systematic review of potential therapeutic use of Lycium Barbarum polysaccharides in disease. Biomed Res Int. 2019;2019:1–18. https://doi.org/10.1155/2019/4615745.
Nardi G. anuário a, Freire C, Megiolaro F, Schneider K, Perazzoli M, et al. anti-inflammatory activity of berry fruits in mice model of inflammation is based on oxidative stress modulation. Pharm Res. 2016;8(5):42–9.
Olatunji OJ, Chen H, Zhou Y. Anti-Ulcerogenic properties of Lycium chinense mill extracts against ethanol-induced acute gastric lesion in animal models and its active constituents. Molecules. 2015;20(12):22553–64. https://doi.org/10.3390/molecules201219867.
Lu Y, Guo S, Zhang F, Yan H, Qian D-W, Wang H-Q, et al. Comparison of functional components and antioxidant activity of Lycium barbarum L. fruits from different regions in China. Molecules. 2019;24(12):2228.
Cui B, Liu S, Lin X, Wang J, Li S, Wang Q, et al. Effects of lycium barbarum aqueous and ethanol extracts on high-fat-diet induced oxidative stress in rat liver tissue. Molecules. 2011;16(11):9116–28. https://doi.org/10.3390/molecules16119116.
Zhang Q, Chen W, Zhao J, Xi W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016;200:230–6. https://doi.org/10.1016/j.foodchem.2016.01.046.
Locatelli C. Lycium barbarum reduces abdominal fat and improves lipid profile and antioxidant status in patients with metabolic syndrome. Oxidative Med Cell Longev. 2017;2017:9763210. https://doi.org/10.1155/2017/9763210.
Rjeibi I, Feriani A, Ben Saad A, Sdayria J, Saidi I, Ncib S, et al. Lycium europaeum extract: a new potential antioxidant source against cisplatin-induced liver and kidney injuries in mice. Oxidative Med Cell Longev. 2018;2018:1–9. https://doi.org/10.1155/2018/1630751.
Wang CC, Chang SC, Inbaraj BS, Chen BH. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010;120(1):184–92. https://doi.org/10.1016/j.foodchem.2009.10.005.
Xue S, Hu X, Zhu L, Nie L, Li G. Protective functions of Lycium barbarum polysaccharides in H2O2-injured vascular endothelial cells through anti-oxidation and anti-apoptosis effects. Biomed Rep. 2019;11(5):207–14. https://doi.org/10.3892/br.2019.1240.
Tang R, Chen X, Dang T, Deng Y, Zou Z, Liu Q, et al. Lycium barbarum polysaccharides extend the mean lifespan of Drosophila melanogaster. Food Funct. 2019;10(7):4231–41. https://doi.org/10.1039/C8FO01751D.
Shan T, Shan T, Liu F, Zheng H, Li G. Effects of Lycium barbarum polysaccharides on the damage to human endometrial stromal cells induced by hydrogen peroxide. Mol Med Rep. 2017;15(2):879–84. https://doi.org/10.3892/mmr.2016.6080.
Luo Q, Cui X, Yan J, Yang M, Liu J, Jiang Y, et al. Antagonistic effects of Lycium barbarum polysaccharides on the impaired reproductive system of male rats induced by local subchronic exposure to 60Co-γ irradiation. Phytother Res. 2011;25(5):694–701. https://doi.org/10.1002/ptr.3314.
Shi G-J, Zheng J, Wu J, Qiao H-Q, Chang Q, Niu Y, et al. Beneficial effects of Lycium barbarum polysaccharide on spermatogenesis by improving antioxidant activity and inhibiting apoptosis in streptozotocin-induced diabetic male mice. Food Funct. 2017;8(3):1215–26. https://doi.org/10.1039/C6FO01575A.
Varoni MV, Gadau SD, Pasciu V, Baralla E, Serra E, Palomba D, et al. Investigation of the effects of Lycium barbarum polysaccharides against cadmium induced damage in testis. Exp Mol Pathol. 2017;103(1):26–32. https://doi.org/10.1016/j.yexmp.2017.06.003.
Ren F, Fang Q, Feng T, Li Y, Wang Y, Zhu H, et al. Lycium barbarum and Laminaria japonica polysaccharides improve cashmere goat sperm quality and fertility rate after cryopreservation. Theriogenology. 2019;129:29–36. https://doi.org/10.1016/j.theriogenology.2019.02.011.
Yang D, Jq Z, Yf F. Lycium barbarum polysaccharide attenuates chemotherapy-induced ovarian injury by reducing oxidative stress. J Obstet Gynaecol Res. 2017;43(10):1621–8. https://doi.org/10.1111/jog.13416.
Shi G-J, Zheng J, Han X-X, Jiang Y-P, Li Z-M, Wu J, et al. Lycium barbarum polysaccharide attenuates diabetic testicular dysfunction via inhibition of the PI3K/Akt pathway-mediated abnormal autophagy in male mice. Cell Tissue Res. 2018;374(3):653–66. https://doi.org/10.1007/s00441-018-2891-1.
Zhang C, Wang A, Sun X, Li X, Zhao X, Li S, et al. Protective effects of Lycium barbarum polysaccharides on testis Spermatogenic injury induced by bisphenol a in mice. Evid Based Complement Alternat Med. 2013;2013:690808.
Liang B, Peng L, Li R, Li H, Mo Z, Dai X, et al. Lycium barbarum polysaccharide protects HSF cells against ultraviolet-induced damage through the activation of Nrf2. Cell Mol Biol Lett. 2018;23(1):18. https://doi.org/10.1186/s11658-018-0084-2.
Huang B, Zheng W-K, Xu Z-W, Chen Y-P. Impact of Lycium barbarum polysaccharide on apoptosis in Mycoplasma-infected splenic lymphocytes. Trop J Pharma Res. 2017;16(9):2127–33.
Zhao R, Cai Y, Shao X, Ma B. Improving the activity of Lycium barbarum polysaccharide on sub-health mice. Food Funct. 2015;6(6):2033–40. https://doi.org/10.1039/C4FO01108B.
Li J, Ding Z, Yang Y, Mao B, Wang Y, Xu X. Lycium barbarum polysaccharides protect human trophoblast HTR8/SVneo cells from hydrogen peroxide-induced oxidative stress and apoptosis (Report). Mol Med Rep. 2018;18(3):2581.
Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G, et al. Lycium barbarum Polysaccharides protect human lens epithelial cells against oxidative stress-induced apoptosis and senescence. (Research Article). PLoS ONE. 2014;9(10):e110275.
Liu Q, Li Y, Hu L, Wang D. Lycium barbarum polysaccharides attenuate cisplatin-induced hair cell loss in rat Cochlear Organotypic cultures. Int J Mol Sci. 2011;12(12):8982–92. https://doi.org/10.3390/ijms12128982.
Li H, Li Z, Peng L, Jiang N, Liu Q, Zhang E, et al. Lycium barbarum polysaccharide protects human keratinocytes against UVB-induced photo-damage. Free Radic Res. 2017;51(2):200–10. https://doi.org/10.1080/10715762.2017.1294755.
Cao S, Du J, Hei Q. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp Ther Med. 2017;14(5):4919–27. https://doi.org/10.3892/etm.2017.5127.
Xiao J, Zhu Y, Liu Y, Tipoe GL, Xing F, So K-F. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int J Biol Macromol. 2014;69:73–8. https://doi.org/10.1016/j.ijbiomac.2014.05.034.
Ceccarini MR, Vannini S, Cataldi S, Moretti M, Villarini M, Fioretti B, et al. In vitro protective effects of Lycium barbarum berries cultivated in Umbria (Italy) on human hepatocellular carcinoma cells. Biomed Res Int. 2016;2016:7529521.
Feng L, Xiao X, Liu J, Wang J, Zhang N, Bing T, et al. Immunomodulatory effects of Lycium barbarum polysaccharide extract and its uptake behaviors at the cellular level. Molecules. 2020;25(6):1351. https://doi.org/10.3390/molecules25061351.
Feng Z, Jia H, Li X, Bai Z, Liu Z, Sun L, et al. A Milk-based wolfberry preparation prevents prenatal stress-induced cognitive impairment of offspring rats, and inhibits oxidative damage and mitochondrial dysfunction in vitro. Neurochem Res. 2010;35(5):702–11. https://doi.org/10.1007/s11064-010-0123-5.
Cheng D, Kong H. The effect of Lycium Barbarum polysaccharide on alcohol-induced oxidative stress in rats. Molecules. 2011;16(3):2542–50. https://doi.org/10.3390/molecules16032542.
Wang K, Xiao J, Peng B, Xing F, So K-F, Tipoe GL, et al. Retinal structure and function preservation by polysaccharides of wolfberry in a mouse model of retinal degeneration. Sci Rep. 2014;4:7601.
Varoni MV, Pasciu V, Gadau SD, Baralla E, Serra E, Palomba D, et al. Possible antioxidant effect of Lycium barbarum polysaccharides on hepatic cadmium-induced oxidative stress in rats. Environ Sci Pollut Res. 2017;24(3):2946–55. https://doi.org/10.1007/s11356-016-8050-x.
Zheng G, Ren H, Li H, Li X, Dong T, Xu S, et al. Lycium barbarum polysaccharide reduces hyperoxic acute lung injury in mice through Nrf2 pathway. Biomed Pharmacother. 2019;111:733–9. https://doi.org/10.1016/j.biopha.2018.12.073.
Ni H, Wang G, Xu Y, Gu X, Sun C, Li H. Lycium barbarum polysaccharide alleviates IL-1beta-evoked chondrogenic ATDC5 cell inflammatory injury through mediation of microRNA-124. Artif Cells Nanomed Biotechnol. 2019;47(1):4046–52. https://doi.org/10.1080/21691401.2019.1673765.
Jing L, Jia XW. Lycium barbarum polysaccharide arbitrates palmitate-induced apoptosis in MC3T3E1 cells through decreasing the activation of ERSmediated apoptosis pathway. Mol Med Rep. 2018;17(2):2415–21. https://doi.org/10.3892/mmr.2017.8128.
Cai ST, Sun JT, Wei X. Lycium barbarum polysaccharide inhibits NF- κB pathway to reduce the level of inflammatory cytokines in osteoarthritis chondrocytes. Chin J Cell Mol Immunol. 2018;34(11):5.
Liu Y, Lv J, Yang B, Liu F, Tian Z, Cai Y, et al. Lycium barbarum polysaccharide attenuates type II collagen-induced arthritis in mice. Int J Biol Macromol. 2015;78:318–23. https://doi.org/10.1016/j.ijbiomac.2015.04.025.
Gong Z. Development and evaluation of acu-magnetic therapeutic apparel for symptomatic knee osteoarthritis (KOA) relief in the elderly: Institute of Textiles Clothing, Hong Kong Polytechnic University; 2019.
Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr Cartil. 2009;17(8):971–9. https://doi.org/10.1016/j.joca.2009.03.002.
Harman D. The free radical theory of aging: effect of age on serum copper levels. J Gerontol. 1965;20(2):151–3. https://doi.org/10.1093/geronj/20.2.151.
Martin JA, Buckwalter JA. Roles of articular cartilage aging and chondrocyte senescence in the pathogenesis of osteoarthritis. Iowa Orthop J. 2001;21:1–7.
Kuszel L, Trzeciak T, Richter M, Czarny-Ratajczak M. Osteoarthritis and telomere shortening. J Appl Genet. 2015;56(2):169–76. https://doi.org/10.1007/s13353-014-0251-8.
Harbo M, Bendix L, Bay-Jensen A-C, Graakjaer J, Søe K, Andersen TL, et al. The distribution pattern of critically short telomeres in human osteoarthritic knees. Arthritis Res Ther. 2012;14(1):1–9.
Harbo M, Delaisse JM, Kjaersgaard-Andersen P, Soerensen FB, Koelvraa S, Bendix L. The relationship between ultra-short telomeres, aging of articular cartilage and the development of human hip osteoarthritis. Mech Ageing Dev. 2013;134(9):367–72. https://doi.org/10.1016/j.mad.2013.07.002.
Yudoh K, Nguyen VT, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther. 2005;7(2):R380–R91. https://doi.org/10.1186/ar1499.
Martin JA, Klingelhutz AJ, Moussavi-Harami F, Buckwalter JA. Effects of oxidative damage and telomerase activity on human articular cartilage chondrocyte senescence. J Gerontol A Biol Sci Med Sci. 2004;59(4):324–37.
Martin JA, Brown T, Heiner A, Buckwalter JA. Post-traumatic osteoarthritis: the role of accelerated chondrocyte senescence. Biorheology. 2004;41(3–4):479–91.
McCulloch K, Litherland G, Rai T. Cellular senescence in osteoarthritis pathology. Aging Cell. 2017;16:210–8.
Cillero-Pastor B, Caramés B, Lires-Deán M, Vaamonde-García C, Blanco FJ, López-Armada MJ. Mitochondrial dysfunction activates cyclooxygenase 2 expression in cultured normal human chondrocytes. Arthritis Rheum. 2008;58(8):2409–19. https://doi.org/10.1002/art.23644.
Goodwin W, McCabe D, Sauter E, Reese E, Walter M, Buckwalter JA, et al. Rotenone prevents impact-induced chondrocyte death. J Orthop Res. 2010;28(8):1057–63. https://doi.org/10.1002/jor.21091.
Ye W, Zhu S, Liao C, Xiao J, Wu Q, Lin Z, et al. Advanced oxidation protein products induce apoptosis of human chondrocyte through reactive oxygen species-mediated mitochondrial dysfunction and endoplasmic reticulum stress pathways. Fundam Clin Pharmacol. 2017;31(1):64–74. https://doi.org/10.1111/fcp.12229.
Maneiro E, Martín MA, De Andres MC, López-Armada MJ, Fernández-Sueiro JL, Del Hoyo P, et al. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48(3):700–8. https://doi.org/10.1002/art.10837.
Kim J, Xu M, Xo R, Mates A, Wilson GL, Pearsall AW, et al. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthr Cartil. 2009;18(3):424–32. https://doi.org/10.1016/j.joca.2009.09.008.
Li YS, Zhang FJ, Zeng C, Luo W, Xiao WF, Gao SG, et al. Autophagy in osteoarthritis. Joint Bone Spine. 2016;83(2):143–8. https://doi.org/10.1016/j.jbspin.2015.06.009.
Gao T, Guo W, Chen M, Huang J, Yuan Z, Zhang Y, et al. Extracellular vesicles and autophagy in osteoarthritis. Biomed Res Int. 2016;2016:2428915.
Grishko VI, Ho R, Wilson GL, Pearsall AW. Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes. Osteoarthr Cartil. 2009;17(1):107–13. https://doi.org/10.1016/j.joca.2008.05.009.
Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7(3):161–9. https://doi.org/10.1038/nrrheum.2010.213.
Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37(3):614–36. https://doi.org/10.1016/0014-4827(65)90211-9.
Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–22. https://doi.org/10.1016/j.cell.2005.02.003.
Peeper DS, Kuilman T. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9(2):81–94.
He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169(6):1000–11. https://doi.org/10.1016/j.cell.2017.05.015.
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–9. https://doi.org/10.1038/nature16932.
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–6. https://doi.org/10.1038/nature10600.
Martin JA, Ellerbroek SM, Buckwalter JA. Age-related decline in chondrocyte response to insulin-like growth factor-I: the role of growth factor binding proteins. J Orthop Res. 1997;15(4):491–8. https://doi.org/10.1002/jor.1100150403.
van Caam A, Madej W, Thijsen E, Garcia de Vinuesa A, van den Berg W, Goumans M-J, et al. Expression of TGFβ-family signalling components in ageing cartilage: age-related loss of TGFβ and BMP receptors. Osteoarthr Cartil. 2016;24(7):1235–45. https://doi.org/10.1016/j.joca.2016.02.008.
Lepetsos P, Papavassiliou KA, Papavassiliou AG. Redox and NF-κB signaling in osteoarthritis. Free Radic Biol Med. 2019;132:90–100. https://doi.org/10.1016/j.freeradbiomed.2018.09.025.
Choi M-C, Jo J, Park J, Kang HK, Park Y. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells. 2019;8(7):734.
Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im HJ, et al. Articular chondrocytes express the receptor for advanced glycation end products: potential role in osteoarthritis. Arthritis Rheum. 2005;52(8):2376–85. https://doi.org/10.1002/art.21199.
Verzijl N, DeGroot J, Zaken CB, Braun-Benjamin O, Maroudas A, Bank RA, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46(1):114–23. https://doi.org/10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P.
Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1(1):57–65. https://doi.org/10.1046/j.1474-9728.2002.00008.x.
Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritic human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44(6):1304–12. https://doi.org/10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T.
Hoshiyama Y, Otsuki S, Oda S, Kurokawa Y, Nakajima M, Jotoku T, et al. Chondrocyte clusters adjacent to sites of cartilage degeneration have characteristics of progenitor cells. J Orthop Res. 2015;33(4):548–55. https://doi.org/10.1002/jor.22782.
Harbo M, Bendix L, Bay-Jensen A-C, Graakjaer J, Søe K, Andersen TL, et al. The distribution pattern of critically short telomeres in human osteoarthritic knees. Arthritis Res Ther. 2012;14(1):R12.
Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775–81. https://doi.org/10.1038/nm.4324.
Zhou HW, Lou SQ, Zhang K. Recovery of function in osteoarthritic chondrocytes induced by p16INK4a-specific siRNA in vitro. Rheumatology (Oxford). 2004;43(5):555–68. https://doi.org/10.1093/rheumatology/keh127.
Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997;40(11):2065–74. https://doi.org/10.1002/art.1780401120.
Gao SG, Zeng C, Li L, Luo W, Zhang F, Tian J, et al. Correlation between senescence-associated beta-galactosidase expression in articular cartilage and disease severity of patients with knee osteoarthritis. Int J Rheum Dis. 2016;19(3):226–32. https://doi.org/10.1111/1756-185X.12096.
Scanzello CR, Loeser RF. Inflammatory activity in symptomatic knee osteoarthritis: not all inflammation is local. Arthritis Rheumatol. 2015;67(11):2797.
Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64(9):1263–7. https://doi.org/10.1136/ard.2004.025270.
Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. Inhibition of TGF-[beta] signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis.(ARTICLES) (transforming growth factor [beta])(Report). Nat Med. 2013;19(6):704.
Millerand M, Berenbaum F, Jacques C. Danger signals and inflammaging in osteoarthritis. Clin Exp Rheumatol. 2019;37(Suppl 120):48–56.
Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, et al. Identification of senescent cells in the bone microenvironment. J Bone Miner Res. 2016;31(11):1920–9. https://doi.org/10.1002/jbmr.2892.
Attur M, Statnikov A, Aliferis C, Li Z, Krasnokutsky S, Samuels J, et al. Inflammatory genomic and plasma biomarkers predict progression of symptomatic knee OA (SKOA). Osteoarthr Cartil. 2012;20:S34–S5. https://doi.org/10.1016/j.joca.2012.02.562.
Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17(4):359–65. https://doi.org/10.1016/j.coi.2005.06.002.
Tang D, Kang R, Zeh HJ III, Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011;14(7):1315–35. https://doi.org/10.1089/ars.2010.3356.
Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, et al. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 2011;25(20):2125–36. https://doi.org/10.1101/gad.17276711.
Jung J-W, Lee S, Seo M-S, Park S-B, Kurtz A, Kang S-K, et al. Histone deacetylase controls adult stem cell aging by balancing the expression of polycomb genes and jumonji domain containing 3. Cell Mol Life Sci. 2010;67(7):1165–76. https://doi.org/10.1007/s00018-009-0242-9.
Jiang SS, Chen C-H, Tseng K-Y, Tsai F-Y, Wang MJ, Chang IS, et al. Gene expression profiling suggests a pathological role of human bone marrow-derived mesenchymal stem cells in aging-related skeletal diseases. Aging. 2011;3(7):672–84. https://doi.org/10.18632/aging.100355.
Park M, Moon SJ, Baek J, Lee E, Jung K, Kim E, et al. Metformin augments anti-inflammatory and Chondroprotective properties of mesenchymal stem cells in experimental osteoarthritis. J Immunol. 2019;203(1):127–36. https://doi.org/10.4049/jimmunol.1800006.
Tong W, Geng Y, Huang Y, Shi Y, Xiang S, Zhang N, et al. In vivo identification and induction of articular cartilage stem cells by inhibiting NF-κB signaling in osteoarthritis. Stem Cells. 2015;33(10):3125–37. https://doi.org/10.1002/stem.2124.
Ma ZF, Zhang H, Teh SS, Wang CW, Zhang Y, Hayford F, Wang L, Ma T, Dong Z, Zhang Y, Zhu Y. Goji Berries as a Potential Natural Antioxidant Medicine: An Insight into Their Molecular Mechanisms of Action. Oxid Med Cell Longev. 2019;2019:2437397. https://doi.org/10.1155/2019/2437397.
Gao Y, Wei Y, Wang Y, Gao F, Chen Z. Lycium Barbarum: a traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017;8(6):778–91. https://doi.org/10.14336/AD.2017.0725.
Zixuan W, Yaqi L, Huiqing G, Zekun Z, Yajie W, Changhua M. Research Progress on extraction methods of polysaccharide from three varieties of Chinese wolfberry. Food Res Dev. 2019;40(1):7.
Zhang Z, Zhou Y, Fan H, Zhao Y, Zhan X, Yang L, et al. Effects of Lycium barbarum Polysaccharides on health and aging of C. elegans depend on daf-12/daf-16. Oxid Med Cell Longevity. 2019;2019. https://doi.org/10.1155/2019/6379493.
Yang L, Gao Z, Lv Q, Zhao Q, Li L, Cao X, et al. Lycium barbarum polysaccharide enhances development of previously-cryopreserved murine two-cell embryos via restoration of mitochondrial function and down-regulated generation of reactive oxygen species. J Reprod Dev. 2019;65(2):163–70. https://doi.org/10.1262/jrd.2018-104.
Xia G, Xin N, Liu W, Yao H, Hou Y, Qi J. Inhibitory effect of Lycium barbarum polysaccharides on cell apoptosis and senescence is potentially mediated by the p53 signaling pathway (Report). Mol Med Rep. 2014;9(4):1237.
Yi R, Liu X-M, Dong Q. A study of Lycium barbarum polysaccharides (LBP) extraction technology and its anti-aging effect. Afr J Tradition Complement Altern Med. 2013;10(4):171–4.
G. Xia, J. Zhu, J. Song, J. Qin, X. Han and W. Liu, "Effect of Astragalus, Ganoderm A Lucidum and Lycium Polysacchride on Zebrafish Senescence," 2012 International Conference on Biomedical Engineering and Biotechnology. 2012;1010-11. https://doi.org/10.1109/iCBEB.2012.166.
Shan X, Zhou J, Ma T, Chai Q. Lycium barbarum polysaccharides reduce exercise-induced oxidative stress. Int J Mol Sci. 2011;12(2):1081–8. https://doi.org/10.3390/ijms12021081.
Liang B, Jin M, Liu H. Water-soluble polysaccharide from dried Lycium barbarum fruits: isolation, structural features and antioxidant activity. Carbohydr Polym. 2011;83(4):1947–51. https://doi.org/10.1016/j.carbpol.2010.10.066.
Li XM, Ma YL, Liu XJ. Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. J Ethnopharmacol. 2007;111(3):504–11. https://doi.org/10.1016/j.jep.2006.12.024.
Li B, Yang C, Zou Z, Fu G, Yu J, Wang J. Study of the anti-mutagenic effect of the crude Lycium barbarum polysaccharide. Occup Health. 2006;22(11):3.
Hong-Bin D, Da-Peng CUI, Jian-Ming J, Yan-Chun F, Nian-Sheng CAI, Dian-Dong LI. Inhibiting Effects of Achyranthes Bidentata Polysaccharide and Lycium Barbarum Polysaccharide on Nonenzyme Glycation in D-galactose Induced Mouse Aging Model. Biomed Environ Sci. 2003;16(3):267–75.
Zhang L, Gu J, Chen Y, Zhang L. A study on four antioxidation effects of lycium barbarum polysaccharides in vitro. Afr J Tradit Complement Altern Med. 2013;10(6):494–8. https://doi.org/10.4314/ajtcam.v10i6.18.
Pan H, Zhao Y, Wang N. Pretreatment of Lycium barbarum polysaccharide reduces H 2 O 2 -induced myocardial apoptosis. Int J Clin Exp Med. 2017;10(5):7981–8.
Zhou J, Pang H, Li W, Liu Q, Xu L, Liu Q, et al. Effects of Lycium barbarum polysaccharides on apoptosis, cellular adhesion, and oxidative damage in bone marrow mononuclear cells of mice exposed to ionizing radiation injury. Biomed Res Int. 2016;2016:4147879.
Hyelin Jeon & Gun-Il Im. Autophagy in osteoarthritis, Connective Tissue Research. 2017;58(6):497-508. https://doi.org/10.1080/03008207.2016.1240790.
Almonte-Becerril M, Navarro-Garcia F, Gonzalez-Robles A, Vega-Lopez MA, Lavalle C, Kouri JB. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of osteoarthritis within an experimental model. Apoptosis. 2010;15(5):631–8. https://doi.org/10.1007/s10495-010-0458-z.
Zhang Y, Vasheghani F, Li Y-H, Blati M, Simeone K, Fahmi H, et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann Rheum Dis. 2015;74(7):1432–40. https://doi.org/10.1136/annrheumdis-2013-204599.
Choi W-S, Lee G, Song W-H, Koh J-T, Yang J, Kwak J-S, et al. The CH25H–CYP7B1–RORα axis of cholesterol metabolism regulates osteoarthritis. Nature. 2019;566(7743):254–8. https://doi.org/10.1038/s41586-019-0920-1.
O’Donnell C, Migliore E, Grandi FC, Koltsov J, Lingampalli N, Cisar C, et al. Platelet-rich plasma (PRP) from older males with knee osteoarthritis depresses chondrocyte metabolism and upregulates inflammation. J Orthop Res. 2019;37(8):1760–70. https://doi.org/10.1002/jor.24322.
Attur MG, Palmer GD, Al-Mussawir HE, Dave M, Teixeira CC, Rifkin DB, et al. F-spondin, a neuroregulatory protein, is up-regulated in osteoarthritis and regulates cartilage metabolism via TGF-β activation. FASEB J. 2009;23(1):79–89. https://doi.org/10.1096/fj.08-114363.
Acknowledgements
The authors would like to sincerely acknowledge the contribution of Dr. Marianne Lauwers for her professional English language editing service to this manuscript.
Funding
This work was supported by Research Grants Council of Hong Kong Early Career Scheme (PolyU 251008/18 M), PROCORE-France/Hong Kong Joint Research Scheme (F-PolyU504/18) and also Health and Medical Research Fund Scheme (01150087#, 15161391#, 16172691#). The funders had no role in study design and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
CYW, XLW and HKK conceived this review. JGN conducted literature search and systemic review. All the authors participated in the analysis and interpretation of the data. JGN, MTA and CYW prepared the draft of the manuscript, which was revised by HKK. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest relevant to this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ni, J., Au, M., Kong, H. et al. Lycium barbarum polysaccharides in ageing and its potential use for prevention and treatment of osteoarthritis: a systematic review. BMC Complement Med Ther 21, 212 (2021). https://doi.org/10.1186/s12906-021-03385-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-021-03385-0