Abstract
Background
Dysregulation of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) form the basis of immune-mediated inflammatory diseases. Vitex trifolia L. is a medicinal plant growing in countries such as China, India, Australia and Singapore. Its dried ripe fruits are documented in Traditional Chinese Medicine to treat ailments like rhinitis and dizziness. Its leaves are used traditionally to treat inflammation-related conditions like rheumatic pain.
Objective
This study aimed to investigate the effects of V. trifolia leaf extracts prepared by different extraction methods (Soxhlet, ultrasonication, and maceration) in various solvents on cytokine production in human U937 macrophages, and identify phytoconstituents from the most active leaf extract.
Methods
Fresh leaves of V. trifolia were extracted using Soxhlet, ultrasonication, and maceration in hexane, dichloromethane, methanol, ethanol or water. Each extract was evaluated for its effects on TNF-α and IL-1β cytokine production by enzyme-linked immunosorbent assay in lipopolysaccharide-stimulated human U937 macrophages. The most active extract was analyzed and further purified by different chemical and spectroscopic techniques.
Results
Amongst 14 different leaf extracts investigated, extracts prepared by ultrasonication in dichloromethane and maceration in ethanol were most active in inhibiting TNF-α and IL-1β production in human U937 macrophages. Further purification led to the isolation of artemetin, casticin, vitexilactone and maslinic acid, and their effects on TNF-α and IL-1β production were evaluated. We report for the first time that artemetin suppressed TNF-α and IL-1β production. Gas chromatography-mass spectrometry analyses revealed the presence of eight other compounds. To the best of our knowledge, this is the first report of butylated hydroxytoluene, 2,4-di-tert-butylphenol, campesterol and maslinic acid in V. trifolia leaf extracts.
Conclusions
In conclusion, leaf extracts of V. trifolia obtained using different solvents and extraction methods were successfully investigated for their effects on cytokine production in human U937 macrophages. The findings provide scientific evidence for the traditional use of V. trifolia leaves (a sustainable resource) and highlight the importance of conservation of medicinal plants as resources for drug discovery. Our results together with others suggest further investigation on V. trifolia and constituents to develop novel treatment strategies in immune-mediated inflammatory conditions is warranted.
Similar content being viewed by others
Background
Inflammation, the body’s natural defence mechanism against injury and infection, is harmful when excessive. Chronic inflammation is known as the driving force of the pathogenesis of immune-mediated inflammatory diseases such as inflammatory bowel disease, rheumatoid arthritis and psoriasis [1]. Although distinct in their clinical manifestations, these immune-mediated inflammatory diseases share common pathogenesis pathways, one of which is the dysregulation of cytokines, for example the overproduction of pro-inflammatory cytokines TNF-α and IL-1β [2, 3]. Current treatment approaches of inflammation include the use of non-steroidal anti-inflammatory drugs and glucocorticoids, as well as novel biologic agents that target specific molecules such as TNF-α and IL-1β [4,5,6]. However, long-term use of existing repertoire of drugs is accompanied with risks of serious adverse side effects, including opportunistic infections, malignancies, anaphylactic reactions, iatrogenic Cushing’s syndrome and osteroporosis [5]. Moreover, novel biologic agents targeting specific molecules though effective come with high costs. Hence, the need to discover alternative anti-inflammatory compounds remains relevant.
Medicinal plants have been documented to treat diverse human ailments for thousands of years and offer a rich resource of novel therapeutics. Vitex trifolia L. var. trifolia (V. trifolia) from the Verbenaceae family is a deciduous plant mainly found in the coastal areas of Pacific-Asian regions including countries such as China, India, Australia and Singapore [7]. Commonly known as Panikisanbhalu (Hindi), three-leaf chaste tree (English) and 三叶蔓荆 (Chinese), V. trifolia is traditionally used for various inflammatory ailments. Dried ripe fruits of V. trifolia (also known as Fructus viticis) are well documented in Traditional Chinese Medicine to treat ailments like inflammation of the eye, headache, blurred vision, rhinitis, and common cold [8, 9]. Leaves of V. trifolia are also used in traditional medicine to treat inflammatory conditions, such as ciguatera fish poisoning in the Pacific region [10]. The leaves are traditionally made into decoction for oral inflammation, or externally applied as a poultice for rheumatic pain and sprains [11]. The flowers are administered orally as infusion for treating intermittent fever accompanied by vomiting and thirst, while the stems are used for dysentery [11, 12]. The roots are used as antiemetic, expectorant and believed to help relieve fever [13, 14]. Several phytochemicals reported in the leaf extracts of V. trifolia include flavonoids, such as casticin [15], vitexin [16] and luteolin [17], and terpenes such as eucalyptol and caryophyllene [18]. A number of studies have been published on the anti-inflammatory effects of V. trifolia leaf extracts using various rat experimental models, such as Carrageenan induced paw edema rat model [19,20,21,22], and RAW264.7 mouse cell lines induced with lipopolysaccharide [10, 11, 23]. These studies focused on investigating aqueous and ethanol leaf extracts prepared using decoction, Soxhlet or maceration. There is limited information on the different extraction methods and solvents on V. trifolia, as well as on its inflammatory activity in human macrophages. Human monocytic cell lines such as U937 differentiated with inflammatory stimuli like phorbol 12-myristate 13-acetate (PMA) offer a model for studying macrophage function [24,25,26]. We have previously reported in an ethnobotanical survey that V. trifolia was one of the fresh medicinal plants commonly used in Singapore [27] and leaf extracts of V. trifolia prepared by different extraction methods and solvents exhibited promising anti-proliferative activity in multiple cancer cell lines [28]. This study aimed to investigate the effects of various V. trifolia leaf extracts prepared by different extraction methods namely Soxhlet, ultrasonication, and maceration in various solvents, on cytokine production in PMA-differentiated U937 macrophages, and to isolate and identify phytoconstituents from the most active leaf extract.
Methods
Reagents
Analytical grade solvents (acetone, dichloromethane, ethanol, ethyl acetate, hexane and methanol) and HPLC-grade methanol and acetonitrile were purchased from Tedia (Fairfield, USA). Water was processed by Milli-Q filter (Millipore Corporation, Billerica, USA). Phorbol 12-myristate 13-acetate (PMA) and lipopolysaccharide (LPS) were from Sigma-Aldrich (USA), dexamethasone, dimethyl sulfoxide (DMSO) were from Sigma-Aldrich (USA), Merck (USA), Hospira (Australia) and MP Biomedical Inc. (USA) respectively. Chemical standards for artemetin, casticin and vitexilactone were from ChemFaces (China), while α-amyrin, β-amyrin, butylated hydroxytoluene (BHT), campesterol, 2,4-Di-tert-butylphenol, maslinic acid, phytol, β-sitosterol, and stigmasterol were from Sigma-Aldrich (USA).
Plant source and preparation of plant extracts
Fresh, healthy and mature leaves of V. trifolia were harvested from Singapore (Leeward Pacific Pte. Ltd) for extraction. A voucher specimen of V. trifolia (VT-0101) was deposited at the Department of Pharmacy Herbarium in National University of Singapore. The plant was identified by Mr. Lua Hock Keong from National Parks Board and by checking with The Plant List [29] and identified with reference to the “World Checklist of Selected Plant Families” [30]. Leaves were washed, air dried and blended using a dry grinder, and extracted using Soxhlet, ultrasonication or maceration in hexane, dichloromethane, 70% v/v methanol, 70% v/v ethanol and water. The extracts were concentrated under vacuum and stored at 25 °C.
Isolation of chemical constituents from V. trifolia leaf extracts
The dried maceration ethanol crude leaf extract was dissolved in water and partitioned with n-hexane, dichloromethane, and butanol; these fractions were analyzed for their effects on cell viability and cytokine production. The resultant dichloromethane fraction was then subjected to column chromatography over silica gel 60 using hexane, dichloromethane and methanol to give sub-purified fraction A1, which was also analyzed for its effects on cell viability and cytokine production.
The ultrasonication dichloromethane crude leaf extract was subjected to column chromatography over silica gel 60 using hexane, dichloromethane and methanol to yield various fractions. One fraction was subjected to semi-preparative HPLC (Agilent 1260 Series HPLC System, Agilent Technologies; ZORBAX SB-C18 column (5 μm, 9.4 × 250 mm; flow rate, 2 ml/min; temperature, 25 °C; detection, UV absorption at 254 nm). For the mobile phase, an initial gradient of 5% acetonitrile and 95% water was set and increased to 20% acetonitrile and 80% water for 5 min, followed by 40% acetonitrile and 60% water for 5 min, and then to 100% acetonitrile for 20 min and finally held at 100% acetonitrile for 15 min to isolate casticin, artemetin and vitexilactone. Maslinic acid was purified on silica gel column using dichloromethane:methanol (99:1) as elution solvent.
Chemical analyses using gas chromatography-mass spectrometry (GC-MS), nuclear magnetic resonance (NMR) spectroscopy, and liquid chromatography-mass spectrometry (LC-MS)
Concentrations of 5 mg/mL for leaf extract or fractions, or 0.1 mg/mL for commercial standards, were prepared and 1 μL was injected into the GC-MS (Agilent 7890 gas chromatograph with 5975C MSD, USA) with splitless injection mode at 230 °C. An Agilent DB-5MS column (30 m × 0.025 μm, 0.25 mm internal diameter, 0.25 μm film thickness) was used with helium carrier gas. The extracts and fractions were analyzed with a temperature program of an initial temperature of 80 °C for 6 min and increased at a rate of 2 °C/min to 280 °C, which was maintained for 10 min before the run ended. Compounds separated from GC-MS were identified using National Institute of Standards and Technology (NIST) Mass Spectral library versions 27 and 247 (NIST, USA) and Wiley Mass Spectral Database Version 7 (John Wiley & Sons, USA).
The NMR spectra were obtained using a Bruker DRX 400 MHz spectrometer 1H at 400 MHz; 13C at 100 MHz (Fallanden, Switzerland) in deuterated chloroform and tetramethylsilane as an internal reference.
LC-MS of isolated compounds and standards at 0.1 mg/mL were performed using a 2000 QTRAP (Applied Biosystems, Foster City, USA). Electrospray ionization mass spectra (ESI-MS) were recorded in positive and negative modes. The HPLC (Agilent-1100 LC Binary, Santa Clara, USA) was programmed with an isocratic gradient solvent system of 98% acetonitrile and 2% water, and a flow rate of 200 μL/min for 5 min. The mass-per-charge ratios (m/z) scanned ranged from 50 to 1000.
General cell culture
The human monocytic cell line U937 (CRL-1593.2; ATCC) was cultured in RPMI-1640 medium (ThermoScientific, USA) supplemented with 10% v/v heat-inactivated fetal bovine serum (ThermoScientific, USA) in 5% v/v CO2 incubator at 37 °C in a humidified atmosphere.
For macrophage differentiation, U937 cells grown in in RPMI-1640 medium supplemented with 2% v/v fetal bovine serum were treated with 5 ng/mL PMA for 24 h, after which the cells were washed with PBS [24,25,26]. It is well known that excessive use of PMA in differentiating the cells could induce genetic over-expression and this may potentially mask the effects induced by plant extracts. We have looked at the effect of different PMA concentrations selected from published literature [24,25,26] in the presence and absence of LPS stimulation on cytokine production, and we found that PMA at 5 ng/mL was able to ensure optimal monocyte differentiation and induce cytokine production. These PMA-differentiated U937 macrophages were also referred to as U937 macrophages in this study.
The leaf extracts and chemical standards were dissolved in DMSO and diluted to the desired concentration before addition to cells. The concentration of DMSO in these dilutions was restricted to no more than 0.4% to minimize potential effects of the solvent on cell growth. To examine the effect of leaf extract, fraction, or standard compound, U937 macrophages were incubated for 6 h with the appropriate agent, and then activated with 50 ng/mL LPS overnight. For investigation of BHT effect on cytokine production, U937 macrophages were incubated with BHT for 6 h. To examine the effect of MCC950 or sulfasalazine on BHT-induced cytokine level, U937 macrophages were incubated with MCC950 or sulfasalazine for 24 h, washed with PBS, and incubated with BHT for 6 h. Cell supernatant was collected and measured for the cytokine level by enzyme-linked immunosorbent assay (ELISA).
Determination of cell viability by WST-1 assay
Exponentially growing cells were plated in 96-well plates at 3 × 104 cells/100 μL in RPMI medium supplemented with 2% v/v fetal bovine serum, treated with PMA for 24 h to differentiate into macrophages and washed with PBS. These differentiated U937 macrophage cells were treated with the appropriate agent (either crude extract, fraction, compound, or DMSO) for 48 h. Untreated differentiated cells were used as controls. After 48 h, the media was aspirated and replaced with 10% v/v WST-1 (Roche, Switzerland) for 1 h. The formazan dye produced was quantified at 440 nm against a reference wavelength of 650 nm using a microplate reader (Tecan infinite M200 PRO, Switzerland). Cell viability was expressed as a percentage of the control cells. The IC50 value from cell viability assay was used as a parameter for anti-proliferative potency [31,32,33] while the IC20 value was taken as an indicator for non-toxic dose of test sample [32, 34]. The IC50 and IC20 values were determined using GraphPad Prism 5 (GraphPad Software, Inc., USA). The results were generated from three independent experiments and each experiment was performed in 5 replicates.
Evaluation of cytokine production by ELISA assay
Cells were plated in 6-well plates (Costar, USA) at 1 × 106 cells per well in RPMI medium supplemented with 2% fetal bovine serum, treated with PMA for 24 h to differentiate into macrophages and washed with PBS. These differentiated cells were incubated with the appropriate extracts, fractions or compounds as described above. Cell supernatant was collected and analysed for cytokine level using a two-site sandwich ELISA kit from Quantikine (Minneapolis, USA) according to manufacturer’s instructions. Briefly, standards and samples were added to wells pre-coated with antibodies for 2 h, washed, and incubated with cytokine conjugate for 1 h. After washing, substrate solution was added for 20 min, followed by stop solution. The cytokine level present was quantified at 450 nm against a reference wavelength of 540 nm using a microplate reader (Tecan infinite M200 PRO, Switzerland) and absolute concentrations of cytokines were interpolated from their respective standard curves. Standard curves were achieved using standard concentrations of the human IL-1β and TNF-α based on instructions in the Quantikine (Minneopolis, USA) kits. The results were generated from three independent experiments.
Statistical analysis
Statistical analyses were performed by Statistical Package for the Social Sciences (International Business Machines Corporation, USA). Welch Analysis of Variances (ANOVA) followed by Games-Howell post-hoc test were used. A p value < 0.05 was considered significant (denoted as *).
Results
Evaluation of V. trifolia crude leaf extracts for cytokine production
Crude leaf extracts of V. trifolia prepared by Soxhlet, ultrasonication, and maceration in various solvents (hexane, dichloromethane, methanol, ethanol, or water) were first evaluated for their potential cytotoxicity in PMA-differentiated U937 macrophages using WST-1 cell viability assay. Visually, unstimulated U937 cells grew as single cell suspension, while PMA-treated U937 cells adhered tightly to the plastic culture plate, showed some cellular aggregation and appeared macrophage-like. The mean IC20 and IC50 values of the crude leaf extracts, which refer to the concentrations of extracts required to inhibit 20 and 50% growth of the differentiated cells respectively, are shown in Table 1. Generally, the IC50 values of methanol, ethanol and water leaf extracts were relatively higher than those of hexane and dichloromethane leaf extracts, regardless of extraction method. Among the different extraction methods using methanol, ethanol and water as solvents, maceration methanol leaf extract showed the smallest IC50 of 84.8 ± 6.6 μg/mL, while Soxhlet water leaf extract exhibited the largest IC50 value of 684.5 ± 99.0 μg/mL (Table 1). Among the different extraction methods using hexane and dichloromethane as solvents, ultrasonication dichloromethane leaf extract showed the smallest IC50 of 3.2 ± 0.1 μg/mL while Soxhlet dichloromethane extract displayed the largest IC50 of 47.6 ± 0.7 μg/mL (Table 1).
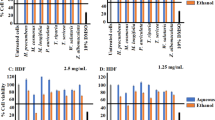
Figure 1 shows in the absence of any treatment, U937 cells produced very low levels of TNF-α and IL-1β (Fig. 1a and b). Treatment with PMA significantly elevated the production of TNF-α and IL-1β in U937 macrophages. Stimulation of PMA-differentiated cells (also referred here as U937 macrophages) with LPS further doubled the production of both TNF-α and IL-1β compared to PMA treatment only (p < 0.05) (Fig. 1a and b). These levels of cytokine secretion are consistent to published reports using U937 as a model system [24,25,26]. As expected, the increased TNF-α and IL-1β levels were abolished upon pre-incubation of cells with dexamethasone (p < 0.05) (Fig. 1a and b), a corticosteroid known to alleviate inflammatory conditions. A criterion for investigating the inflammatory effects of the leaf extracts was that the concentration of leaf extracts used should be the highest one in which the cells remained viable. We chose to use the IC20 values of the extracts [32,33,34], which represented the concentration at which at least 80% of the cell population was alive. Based on Table 1 and our observations of cells treated with different concentrations of extracts, each extract was assayed at 100 μg/mL (for methanol, ethanol and water extracts) or 2 μg/mL (for hexane and dichloromethane extracts) to determine the levels of TNF-α and IL-1β in the supernatant by ELISA.
Effects of V. trifolia crude leaf extracts on TNF-α and IL-1β production by human U937 macrophages. a, b Absolute production of TNF-α (a) and (b) IL-1β by human U937 cells in the presence or absence of PMA, LPS and dexamethasone. c-f Fold change of (c, e) TNF-α and (d, f) IL-1β production relative to control in the supernatant of human U937 macrophages. Cells were pre-incubated for 6 h with 100 μg/mL (c, d) or 2 μg/mL (e, f) of the appropriate crude leaf extract, followed by LPS stimulation. Sox, Soxhlet; Ult, ultrasonication; Mac, maceration; W, water; E, ethanol; M, methanol; D, dichloromethane; H, hexane. Cytokine production by cells not treated with any extract (ie. none) was taken as 1. Dexamethasone (Dex) at 64.4 ng/mL was used as positive control. The data are presented as mean fold change ± SD of three independent experiments performed in duplicates. *p < 0.05
We first studied the methanol, ethanol and water leaf extracts prepared by Soxhlet, ultrasonication, or maceration on cytokine levels in U937 macrophages. Pre-treatment of U937 macrophages with all except Soxhlet water leaf extract significantly inhibited the production of TNF-α and IL-1β by 60–80% compared to untreated macrophages (p < 0.05) (Fig. 1c and d). Maceration ethanol leaf extract was overall most effective in suppressing TNF-α and IL-1β production in U937 macrophages amongst the extracts investigated, while Soxhlet water leaf extract was least effective (Fig. 1c and d).
We next studied the hexane and dichloromethane leaf extracts prepared by Soxhlet, ultrasonication, or maceration. Pre-treatment of U937 macrophages with the ultrasonication dichloromethane leaf extracts significantly inhibited TNF-α production by 40% compared to untreated macrophages (p < 0.05), while the other leaf extracts did not significantly affect TNF-α level at the concentration tested (Fig. 1e). Pre-treatment of U937 macrophages with all except Soxhlet dichloromethane leaf extract significantly inhibited IL-1β production by 40–80% compared to untreated macrophages (p < 0.05) (Fig. 1f). Ultrasonication dichloromethane leaf extract was overall most effective in suppressing TNF-α and IL-1β levels amongst these extracts, while Soxhlet dichloromethane leaf extract was least effective (Fig. 1e and f).
We further examined the effects of varying concentrations of ultrasonication dichloromethane leaf extract and maceration ethanol leaf extract on cytokine production. The ultrasonication dichloromethane leaf extract reduced TNF-α and IL-1β levels in a concentration-dependent manner, with much smaller IC50 values of 4.7 ± 0.9 μg/mL and 1.2 ± 0.2 μg/mL respectively (Fig. 2a and b). The maceration ethanol leaf extract also inhibited TNF-α and IL-1β level in a concentration-dependent manner, with IC50 values of 43.6 ± 3.9 μg/mL and 29.2 ± 2.3 μg/mL respectively (Fig. 2c and d). Given the effectiveness of both maceration ethanol and ultrasonication dichloromethane leaf extracts, we chose both extracts for further investigation to identify the active phytoconstituents.
Effects of crude leaf extracts and purified fractions of V. trifolia on TNF-α and IL-1β production by human U937 macrophages. Fold change of TNF-α (a, c, e, g) and IL-1β (b, d, f, h) production relative to control in the supernatant of human U937 macrophages measured by ELISA. Cells were pre-incubated for 6 h with the appropriate extracts or fractions (10 μg/mL in (e, f); 2 μg/mL in (g, h)), followed by LPS stimulation. Fractions in (e, f) were purified from maceration ethanol crude extract, while A1 fraction was purified from the dichloromethane fraction in (e, f). Cytokine production by cells not treated with any extract or fraction (ie. none) was taken as 1. Dexamethasone (Dex) at 64.4 ng/mL was used as a positive control. The data are presented as mean fold change ± SD of three independent experiments performed in duplicates. *p < 0.05
Isolation and identification of compounds in V. trifolia leaf extracts
Gas chromatography-mass spectrometry (GC-MS) analysis was performed on both V. trifolia maceration ethanol and ultrasonication dichloromethane crude leaf extracts to characterise their components. Preliminary identification of each compound was based on comparing the mass spectrometric data with the NIST and WILEY reference libraries. The identities of eight putative compounds were confirmed by comparison with the mass spectrometric data of commercial standards, and they are: butylated hydroxytoluene (BHT), 2,4-di-tert-butylphenol, phytol, campesterol, stigmasterol, β-sitosterol, β-amyrin, and α-amyrin (Fig. 3).
To investigate the active constituents responsible for TNF-α and IL-1β inhibition observed, maceration ethanol crude leaf extract was partitioned using hexane, dichloromethane, n-butanol and water. These fractions were evaluated for their effect on cell viability in U937 macrophages and the results are presented in Table 2. Among these four fractions, dicloromethane fraction showed the smallest IC50 of 21.1 ± 1.0 μg/mL, while water fraction displayed the largest IC50 of 3900.0 ± 450.0 μg/mL (Table 2). The IC50 for dicloromethane fraction (21.1 ± 1.0 μg/mL) was about four times less than the IC50 for maceration ethanol crude leaf extract (84.8 ± 6.6 μg/mL). We next evaluated the effect of pre-treatment of U937 macrophages with these fractions on TNF-α and IL-1β levels, and found that dichloromethane fraction significantly suppressed TNF-α and IL-1β levels by 80% compared to untreated cells (p < 0.05), whereas the other fractions did not show any appreciable difference (Fig. 2e and f).
Purification of dichloromethane fraction over column chromatography yielded a sub-purified fraction A1 that, when tested for its effect on cell viability in U937 macrophages, gave an IC50 of 6.7 ± 0.1 μg/mL, This is about three times lower than IC50 of dichloromethane fraction (21.1 ± 1.0 μg/mL). The sub-purified fraction A1 at 2 μg/ml significantly suppressed the production of TNF-α and IL-1β by almost 80% (p < 0.05) (Fig. 2g and h). In contrast, the dichloromethane fraction at 2 μg/ml showed no observable effect on TNF-α and IL-1β production (Fig. 2g and h). Interestingly, this sub-purified fraction A1 was similar in activity as the ultrasonication dichloromethane crude leaf extract, in terms of its effects on TNF-α and IL-1β production and cytotoxicity in U937 macrophages. Since the ultrasonication dichloromethane crude leaf extract had comparatively more yield, we chose it for subsequent studies.
Further purification of the ultrasonication dichloromethane crude leaf extract led to the isolation and identification of casticin (or vitexicarpin), artemetin, vitexilactone, and maslinic acid (Fig. 3). The chemical structures of these isolated compounds were confirmed by comparing NMR and LC-MS data with published data and their respective commercial standards [35,36,37,38]. Taken together, 12 compounds were identified in V. trifolia leaf extract. To the best of our knowledge, this is the first report of BHT, 2,4-di-tert-butylphenol, campesterol and maslinic acid in leaf extracts of V. trifolia.
Evaluation of compounds for cell viability and cytokine production in U937 macrophages
Artemetin, casticin, vitexilactone, maslinic acid and BHT were first investigated for their effects on cell viability in U937 macrophages. Artemetin, maslinic acid and BHT inhibited the growth of these cells with IC50 values 125.6 ± 15.3 μg/mL (323.4 ± 39.3 μM), 108.8 ± 4.7 μg/mL (230.2 ± 9.9 μM), and 17.0 ± 0.2 μg/mL (77.1 ± 0.9 μM) respectively. The IC20 values were: artemetin 109.5 ± 14.1 μg/mL (281.9 ± 36.3 μM), maslinic acid 64.6 ± 3.0 μg/mL (136.7 ± 6.3 μM), and BHT 14.0 ± 1.5 μg/mL (63.5 ± 6.8 μM). Casticin and vitexilactone did not show any appreciable effect on cell viability of U937 macrophages up to concentrations of 200 μg/mL.
We next evaluated these compounds for their effects on TNF-α and IL-1β production in U937 macrophages. Artemetin at 50 μg/mL and 100 μg/mL reduced TNF-α level to 20 and 30% respectively (Fig. 4a), and its IC50 value of inhibitory effect on TNF-α could not be determined at the highest concentration of 100 μg/mL achievable. The reduction in TNF-α levels by artemetin at 50 μg/mL and 100 μg/mL were not due to decrease in cell viability as we did not observe any significant difference in cell viability at the same concentrations. Artemetin at 50 μg/mL significantly reduced IL-1β level to 60% and the reduction in IL-1β level was concentration-dependent, with an IC50 value (inhibitory effect on IL-1β) of 65.5 ± 7.4 μg/mL (168.6 ± 19.0 μM) (Fig. 4b). Casticin significantly inhibited TNF-α level, with an IC50 value (inhibitory effect on TNF-α) of 7.1 ± 0.3 μg/mL (19.0 ± 0.8 μM) (Fig. 4c). Casticin did not have an appreciable effect on IL-1β level at 2 μg/mL; however casticin at 200 μg/mL reduced IL-1β to nearly 50% (Fig. 4d); its IC50 value of inhibitory effect on IL-1β could not be determined at the highest concentration of 200 μg/mL tested. Vitexilactone at 20 μg/mL reduced TNF-α level by 30% and a more significant inhibition was observed at higher concentration with an IC50 value (inhibitory effect on TNF-α) of 37.5 ± 2.8 μg/mL (99.1 ± 7.4 μM) (Fig. 4e). Similarly, vitexilactone at 20 μg/mL significantly reduced IL1-β level (p < 0.05) and further reduction in IL-1β level was observed at higher concentration, with an IC50 value (inhibitory effect on IL-1β) of 80.6 ± 9.2 μg/mL (212.9 ± 24.3 μM) (Fig. 4f). Maslinic acid suppressed TNF-α level in a concentration-dependent, with an IC50 value (inhibitory effect on TNF-α) of 27.6 ± 1.7 μg/mL (58.4 ± 3.6 μM) (Fig. 4g). On the other hand, maslinic acid significantly increased IL-1β level by 1.7-fold (p < 0.05) at 65 μg/mL (Fig. 4h).
Effects of artemetin, casticin, vitexilactone and maslinic acid on TNF-α and IL-1β production by human U937 macrophages. Fold change of TNF-α (a, c, e, g) and IL-1β (b, d, f, h) production relative to control in the supernatant of human U937 macrophages measured by ELISA. Cells were treated with none or varying concentrations of artemetin (a, b), casticin (c, d), vitexilactone (e, f), and maslinic acid (g, h) for 6 h, followed by LPS stimulation. Cytokine production by cells not treated with compound (ie. none) was taken as 1. The data are presented as mean fold change ± SD of three independent experiments performed in duplicates. *p < 0.05
We next evaluated the effects of BHT. We observed that cells pre-treated with BHT and then stimulated with LPS did not result in any detectable difference in cytokine level. However, in the absence of LPS stimulation, pre-treatment of human U937 macrophages with BHT increased the production of TNF-α and this increase was concentration-dependent, up to 2.4-fold at 15 μg/mL BHT (Fig. 5a). Similarly, BHT elevated IL-1β production in U937 macrophages and the increase was concentration-dependent, up to 4-fold at 15 μg/mL BHT (Fig. 5b). Subsequent studies on the effects of BHT on TNF-α and IL-1β production in human U937 macrophages were evaluated without LPS stimulation. The inflammasomes are a family of multi-protein cytoplasmic sensors that orchestrate the inflammatory response, of which the NRLP3 inflammasome has been more well characterized [39]. The NLRP3 inflammasome inhibitor MCC950 is known to specifically inhibit IL1-β pathway [40]. We asked if pre-treatment of human U937 macrophages with MCC950 could affect the cytokine level induced by BHT. Addition of MCC950 did not alter TNF-α production in BHT-treated U937 macrophages even up to 100 μg/mL MCC950 (Fig. 5c). There was no observable effect on cell viability to U937 macrophages up to 100 μg/mL MCC950. In contrast, IL-1β production by BHT-treated U937 macrophages was significantly reduced (p < 0.05) in the presence of 100 μg/mL MCC950 (Fig. 5d). Therefore, MCC950 reduced IL-1β level but not TNF-α level in BHT-treated U937 macrophages. We next asked if sulfasalazine, a known inhibitor of NF-κB [41], would affect the cytokine level induced by BHT. A significant reduction in TNF-α and IL-1β levels (p < 0.05) was observed at 300 μg/mL sulfasalazine (Fig. 5e and f). There was no observable effect on cell viability to U937 macrophages at 200 μg/mL and 300 μg/mL sulfasalazine.
Effects of BHT on TNF-α and IL-1β production by human U937 macrophage cells. Fold change of TNF-α (a, c, e) and IL-1β (b, d, f) production relative to control in the supernatant of human U937 macrophages measured by ELISA. a, b Cells were treated with none or varying concentrations of butylated hydroxytoluene (BHT) for 18 h. c-f Cells were pre-incubated for 6 h with none, 50 μg/mL or 100 μg/mL MCC950 (c, d), or with 200 μg/mL or 300 μg/mL sulfasalazine (e, f), followed by stimulation with 2 μg/mL of BHT for 18 h. The concentrations of MCC950 and sulfasalazine used were non-toxic to the cells. Cytokine production by cells not treated with compound (ie. none) was taken as 1. The data are presented as mean fold change ± SD of three independent experiments performed in duplicates. *p < 0.05
Discussion
To the best of our knowledge, this is the first study comparing the effects of different types of extraction methods and solvents of V. trifolia leaves on cytotoxicity and cytokine production in human U937 macrophages. We found ultrasonication dichloromethane V. trifolia leaf extract was comparatively most cytotoxic (IC50 3.2 ± 0.1 μg/mL) while Soxhlet water leaf extract was the least cytotoxic (IC50 684.5 ± 99.0 μg/mL) to U937 macrophages (Table 1). Leaf extracts of both maceration ethanol (Fig. 1c and d) and ultrasonication dichloromethane (Fig. 1e and f) were most active in inhibiting TNF-α and IL-1β levels in U937 macrophages. Previous work has shown Soxhlet methanol-derived V.trifolia leaf extract (IC50 6.72 μg/mL) was comparatively less cytotoxic to MCF-7 cells than petroleum-derived leaf extract (IC50 0.41 μg/mL) [42]. In our study, the IC50 of Soxhlet methanol V.trifolia leaf extract in U937 macrophage cells was 145.1 ± 13.7 μg/mL, which is comparatively less toxic than that reported by Garbi et al [42]. Vasanthi and colleagues reported cytotoxicity of Soxhlet hexane V. trifolia leaf extract in MCF-7 and HeLa cells, with both showing an IC50 of 80 μg/mL [43]. We found that the IC50 of Soxhlet hexane V. trifolia leaf extract in U937 macrophages was 5.2 ± 0.5 μg/mL (Table 1), which is comparatively more toxic than that reported by Vasanthi et al [43]. These differences may be due to the different cell lines studied. Separately, cytotoxic activities of V. trifolia aerial extracts in methanol, ethyl acetate and chloroform were evaluated using brine shrimp bioassay method, and the LC50 values were 140 mg/mL, 165 mg/mL and 180 mg/mL, respectively [44]. These LC50 values were much higher than the IC50 values we observed in our study (Table 1), most likely due to the different assays used. Kumar-Roiné et al [10] showed aqueous decoction of V.trifolia leaves inhibited nitric oxide at IC50 of 13.8 mg/mL extract in RAW264.7 murine macrophages, and the aqueous extract had no significant toxicity in LPS-stimulated RAW264.7. In another study, 2.5 mg/mL aqueous leaf extract of V.trifolia suppressed significantly the mRNA production of LPS-induced chemokines C-X-C motif 10 (CXCL-10), C–C motif ligand 3(CCL-3) and cyclo-oxygenase (COX)-2 [23]. Further, 2.5 mg/mL aqueous leaf extract significantly inhibited mRNA production of IL-1β, IL-6, TNF-α and iNOS, elevated IL-10 mRNA, and reduced the protein levels of IL-6 (67.5% inhibition) and TNF-α (10.4% inhibition), and increased IL-10 protein level (3.5-fold) [11]. In contrast to Matsui et al [11], we observed a more pronounced reduction in TNF-α protein in U937 macrophages by maceration ethanol leaf extract (Fig. 2a) and ultrasonication dichloromethane leaf extract (Fig. 2c). This may be due to the different experimental systems used. We noted that our Soxhlet water leaf extract at 100 μg/mL did not result in any significant reduction in TNF-α protein level in U937 macrophages (Fig. 1c). This is in agreement with the observations in murine macrophages by Matsui et al [11].
Further investigation of our most active leaf extracts (ie. maceration ethanol and ultrasonication dichloromethane leaf extracts) led to the isolation and identification of artemetin, casticin, vitexilactone and maslinic acid. In total, 12 compounds were identified in the V. trifolia leaf extracts (Fig. 3). To the best of our knowledge, this is the first report of BHT, 2,4-di-tert-butylphenol, campesterol and maslinic acid in the leaf extracts of V. trifolia. Artemetin and casticin (Fig. 3) have been identified from ethanolic extracts of dried fruits of V. trifolia [45, 46] and methanolic extracts of dried leaves and twigs of V. trifolia [17]. Both artemetin and casticin are reported to have potent lipoxygenase inhibition, with casticin two times more potent than artemetin [47]. Artemetin is shown to have anti-inflammatory activity using various experimental models in rats, including inhibiting carrageenan-induced paw edema, reduced granuloma formation and reduced vascular permeability to intracutaneous histamine [48]. Artemetin can also protect endothelial function by acting as an anti-oxidant and anti-apoptotic agent [49]. We showed that artemetin inhibited the production of both TNF-α and IL-1β (Fig. 4a and b). To the best of our knowledge, this is the first report that shows artemetin can inhibit TNF-α and IL-1β cytokine production in human U937 macrophages. We observed that artemetin inhibited cell viability of U937 macrophages, consistent with several reports showing artemetin inhibited cell viability. Ono et al [50] reported that artemetin showed a GI50 of 2270 ng/mL in human lung cancer PC-12 cells and 2200 ng/mL in human colon cancer HCT116 cells. Artemetin decreased growth of human leukemia HL-60 cells in dose-dependent manner, with IC50 of 39.98 μM after 96 h [51]. Casticin is reported to alleviate airway inflammation by suppressing pro-inflammatory cytokine production such as TNF-α in the lungs and bronchoalveolar lavage fluid in an inflammatory murine model of asthma [52]. Casticin inhibited TNF-α and IL-1β cytokine production in LPS-stimulated mouse macrophages at the range of 3 μM to 10 μM of casticin [53]. Our results showing casticin inhibited TNF-α production (Fig. 4c) are in the similar range reported by Liou et al [53]. Interestingly, we observed that the inhibitory effects of casticin on IL-1β cytokine production in human U937 macrophages were about 100-fold higher (Fig. 4d) than that reported by Liou et al [53]. This may be attributed to the different experimental systems. The molecular mechanism responsible for the anti-inflammatory activity of casticin likely involved NF-κB, AKT and MAPK signaling pathways [53]. In human umbilical vein endothelial cells, casticin significantly decreased vascular inflammation through inhibiting ROS-NF-κB pathway [54].
Vitexilactone, a labdane-type diterpenoid (Fig. 3), has been isolated from the fruits of V. trifolia and V. agnus-castus [55, 56]. It was reported to induce adipogenesis in 3T3-L1 preadipocytes [57]. Fang and colleagues showed that in HEK293 cell line, vitexilactone D inhibited TNF-α induced NF-κB activation [58]. In our study, we found vitexilactone inhibited the production of TNF-α and IL-1β (Fig. 4e and f). We did not observe any appreciable cytotoxicity of vitexilactone in human U937 macrophages, similar to a report indicating vitexilactone up to 100 μM had negligible cytotoxicity in mouse 3T3-L1 preadipocytes [57]. The phytoconsitutents β-sitosterol, campesterol, stigmasterol and phytol (Fig. 3) identified in our leaf extracts are known to have anti-inflammatory properties amongst other biological activities, such as anti-oxidant and anti-angiogenic [59, 60]. Both β-sitosterol and stigmasterol have been previously isolated in V. trifolia leaf extracts [61]. As far as we are aware, this is the first report of campesterol in V. trifolia leaf extracts. Campesterol is found in other Vitex species, such as leaves of V. agnus-castus [62].
Maslinic acid, an oleanane-type triterpenoid (Fig. 3), has been isolated from V. negundo, V. altissima, and V. agnus-castus [63]. Maslinic acid is reported to reduce neuroinflammation in cultured rat cortical astrocytes by inhibiting nitric oxide and TNF-α mRNA and protein levels through NF-κB signaling pathway [64]. The secretion of the inflammatory cytokines IL-6 and TNF-α from LPS-stimulated murine macrophages were significantly reduced (p < 0.01) by 50 μM and 100 μM of maslinic acid [65]. In THP-1 cells, maslinic acid enhanced the recruitment of macrophages by elevating the production of IL-8, IL-1α, and IL-1β [66]. Maslinic acid suppressed TNF-α production in RAW264.7 cells, and maslinic acid had anti-inflammatory effects in carrageenan-induced paw edema model, as well as anti-arthritis effects in mice models of arthritis [67]. In our study, maslinic acid suppressed TNF-α and enhanced IL-1β in human U937 macrophages (Fig. 4g and h), which is in alignment with observations by others [64,65,66,67]. The other two terpenoids identified in our study, α-amyrin and β-amyrin (Fig. 3), are known to have anti-inflammatory activities [68], and have been previously reported in V. trifolia leaves [16].
The phenolic compound 2,4 di-tert-butylphenol (Fig. 3) is typically used as an intermediate in preparing UV stabilizers and antioxidants, and in the manufacture of pharmaceuticals and fragrances [69]. It is found naturally occurring in nature, for example in lactic acid bacteria Lactococcus sp. [70], leaves of Pereskia bleo (Kunth) [71], and roots of Humboldtia unijuga [72]. To the best of our knowledge, this is the first report of 2,4-di-tert-butylphenol in V. trifolia leaf extracts. Besides its antioxidant property, 2,4 di-tert-butylphenol also has antifungal, antitumor activities and anti-inflammatory activities [70, 72]. Another naturally occurring phenolic compound is BHT (Fig. 3), which can be found in freshwater phytoplankton, including a green alga and three cyanobacteria [73], and plants such as Cytisus triflorus [74], Mesembryanthemum crystallinum [75] and seeds of Trichilia emetic commonly known as natal mahogany [76]. Originally found as a synthetic antioxidant, BHT has been extensively used in food industry, petroleum products and rubber [76]. Typically used as food preservative, BHT has been restricted in its use as it may be toxic at higher concentrations; indeed, BHT is applied as an inducer for animal lung tumor models [77]. To the best of our knowledge, this is the first report of BHT in V. trifolia leaf extracts. Our findings that BHT increased TNF-α and IL-1β cytokine production in human U937 macrophages (Fig. 5) suggest BHT exert pro-inflammatory effects. To the best of our knowledge, this is the first report showing the pro-inflammatory effects of BHT on TNF-α and IL-1β protein levels in human U937 macrophages. Murakami et al [78] studied LPS-stimulated murine macrophage RAW264.7 cells treated with BHT and did not find any significant difference in TNF-α mRNA expression compared with non-treated control cells. Their study on BHT was conducted in the presence of LPS, in contrast to our study performed in the absence of LPS, which could account for the difference in observations. The presence of LPS likely masked any increase in cytokine signal elicited by BHT; indeed, we noted that cells pre-treated with BHT and then stimulated with LPS did not result in any alteration in cytokine level. Our results along with other published literature point to the pro-inflammatory effects of BHT. Increased cyclooxygenase-1 and cyclooxygenase-2 expression, and increased inflammatory cell infiltration were observed in lung tumor formation in BALB mice models caused by BHT administration following an initiating agent [77]. There was elevated production of pro-inflammatory mediators such as prostaglandin, and increased translocation of 5-lipoxygenase from the cytosol to the membrane which could be partially inhibited by celecoxib, an inhibitor of cyclooxygenase-2 enzyme [79]. Future work will include using in vivo models such as a carrageenan-induced paw-edema model to validate the activity of the individual compounds identified. Further, the physiological significance of these individual compounds may be evaluated in monocytes freshly extracted from rats, and performing western blot analyses.
Conclusions
In conclusion, leaf extracts of V. trifolia obtained using different solvents and extraction methods were successfully investigated for their effects on cytokine production in human U937 macrophages. The findings provide scientific evidence for the traditional use of V. trifolia leaves (a sustainable resource) and highlight the importance of conservation of medicinal plants as resources for drug discovery. Our results together with others suggest further investigation on V. trifolia and constituents to develop novel treatment strategies in immune-mediated inflammatory conditions is warranted.
Availability of data and materials
Data are available on request due to privacy or other restrictions.
Abbreviations
- BHT:
-
Butylated hydroxytoluene
- Dex:
-
Dexamethasone
- DCM:
-
Dichloromethane
- DMSO:
-
Dimethyl sulfoxide
- ELISA:
-
Enzyme-linked immunosorbent assay
- GC-MS:
-
Gas chromatography-mass spectrometry
- HPLC:
-
High performance liquid chromatography
- IC50 :
-
Inhibitory concentration required for 50% inhibition of activity
- IC20 :
-
Inhibitory concentration required for 20% inhibition of activity
- IL-1β:
-
Interleukin-1β
- LC-MS:
-
Liquid chromatography-mass spectrometry
- LPS:
-
Lipopolysaccharide
- Mac:
-
Maceration
- NLRP3:
-
Nod-like receptor protein family pyrin domain containing 3
- PBS:
-
Phosphate buffered saline
- PMA:
-
Phorbol 12-myristate 13-acetate
- Sox:
-
Soxhlet
- TNF-α:
-
Tumor necrosis factor-α
- US:
-
Ultrasonication
- WST-1:
-
Water soluble tetrazolium salt
References
Catrina AI, Joshua V, Klareskog L, Malmström V. Mechanisms involved in triggering rheumatoid arthritis. Immunol Rev. 2016;269(1):162–74.
Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491.
David T, Ling SF, Baron A. Genetics of immune-mediated inflammatory disease. Clin Exp Immunol. 2018;193(1):3–12.
Kuek A, Hazleman BL, Ostor AJK. Immune-mediated inflammatory diseases (IMD) and biologic therapy: a medical revolution. Postgrad Med J. 2007;83:251–60.
Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–32.
Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis. 2018;77:175–87.
Chan EWC, Baba S, Chan HT, Kainuma M, Tangah J. Medicinal plants of sandy shores: a short review on Vitex trifolia L. and Ipomoea pes-caprae (L.) R. Br. Indian J Nat Prod Resour. 2016;7(2):107–15.
The State Pharmacopoeia Commission of People’s Republic of China. Pharmacopoeia of the People’s Republic of China, vol. 1. Beijing: China Medical Science Press; 2010.
Zhao ZZ, Xiao PG. Verbenaceae: Vitex trifolia L. In: Zhao ZZ, Xiao PG, editors. Encyclopedia of medicinal plants, vol. 2. Shanghai: Shanghai World Publishing Corporation; 2010. p. 514–7.
Kumar-Roiné S, Matsui M, Reybier K, Darius HT, Chinain M, Pauillac S, Laurent D. Ability of certain plant extracts traditionally used to treat ciguatera fish poisoning to inhibit nitric oxide production in RAW 264.7 macrophages. J Ethnopharmacol. 2009;123(3):369–77.
Matsui M, Kumar-Roine S, Daruis HT, Chinain M, Laurent D, Pauillac S. Characterisation of the anti-inflammatory potential of Vitex trifolia L. (Labiatae), a multipurpose plant of the Pacific traditional medicine. J Ethnopharmacol. 2009;126(3):427–33.
Hernandez MM, Heraso C, Villarreal ML, Vargas-Arispuro, Aranda E. Biological activities of crude plant extracts from Vitex trifolia L. (Verbenaceae). J Ethnopharmacol. 1999;67(1):37–44.
Kulkarni LA. Pharmacological review on Vitex trifolia Linn. (Verbaneaceae). Pharmacologyonline. 2011;3:858–63.
Manjunatha BK, Vidya SM. Hepatoprotective activity of Vitex trifolia against carbon tetrachloride-induced hepatic damage. Indian J Pharm Sci. 2008;70(2):241–5.
Alam G, Wahyuono S, Ganjar IG, Hakim L, Timmerman H, Verpoorte R. Tracheospasmolytic activity of viteosin-A and vitexicarpin isolated from Vitex trifolia. Planta Med. 2002;68(11):1047–9.
Tiwari N, Thakur J, Saikia D, Gupta MM. Antitubercular diterpenoids from Vitex trifolia. Phytomedicine. 2013;20(7):605–10.
Huang MY, Zhong LJ, Xie JM, Wang F, Zhang YH. A new taraxastane-type triterpene from Vitex trifolia var. simplicifolia. Helv Chim Acta. 2013;96(11):2040–5.
Suksamrarn A, Werawattanametin K, Brophy JJ. Variation of essential oil constituents in Vitex trifolia species. Flavour Frag J. 1991;6(1):97–9.
Mustarichie R, Sumiwi SA, Hanifah AT. ED50 from anti-inflammatory properties of Vitex trifolia L. ethanol extract. Natl J Physiol Pharm Pharmacol. 2018;8(5):719–25.
Ankalikar A, Viswanathswamy AH. Effect of leaves of Vitex trifolia Linn on different stages of inflammation. Indian J Pharm Educ. 2017;51(3):461–71.
Pfuzia A, Devi RKB, Sharatchandra KH, Debashree BN, Banylla SN, Monica KHS. Studies on the anti-inflammatory effect of the aqueous extract of the leaves of Vitex trifolia L. in albino rats. Int J Pharm Bio Sci. 2013;4(2):588–93.
Goverdham P, Bobbala D. Anti-nociceptive and anti-inflammatroy effects of the leaf extract of Vitex trifolia Linn in experimental animals. Ethnobotanical Leaflets. 2009;13:65–72.
Matsui M, Adib-Conquy M, Coste A, Kumar-Roiné S, Pipy B, Laurent D, Pauillac S. Aqueous extract of Vitex trifolia L. (Labiatae) inhibits LPS-dependent regulation of inflammatory mediators in RAW 264.7 macrophages through inhibition of nuclear factor kappa B translocation and expression. J Ethnopharmacol. 2012;143(1):24–32.
Garrelds IM, van Hal PT, Haakmat RC, Hoogsteden HC, Saxena PR, Zijlstra FJ. Time dependent production of cytokines and eicosanoids by human monocytic leukaemia U937 cells; effects of glucocorticosteroids. Mediat Inflamm. 1999;8(4–5):229–35.
Ortiz-Lazareno PC, Hernandez-Flores G, Dominguez-Rodriguez JR, Lerma-Diaz JM, Jave-Suarez LF, Aguilar-Lemarroy A, Gomez-Contreras PC, Scott-Algara D, Bravo-Cuellar A. MG132 proteasome inhibitor modulates proinflammatory cytokines production and expression of their receptors in U937 cells: involvement of nuclear factor-kappaB and activator protein-1. Immunology. 2008;124(4):534–41.
Chanput W, Peters V, Wichers H. THP-1 and U937 cells. In: Verhoeckx K, Cotter P, López-Expósito I, Kleiveland C, Lea T, Mackie A, Requena T, Swiatecka D, Wichers H, editors. The impact of food bioactives on health: in vitro and ex vivo models, vol. Chapter 14. Cham: Springer; 2015. p. 147–59. https://doi.org/10.1007/978-3-319-16104-4_14.
Siew YY, Zareisedehizadeh S, Seetoh WG, Neo SY, Tan CH, Koh HL. Ethnobotanical survey of usage of fresh medicinal plants in Singapore. J Ethnopharmacol. 2014;155:1450–66.
Siew YY, Yew HC, Neo SY, Seow SV, Lew SM, Lim SW, Lim CSE, Ng YC, Seetoh WG, Ali A, Tan CH, Koh HL. Evaluation of anti-proliferative activity of medicinal plants used in Asian traditional medicine to treat cancer. J Ethnopharmacol. 2019;235:75–87.
The plant list, 2013. Version 1.1. https://theplantlist.org/. Accessed 15 June 2019.
WCSP, 2019. World checklist of selected plant families. Facilitated by the Royal Botanic Gardens, Kew. http://apps.kew.org/wcsp/. Accessed 15 June 2019.
Mgbonyebi OP, Russo J, Russo IH. Antiproliferative effect of synthetic resveratrol on human breast epithelial cells. Int J Oncol. 1998;12(4):865–9.
Jondeau A, Dahbi L, Bani-Estivals MH, Chagnon MC. Evaluation of the sensitivity of three sublethal cytotoxicity assays in human HepG2 cell line using water contaminants. Toxicology. 2006;226(2–3):218–28.
Dourlat J, Liu WQ, Gresh N, Garbay C. Novel 1,4-benzodiazepine derivatives with antiproliferative properties on tumor cell lines. Bioorg Med Chem Lett. 2007;17(9):2527–30.
Sririwichitchai R, Saiai A, Inthanon K, Chomdej S, Wongkham W, Roongruangwongse W. Anti-adipogenesis activities of Zingiber cassumunar Roxb. rhizome extracts on L929 cells evaluated by image-based analysis. Vet Integr Sci. 2018;16(2):35–51.
Kikuchi H, Yuan B, Nishimura Y, Imai M, Furutani R, Kamoi S, Seno M, Fukushima S, Hazama S, Hirobe C, Ohyama K, Hu XM, Takagi N, Hirano T, Toyoda H. Cytotoxicity of Vitex agnus-castus fruit extract and its major component, casticin, correlates with differentiation status in leukemia cell lines. Int J Oncol. 2013;43:1976–84.
Wenkert E, Gottlieb HE. Carbon-13 nuclear magnetic resonance spectroscopy of flavonoid and isoflavonoid compounds. Phytochemistry. 1977;16:1811–6.
Ono M, Yamamoto M, Yanaka T, Ito Y, Nohara T. Ten new labdane-type diterpenes from the fruit of Vitex rotundifolia. Chem Pharm Bull. 2001;49(1):82–6.
Bilia AR, Mendez J, Morelli I. Phytochemical investigations of Licania genus. Flavonoids and triterpenoids from Licania carii. Pharm Acta Helv. 1996;71(1):191–7.
Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328. https://doi.org/10.3390/ijms20133328.
Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O'Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248–55.
Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101(5):1163–74.
Garbi MI, Osman EE, Kabbashi AS, Saleh MS, Yuosof YS, Mahmoud SA, Salam HAA. Cytotoxicity of Vitex trifolia leaf extracts on MCF-7 and Vero cell lines. J Sci Innov Res. 2015;4(2):89–93.
Vasanthi VJ, Radhjeyalakshmi R, Nasrin F. Evaluation of anticancer activity using hexanic extract of Vitex trifolia on two different cancer cell lines. Int J Pharmacognosy Phytochem Res. 2014;6(3):449–53.
El-Kousy S, Mohamed M, Mohamed S. Phenolic and biological activities of Vitex trifolia aerials parts. Life Sci J. 2012;9(2):670–7.
Guan R, Wang D, Yu Z, Wang X, Lan T. Preparative isolation and purification of the active components from Viticis Fructus by high-speed counter-current chromatography. Se Pu. 2010;28(11):1043–7.
Li WX, Cui CB, Cai B, Wang HY, Yao XS. Flavonoids from Vitex trifolia L. inhibit cell cycle progression at G2/M phase and induce apoptosis in mammalian cancer cells. J Asian Nat Prod Res. 2005;7(4):615–26.
Choudhary MI, Azizuddin, Jalil S, Nawaz SA, Khan KM, Tareen RB, Atta-ur-Rahman. Antiinflammatory and lipoxygenase inhibitory compounds from Vitex agnus-castus. Phytother Res. 2009;23(9):1336–9.
Sertié JA, Basile AC, Panizza S, Matida AK, Zelnik R. Anti-inflammatory activity and sub-acute toxicity of artemetin. Planta Med. 1990;56(1):36–40.
Grossini E, Marotta P, Farruggio S, Sigaudo L, Qoqaiche F, Raina G, de Giuli V, Mary D, Vacca G, Pollastro F. Effects of artemetin on nitric oxide release and protection against peroxidative injuries in porcine coronary artery endothelial cells. Phytother Res. 2015;29(9):1339–48.
Ono M, Yanaka T, Yamamoto M, Ito Y, Nohara T. New diterpenes and norditerpenes from the fruits of Vitex rotundifolia. J Nat Prod. 2002;65(4):537–41.
Ko WG, Kang TH, Lee SJ, Kim NY, Kim YC, Sohn DH, Lee BH. Polymethoxyflavonoids from Vitex rotundifolia inhibit proliferation by inducing apoptosis in human myeloid leukemia cells. Food Chem Toxicol. 2000;38(10):861–5.
Liou CJ, Cheng CY, Yeh KW, Wu YH, Huang WC. Protective effects of casticin from Vitex trifolia alleviate eosinophilic airway inflammation and oxidative stress in a murine asthma model. Front Pharmacol. 2018;9:635. https://doi.org/10.3389/fphar.2018.00635.
Liou CJ, Len WB, Wu SJ, Lin CF, Wu XL, Huang WC. Casticin inhibits COX-2 and iNOS expression via suppression of NF-κB and MAPK signaling in lipopolysaccharide-stimulated mouse macrophages. J Ethnopharmacol. 2014;158:310–6.
Lee SM, Lee YJ, Kim YC, Kim JS, Kang DG, Lee HS. Vascular protective role of vitexicarpin isolated from Vitex rotundifolia in human umbilical vein endothelial cells. Inflammation. 2012;35(2):584–93.
Wu J, Zhou T, Zhang S-W, Zhang X-H, Xuan L-J. Cytotoxic terpenoids from the fruits of Vitex trifolia L. Planta Med. 2009;7:367–70.
Ono M, Eguchi K, Konoshita M, Furusawa C, Sakamoto J, Yasuda S, Ikeda T, Okawa M, Kinjo J, Yoshimitsu H, Nohara T. A new diterpenoid glucoside and two new diterpenoids from the fruit of Vitex agnus-castus. Chem Pharm Bull. 2011;59(3):392–6.
Nishina A, Itagaki M, Sato D, Kimura H, Hirai Y, Phay N, Makishima M. The rosiglitazone-like effects of vitexilactone, a constituent from Vitex trifolia L. in 3T3-L1 preadipocytes. Molecules. 2017;22(11):2030. https://doi.org/10.3390/molecules22112030.
Fang SM, Liu R, Li L, Yao JL, Liu EW, Fan GW, Zhang H, Gao XM. Anti-inflammatory diterpenes from the fruits of Vitex trifolia L. var. simplicifolia Cham. J Asian Nat Prod Res. 2018;12:1–7.
Plat J, Baumgartner S, Vanmierlo T, Lütjohann D, Calkins KL, Burrin DG, Guthrie G, Thijs C, Te Velde AA, Vreugdenhil ACE, Sverdlov R, Garssen J, Wouters K, Trautwein EA, Wolfs TG, van Gorp C, Mulder MT, Riksen NP, Groen AK, Mensink RP. Plant-based sterols and stanols in health & disease: “consequences of human development in a plant-based environment?”. Prog Lipid Res. 2019;74:87–102.
Lampronti I, Dechecchi MC, Rimessi A, Bezzerri V, Nicolis E, Guerrini A, Tacchini M, Tamanini A, Munari S, D’Aversa E, Santangelo A, Lippi G, Sacchetti G, Pinton P, Gambari R, Agostini M, Cabrini G. β-Sitosterol reduces the expression of chemotactic cytokine genes in cystic fibrosis bronchial epithelial cells. Front Pharmacol. 2017;8:236. https://doi.org/10.3389/fphar.2017.00236.
Ganapaty S, Vidyadhar KN. Phytoconstituents and biological activities of Vitex - a review. J Nat Remedies. 2008;5(2):75–95.
Ibrahim N, Shalaby A, Farag R, Elbaroty G, Hassa E. Chemical composition and biological evaluation of Vitex agnus-castus L. Med Aromatic Plant Sci Biotech. 2009;3(Special Issue 1):27–31.
Yao J-L, Fang S-M, Liu R, Oppong MB, Lu E-W, Fan G-W, Zhang H. A review on the terpenes from genus Vitex. Molecules. 2016;21:1179. https://doi.org/10.3390/molecules21091179.
Huang L, Guan T, Qian Y, Huang M, Tang X, Li Y, Sun H. Anti-inflammatory effects of maslinic acid, a natural triterpene, in cultured cortical astrocytes via suppression of nuclear factor-kappa B. Eur J Pharmacol. 2011;672:169–74.
Márquez Martín A, de la Puerta Vázquez R, Fernández-Arche A, Ruiz-Gutiérrez V. Supressive effect of maslinic acid from pomace olive oil on oxidative stress and cytokine production in stimulated murine macrophages. Free Radic Res. 2006;40(3):295–302.
Sánchez-Quesada C, López-Biedma A, Gaforio JJ. Maslinic acid enhances signals for the recruitment of macrophages and their differentiation to M1 state. Evid Based Complement Alternat Med. 2015;2015:654721. https://doi.org/10.1155/2015/654721.
Fukumitsu S, Villareal MO, Fujitsuka T, Aida K, Isoda H. Anti-inflammatory and anti-arthritic effects of pentacyclic triterpenoids maslinic acid through NF-κB inactivation. Mol Nutr Food Res. 2016;60(2):399–409.
Vázquez LH, Palazon J, Navarro-Ocaña A. The pentacyclic triterpenes a, b-amyrins: a review of sources and biological activities. In: Rao V, editor. Phytochemicals - a global perspective of their role in nutrition and health; 2012. ISBN: 978-953-51-0296-0, InTech, Available from: http://www.intechopen.com/books/phytochemicals-a-global-perspective-of-their-role-in-nutrition-andhealth/the-pentacyclic-triterpenes-amyrins-a-review-of-sources-and-biological-activities.
Choi SJ, Kim JK, Kim HK, Harris K, Kim C-J, Park GG, Park C-S, Dong-Hoon Shin D-H. 2,4-Di-tert-butylphenol from sweet potato protects against oxidative stress in PC12 cells and in mice. J Med Food. 2013;16(11):977–83.
Varsha KK, Devendra L, Shilpa G, Priya S, Pandey A, Nampoothiri KM. 2,4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int J Food Microbiol. 2015;211:44–50.
Malek SNA, Shin SK, Wahab NA, Yaacob H. Cytotoxic components of Pereskia bleo (Kunth) DC. (Cactaceae) leaves. Molecules. 2009;14(5):1713–24.
Nair RVR, Jayasree DV, Biju PG, Baby S. Anti-inflammatory and anticancer activities of erythrodiol-3-acetate and 2,4-di-tert-butylphenol isolated from Humboldtia unijuga. Nat Prod Res. 2018. https://doi.org/10.1080/14786419.2018.1531406.
Babu B, Wu JT. Production of natural butylated hydroxytoluene as an antioxidant by freshwater phytoplankton. J Phycol. 2008;44(6):1447–54.
Aourahoun KA, Fazouane F, Benayad T, Bettache Z, Denni N. The synthetic antioxidant butylated hydroxytoluene, a naturally occurring constituent of the broom Cytisus triflorus L’Hérit. J Nat Prod. 2014;7:58–64.
Ibtissem B, Imen M, Souad S. Dosage of 2,6-Bis (1.1-Dimethylethyl)-4-Methylphenol (BHT) in the plant extract Mesembryanthemum crystallinum. J Biomed Biotechnol. 2010:142486. https://doi.org/10.1155/2010/142486.
Usman A, Mohammad RH, Raheem D. Isolation and characterization of naturally occurring butylated hydroxytoluene from Trichilia emetica whole seeds. J Nat Prod Resour. 2016;2(2):68–70.
Bauer AK, Dwyer-Nield LD, Hankin JA, Murphy RC, Malkinson AM. The lung tumor promoter, butylated hydroxytoluene (BHT), causes chronic inflammation in promotion-sensitive BALB/cByJ mice but not in promotion-resistant CXB4 mice. Toxicology. 2001;169:1–15.
Murakami Y, Kawata A, Katayama T, Fujisawa S. Anti-inflammatory activity of the artificial antioxidants 2-tert-butyl-4-methoxyphenol (BHA), 2,6-di-tert-butyl-4-methylphenol (BHT) and 2,4,6-tri-tert-butylphenol (TBP), and their various combinations. In Vivo. 2015;29(2):197–206.
Kisley LR, Barrett BS, Dwyer-Nield LD, Bauer AK, Thompson DC, Malkinson AM. Celecoxib reduces pulmonary inflammation but not lung tumorigenesis in mice. Carcinogenesis. 2002;23(10):1653–60.
Acknowledgements
We thank Mdm Lee Ying from Leeward Pacific for her collaboration and support, and Mr. Lua Hock Keong from National Parks Board for his support.
Funding
This study is supported by the National University of Singapore - Leeward Pacific Pte Ltd. research collaboration grants R-148-000-172-592 and R-148-000-140-592, Lee Foundation Grant R-184-000-290-720, and the National University of Singapore President’s Graduate Fellowship (to WHN). The funders had no involvement in the design of the study and collection, analysis and interpretation of data, as well as writing the manuscript for publication.
Author information
Authors and Affiliations
Contributions
HLK and CHT conceptualized, designed the study and corrected the manuscript; SYN designed and carried out the experiments, analysed data and corrected the manuscript; HNW carried out the experiments, analysed data, and drafted the manuscript; DS, HCY, ZYQ, XRCT, SYH, KYCY carried out the experiments and analysed data. All the authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wee, HN., Neo, SY., Singh, D. et al. Effects of Vitex trifolia L. leaf extracts and phytoconstituents on cytokine production in human U937 macrophages. BMC Complement Med Ther 20, 91 (2020). https://doi.org/10.1186/s12906-020-02884-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-020-02884-w