Abstract
Background
Traditional Chinese medicine injections (TCMIs) have been widely used to treat severe and acute diseases due to their high bioavailability, accurate curative effect, and rapid effect. However, incidence rates of adverse drug reactions (ADRs) of TCMIs have also increased in recent years. Xueshuantong injection (XSTI) is a commonly-used TCMI comprised of Panax notoginseng total sapiens for the treatment of stroke hemiplegia, chest pain, and central retinal vein occlusion. Its safety remains uncelar. Therefore, post-marketing safety of XSTI was studied in this research.
Methods
In present study, post-marketing safety surveillance and re-evaluation of XSTI were reported. Thirty thousand eight hundred eighty-four patients in 33 hospitals from 7 provinces participated in this study. Incidence rate, most common clinical manifestations, types, severity, occurrence time, and disposal of ADRs were calculated.
Results
Incidence rate of ADR of XSTI was 4.14‰ and the most common clinical manifestations were skin and its appendages damage. Type A accounts for 95.49% of ADRs of XSTI and most of them (41.41%) were occurred within 24 h after receiving XSTI treatment. Severities of most ADRs of XSTI were moderate reactions (86.72%). Main disposition of ADRs of XSTI was drug withdrawal and symptomatic treatment (54.69%).
Conclusions
Our data provide basis for improvement of instructions of XSTI and clinical safety of XSTI. Post-marketing surveillance of TCMIs in this study is a powerful tool to identify types and manifestations of ADRs to improve safety and effectiveness of drugs in clinical applications.
Trial registration
This protocol has international registration in China clinical trial registration center (ChiCTR~OPC~ 14,005,718) at December 22, 2014.
Similar content being viewed by others
Background
Traditional Chinese medicine injection (TCMI) is made by modern technologies and scientific methods to extract and purify effective substances from herbs (or decoction pieces). Compared with other traditional Chinese medicine formulations, injection has advantages of high bioavailability, rapid, and accurate curative effect. Therefore, TCMI is widely used to treat many severe and acute diseases [1,2,3,4,5,6,7]. In recent years, with the widespread use of TCMIs, the incidences of adverse drug reactions (ADRs)/adverse drug events (ADEs) has gradually increased [8,9,10]. However, safety profile of most TCMIs remains largely unknown currently.
Xueshuantong injection (lyophilized) (XSTI) is a standardized herbal preparation and has been collected by “2012 national essential drugs list” and People’s Republic of China Pharmacopoeia, respectively. Notoginseng total saponins, isolated from the root and rhizome of P. notoginseng, is the main component of XSTI. XSTI is generally used for treatment of cardiovascular and cerebrovascular disease [11]. Total revenue of XSTI in Chinese market in 2013 was over $700 million [12]. Therefore, the enormous consumption requires stricter and accurate evidence on its safety. However, many reports on the ADRs of XSTI were case reports and there is still lack large sample and high level evidence-based basis for safety of XSTI. Till now, evaluation on post-marketing safety of XSTI has not been reported. Therefore, ADRs/ADEs of XSTI were studied in this research using hospital centralized monitoring method.
Hospital centralized monitoring also known as real world study (RWS), is an observational research method by recording detailed ADRs of drugs within a certain range of a hospital or an area in a certain period of time. It is attracting more and more attention in field of global clinical epidemiology due to its broad range of inclusion and exclusion criteria, comprehensive coverage of population, and authenticity [13,14,15]. A new hospital centralized monitoring method based on hospital information system (HIS) system was established in. our previous study on post-market clinical safety evaluation of TCMI [16]. In present research, post marketing safety (including incidence rate, types, severities, and other information of ADRs/ADEs) of XSTI with 30,884 cases by employing an improved method of hospital-centralized monitoring. This research is the first post-marketing ADRs/ADEs study of XSTI with large scale and multi-center and can provide essential basis for safe clinical use of XSTI.
Methods
Inclusion and exclusion criteria
Inclusion criteria: patients who used XSTI.
Exclusion criteria: patients who did not use XSTI.
Subjects
A total of 30,884 in-patients received XSTI from 33 hospitals in 7 provinces participated in this study between January 1, 2015 and December 31, 2016.
Drug
All three product specification (100 mg、150 mg and 250 mg per bottle) of XSTI were manufactured by Guangxi Wuzhou Pharmaceutical Co., Ltd. (Wuzhou, Guangxi, China). All drugs used in this research were sold on the market and in conformity with the standard of Ministry of Public Health of China.
Method design
This study was not a randomized controlled trial but a centralized monitoring study in hospital and all data were collected from clinical daily treatment without any intervention. Thus this study was not designed entirely according to CONSORT guidelines. We designed the monitoring data collection and quality control method according to other hospital centralized monitoring methods [13,14,15,16].
Method of monitoring data collection
The monitoring data were from two parts: monitoring table and hospital information system / laboratory information management system (HIS/LIS). Information in front page of the medical record, doctor’s orders and results of laboratory examination were extracted from HIS/LIS system after being approved by ethics committee. To ensure the safety of the patient’s personal information all monitors have been trained on information confidentiality. Monitoring table consists of Table A (basic monitoring information including daily dose, frequency, drug combination, etc.) and Table B (ADR/ADE information). Table A was filled by pharmacists within 5 days after the end of medication by “face-to-face” observation. Monitoring Table B was filled once ADR/ADE, especially serious ADR/ADE such as anaphylactic shock, severe allergic reactions, severe mucocutaneous lesions, liver damage, renal damage, and death, was happened. Accordance to requirements of “National ADR Reporting and Monitoring Management Measures”, all serious ADRs/ADEs were further investigated by a panel consisting of head of organizer of the project and staffs from sub center and manufacturing enterprise. “Adverse Drug Reaction / Event Report” was written and submitted to official website according to the rules of the CFDA. The overall data collection flow chart is shown in Fig. 1 and ADRs/ADEs processing process is shown in Fig. 2.
Method of monitoring quality control
In order to guarantee the objectivity and accuracy of ADR results, unified training on monitoring plan and ADRs/ADEs judgment was carried out for monitoring personnel and a three-grade evaluation of ADRs / ADEs and third party quality control were conducted in this study. The detailed monitoring process is shown in Fig. 2. Strict selection criteria were set for the screening of participating hospital. Primary quality control monitoring hospital included comprehensive hospital and traditional Chinese medicine hospital. Sub-center monitoring hospitals were all three grade hospital in China and have organized or participated in the evaluation of drug safety. All participating hospitals had a team of clinical pharmacists and collected at least 500 cases within 1 years. There were 7 sub centers in total, and each sub center was responsible for 5–6 hospitals. A contract research organization (CRO) company (Shanghai Yongzheng medical science and Technology Co., Ltd.), was employed to carry on quality management of the study (Fig. 3). Reliability of monitoring reports and research progress of each monitoring hospitals and monitoring centers regularly were judged by CRO company. The hospitals which couldn’t complete the monitoring progress on time or their monitoring reports were judged as unqualified more than three times were refused to continue to participate into the research project. The sub center was eliminated when more than half of its monitoring hospitals were eliminated.
Correlation assessment between ADRs and ADEs
Correlation assessment between ADRs and ADEs was conducted according to method recommended by CFDA evaluation center of adverse reactions. All ADRs/ADEs were preliminarily classified on basis of their definitions, respectively. ADR is unrelated or unexpected adverse reaction to medication purpose when using approved drugs within normal dosage. It does not include reactions caused by accidental or intentional drug overdoses or improper medications. ADE refers to any injury occurred during drug administration period, whether or not drug usage is the cause of injury. ADR is a special type of ADE for which the causative relationship between drug usage and adverse reaction is identified. Relevance assessment is divided into 6 grades: (1) Certain: the sequence between medication and ADRs’ occurrence is reasonable. ADRs could be stopped or quickly reduced or turn better after drug withdrawal. Alternatively, ADRs would be occured again or significantly worse when drug was re-administered. It could also be supported by literatures. Notably, primary disease and other factors should be ruled out. (2) Probable: there is no history of repeating medication, others are same as “Certain”. If the investigated drug was administrated in combination with other drugs, the probability of ADR caused by combined drugs could be excluded. (3) Possible: there is close relationship between medication and ADEs’ occurrence. It is coincided with common type of ADRs, but there is no reaction data after drug withdrawal, or there are more than one drug leading to ADRs/ADEs, or causative factors of primary disease could not be ruled out. (4) Unlikely: there was no close relationship between medication and ADEs’ occurrence. The reactions do not link to ADRs/ADEs of the investigated drug. Reactions during development of primary disease may display similar clinical manifestations. (5) Pending: There are missing contents of “Monitoring Information Form” and evaluation will not be completed until the supplementary specifications are provided. Thus, it is difficult to determine relationship between cause and effect due to absence in documentation. (6) Unassessable: many items in the “Monitoring Information Form” are unavailable. It is unable to analyze relationship between cause and effect because missing items could not be supplemented [2, 17, 18].
Results
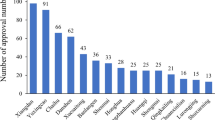
Number of cases of XSTI in each hospital
In this study, a total of 30,884 cases received XSTI from 33 hospitals participated in the monitoring assessment. The number of ADE cases in 33 monitoring hospitals is shown in Table 1.
Association assessment of adverse reactions
In this study, 128 cases were grouped as “probable and possible “. Results of relevance evaluation were shown in Table 2.
Incidence rate and manifestations of ADRs
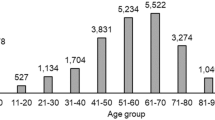
The ADR incidence of XSTI was 4.14‰. The clinical manifestations were 236 times. The most common clinical manifestations were skin and its appendages damage (52.97%), systemic injury (9.32%), and central and peripheral nervous system damage (8.90%). Statistics analysis were shown in Table 3 in detail.
Types of ADRs
ADRs are classified into three types (types A, B, and C) by WHO. Type A reaction caused by the enhancement of pharmacological effect of drugs is dose-related and able to be predicted. Type B reaction is abnormal reaction unrelated to normal pharmacological effect. It is not able to be detected by conventional toxicological screening and hard to make prediction. Type C reaction refers to abnormal reaction other than types A and B. According to above classification, all 128 cases of ADRs were divided into type A, B, and C, respectively as shown in Table 4.
Time of occurrence of ADRs
Time of occurrence of ADRs after injection of XSTI is shown in Table 5 and Table 6. According to the results, most ADRs of XST were occurred rapidly and nearly half cases of ADRs were appeared on the first day of injection (41.41%). There is 25.78% of ADRs were happened 2~ 4 days after injection.
Severity of ADRs
As shown in Table 7, severity of ADRs were classified into three grades: mild (symptoms or signs can be felt and stopping medication or special treatment is no necessary), moderate (symptoms and signs are tolerable and there is no effect on daily life but special treatment is necessary), and severe (symptoms and signs are intolerable and drug withdrawal and special treatment are needed). Our results showed that most cases (86.72%) were graded into moderate reactions and 11 cases were classified as mild. In addition, among 6 cases with severe ADRs, 3 cases had severe symptoms, including rash, flushing, shivering, palpitation, high fever, dyspnea, and convulsions.
Disposal of ADRs
In most cases, ADRs need special treatments such as reducing times and dose of usage, withdrawal, symptomatic treatment, or combined together. Special treatments used in this study were listed as follows: withdrawal and symptomatic treatment (54.69%), withdrawal (30.47%), symptomatic treatment (7.03%) (Table 8). In addition, 1.56% of the ADRs did not get any special treatment.
Recovery of ADRs
Among of patients with ADRs, 80 cases were cured, 48 cases got improved, and there was no sequelae and death. Upon recovery time, 12, 26, 17, and 70 cases were improved within 1 h (9.38%), 1 ∼ 6 h (20.31%), 6 ∼ 24 h (13.28%), or over 24 h (54.69%), respectively. In addition, 3 cases were not recorded in detail.
Discussion
The incidence of ADRs of XSTI was 4.14‰, which was an “occasionally” “level and severe ADRs was 0.19 ‰, which was a “rare” grade and 95.49% of ADRs in this research were type A which can be predicted. 42.97% of ADRs were recovered in 24 h and there was no sequelae and death. Therefore, XSTI is safe in clinical use according to the incidence, type and recovery of ADRs in this study. Most of ADRs were anaphylaxis which indicated that safety monitoring should be in progress promptly. In addition, ADRs of XSTI could happened throughout the whole course of treatment after administration which indicated that the whole process needs safety monitoring. So far, the description of the XSTI used in the study is not clear about ADRs. The results of this study make clear the manifestations of ADRs of XSTI, which provide a high level evidence-based basis for the improvement of the instructions. In this research, manifestations of ADRs were studied and the most common ADR manifestation of XSTI was skin and its appendages damages which were consistent with other published reports [19]. However, there is no suitable medicine to alleviate the ADRs of cutaneous systems. The main preventive measures by far is washing infusion tube before the injection of different injections to reduce the incidence of ADRs during the combined use of XSTI and other injections. It is generally believed that the skin and its appendages damages were caused by allergies. There is a study using P815 cell degranulation model to screen components of XSTJ [20]. Results showed that XSTI promoted the P815 cell degranulation and the effect may have related to Ginsenoside Rb1 and Rg1. Besides, impurities are difficult to remove in purification and refining processes due to the complex components in traditional Chinese medicine injections, which may also cause anaphylaxis [21,22,23]. However, few studies on the anaphylaxis mechanism of XSTI or panax notoginseng saponins and further investigation are needed to explore the ADR mechanism of XSTI. In addition, this study found some ADRs beyond instruction.
By comparison of four methods (hospital-centralized monitoring method, spontaneous reporting method, literature research method, and medical record review method) in our previous study on post-market clinical safety evaluation of TCMI and other reports, hospital centralized monitoring is a scientific, advanced, and feasible tool to assess clinical safety of TCM injection receiving approval [16]. In this study, monitoring method was improved on the basis of classic hospital-centralized monitoring. First, HIS/LIS system was utilized in the study. To analyze the impact factors of the ADRs/ADEs caused by XSTI, a large number of information associated with ADRs/ADEs were collected. The data collected in the research were composed of general information, medication information and laboratory test data. The general information and medication information such as patients’ ID number, gender, age, height, weight, admission diagnosis, allergic history, nationality, dosage, combined administration were mainly collected by monitoring table, while the laboratory test data such as blood routine, urine routine, faecal routine, liver function, renal function, thrombus and hemostasis and other inspection results were all extracted from HIS/LIS. Besides, the missing information in some of the monitoring tables were also supplemented by the HIS system. Secondly, majority of data collection and analyses in this study was performed by clinical pharmacists. Pharmacist plays important role in rational drug applications and improvement of life quality of patients. In one hand, clinical pharmacists are familiar with the treatment process of ADR/ADEs, on the other hand, they can put more concentration on the research by comparison with clinician. Altogether, pharmacist is the best candidate for ADE surveillance. Last but not least, strict quality control method was designed in this multi-center research. Three-level quality control method used in this study has been successfully used in our previous post-marketing safety surveillance of Danhong injection. In order to strengthen quality control, third-party quality control, a contract research organization (CRO) company, was employed in this study. Because CRO companies has a large number of professional medical and pharmaceutical experts, they actively participate in many phase II or III clinical trials and undertake supervision as the third party, to guarantee the objectivity of the result in studies. Application of above working model in our study contributed greatly to improve objectivity of results and efficiency of research.
Our study has several shorter. We calculated the incidence, main types, main manifestations and severity classification of ADRs in this article, which reflect the safety of clinical use of XSTI in general, but the main influencing factors of ADRs were not studied in this article. However, all relevant data have been collected and are being analyzed. We will complete this part of study in following researches. In addition, the mechanism of the ADRs in the study is still unknown.
Conclusion
Post-marketing safety surveillance and re-evaluation of XSTI was carried out with 30,884 cases from 33 hospitals in 7 provinces. We obtained incidence rate, types, severities, as well as other information of ADRs/ADEs of XSTI. As far as we know, this research is the first study on the ADR of XSTI using large-scale hospital centralized monitoring method. The results in this study provide a high level evidence-based basis for safety of XSTI. We further founded novel research system and mode of post-marketing safety surveillance and re-evaluation of TCMIs, which also provides a method to dramatically improve rationality and safety of clinical applications of TCMIs.
Abbreviations
- ADE:
-
Adverse Drug Event
- ADR:
-
Adverse Drug Reaction
- CRO:
-
Contract Research Organization
- HIS:
-
Hospital Information System
- LIS:
-
Laboratory Information management System
- TCMIs:
-
Traditional Chinese medicine injections
- XSTI:
-
Xueshuantong injection
References
National Pharmacopoeia Committee, Chinese Pharmacopoeia. Part 1. Beijing: Chemical Industry Press; 2005: Appendix 13.
Ren D-Q, Zhang B-L. Clinical application guide of TCM injections, People’s health publishing house. China: Beijing; 2011.
Li B, Wang Y, Lu J, et al. Evaluating the effects of Danhong injection in treatment of acute ischemic stroke: study protocol for a multicenter randomized controlled trial. Trials. 2015, 9;16:561.
Liu Y, Huang Y, Zhao C, et al. Salvia miltiorrhiza injection on pulmonary heart disease: a systematic review and meta-analysis. Am J Chin Med. 2014;42(6):1315–31.
Yang H, Zhang W, Huang C, et al. A novel systems pharmacology model for herbal medicine injection: a case using Reduning injection. BMC Complement Altern Med. 2014, 4;14:430.
Luo J, Shang Q, Han M, et al. Traditional Chinese medicine injection for angina pectoris: an overview of systematic reviews. Am J Chin Med. 2014;42(1):37–59.
Fu S, Zhang J, Menniti-Ippolito F, et al. Huangqi injection (a traditional Chinese patent medicine) for chronic heart failure: a systematic review. PloS One. 2011, 6;6(5): 19604.
Guo XJ, Ye XF, Wang XX, et al. Reporting patterns of adverse drug reactions over recent years in China: analysis from publications. Expert Opin Drug Saf. 2015;14(2):191–8.
Liao X, Robinson N. Methodological approaches to developing and establishing the body of evidence on post-marketing Chinese medicine safety. Chin J Integr Med. 2013;19(7):494–7.
Wang L, Yuan Q, Marshall G, et al. Adverse drug reactions and adverse events of 33 varieties of traditional Chinese medicine injections on National Essential medicines list (2004 edition) of China: an overview on published literatures. J Evid Based Med. 2010;3(2):95–104.
Wang XM, Wang SX, Wang JX, et al. Neuroprotective effect of xueshuantong for injection (lyophilized) in transient and permanent rat cerebral ischemia model. Evid Based Complement Alternat Med. 2015;2015:134685.
Wang FJ, Wang SX, Chai LJ, et al. Xueshuantong injection (lyophilized) combined with salvianolate lyophilized injection protects against focal cerebral ischemia/reperfusion injury in rats through attenuation of oxidative stress. Acta Pharmacol Sin. 2017;36:1–14.
Zhao Y, Shi C, Huang P. Analysis of clinical use of post-marketing hospital centralized monitoring of Xiyanping injection. Zhongguo Zhong Yao Za Zhi. 2016;41(4):743–7.
Jiang JJ, Xie YM. Discussion on establishment of quality control system for intensive hospital monitoring on traditional Chinese medicine injections. Zhongguo Zhong Yao Za Zhi. 2012;37(18):2689–91.
Li X, Tang J, Meng F, et al. Study on 10 409 cases of post-marketing safety Danhong injection centralized monitoring of hospital. Zhongguo Zhong Yao Za Zhi. 2011;36(20):2783–5.
Li XL, Tang JF, Li WX, et al. Postmarketing safety surveillance and reevaluation of Danhong injection: clinical study of 30888 cases. Evid Based Complement Alternat Med. 2015;2015:610846.
Y.-Y. Wang, A.-P. Lv, and Y.-M. Xie. The key technologies of clinical re-evaluation of post-marketing traditional Chinese medicine, People’s medical publishing house, Beijing, China,2011.
Baars E-W, Jong M, Nierop AF, et al. Savelkoul, “Citrus/cydonia compositum subcutaneous injections versus nasal spray for seasonal allergic rhinitis: a randomized controlled trial on efficacy and safety,”. ISRN Allergy. 2011;2011:836051.
He GF, Dou WM. Zeng retrospective analysis on 697 cases of adverse drug reaction of Xueshuantong preparations. Chin J Pharmacoepidemiol. 2016;11(25):715–8.
Li HC, Wu QY, Fu JT, et al. Study on screening method of allergenic ingredients of TCM injections. Pharmacol Clin Chin Mater Med. 2014;01(30):139–41.
Feng WW, Zhang Y, Tang JF, et al. Combination of chemical fingerprinting with bioassay, a preferable approach for quality control of safflower injection. Anal Chim Acta. 2018,20;1003:56–63.
Zhang L, Ma L, Feng W, et al. Quality fluctuation detection of an herbal injection based on biological fingerprint combined with chemical fingerprint. Anal Bioanal Chem. 2014;406(20):5009–18.
Ren Y, Zhang P, Yan D, et al. A strategy for the detection of quality fluctuation of a Chinese herbal injection based on chemical fingerprinting combined with biological fingerprinting. J Pharm Biomed Anal. 2011;56(2):436–42.
Acknowledgments
The authors want to thank all volunteers in our research team who participated in this study. We also thank academician Boli Zhang and researcher Weiliang Wong for guidance.
Funding
This work was supported by a grant from the National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2012ZX09101201).
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due part of the data is still in analysis but are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
C-XL, TX, PZ and GG were participated in this study as clinical pharmacists and all data were collected by them and C-XL wrote the manuscript. TX, J-HZ and HZ performed the analysis of the data. XL and W-XL were major contributors in writing the manuscript. FM, G-PL, L-YL, JY were major contributors in the data quality control. X-LL and M-JZ designed this research and were corresponding authors of this article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was an observational study. No medical intervention was conducted on the observed objects. The experiment protocol was approved by the Ethical Committee of the First Affiliated Hospital of Henan University of TCM (Approval number 2014HL~ 053). In addition, this protocol has international registration in China clinical trial registration center (ChiCTR~OPC~ 14,005,718). Written informed consents were obtained from all participants.
Consent for publication
There was no individual person’s data in this research.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, C., Xu, T., Zhou, P. et al. Post-marketing safety surveillance and re-evaluation of Xueshuantong injection. BMC Complement Altern Med 18, 277 (2018). https://doi.org/10.1186/s12906-018-2329-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-018-2329-z